Abstract
Purpose
Though perianal fistulas are commonly seen in patients with Crohn’s disease, they can also be seen in patients without inflammatory bowel disease. The purpose of this study was to evaluate MR imaging differences of perianal fistulas in patients with and without Crohn’s disease.
Methods
Our retrospective search from January 2012 to December 2015 of the Radiology database for perianal fistula yielded 207 patients. Only patients with dedicated MR fistula protocol studies were included, whereas patients with previous anal surgery or anastomosis, anorectal tumors, and equivocal findings that could not be definitely assessed as a fistula were excluded. The following features were assessed: anatomic type of fistula (Parks Classification), luminal origin (hour clock position), anal verge distance, signs of acute inflammation, circumference of anus involved by inflammation, presence of rectal inflammation. and abscess.
Results
One hundred and twenty six of 207 patients met inclusion criteria. Of these, 96 (76.2%) had Crohn’s disease and 30 (23.8%) did not. The most common fistulas identified were transphincteric (38.5% of Crohn’s and 50% of non-Crohn’s) and intersphincteric (33.3% of Crohn’s and 35.4% of non-Crohn’s). An abscess was associated in 41 cases, 32 (33.3%) in the Crohn’s group and 9 (30.0%) in the non-Crohn’s group. Rectal inflammation was present in 29 patients with Crohn’s disease (29.2%) and in 2 without Crohn’s (6.7%). This finding was statistically significant (p = 0.0009).
Conclusions
Our study demonstrates that while both groups can have similar MR imaging features, accompanying rectal inflammation was more commonly seen in Crohn’s disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The true prevalence of perianal fistula is unknown, but an estimated incidence in European Union countries accounts for 1.04 to 2.32 per 10,000/ year [1], affecting predominantly young and male patients [2]. Despite being an uncommon process, it can cause a significant morbidity.
Most cases are thought to be idiopathic, but perianal fistulas may also be caused by several inflammatory conditions and events, including pelvic infection, tuberculosis, diverticulitis, trauma during childbirth, pelvic malignancy, and radiation therapy [3]. A very common association is with Crohn’s disease (CD). These cases are frequently seen in radiology practice due to their complexity and propensity for incomplete response to therapy and recurrence [4].
The treatment of perianal fistulas is primarily surgical. When a surgical approach is required, the relationship of the fistula to the sphincter complex must be assessed in order to preserve anal continence and to identify secondary tracts or abscesses that could be a potential source for recurrence [4, 5]. MRI is a well-established imaging modality for evaluating perianal fistulas due its high sensitivity and specificity. It has been demonstrated to delineate additional information that would not be clinically evident to the surgeon, which helps to reduce recurrence rates [5].
Although several studies have demonstrated the features of perianal fistulas on MRI, no large study so far has demonstrated differences on MR imaging features between Crohn’s and non-Crohn’s patients. This may not only have a treatment implication but also may assist in the clinical evaluation of a patient presenting with a perianal fistula, as this may be the first manifestation of inflammatory bowel disease. The purpose of our study is to evaluate imaging features of perianal fistulas comparing patients with and without Crohn’s disease.
Material and methods
This HIPAA compliant study was approved by Institutional Review Board (IRB), and a waiver of informed consented was obtained.
A search on our electronic database was performed to identify cases of perianal fistulas diagnosed by magnetic resonance imaging (MRI) between January 2012 and December 2015. Patients with available clinical records and dedicated MRI fistula protocol were included. Patients with prior anal resection or anastomosis, anorectal tumor, or equivocal imaging findings that could not be definitely assessed as a fistula were excluded; meanwhile, patients previously treated with Seton wires were not excluded. When more than one scan was available for the same patient, we reviewed the data from the oldest exam.
MRI scans were performed either on a 1.5-Tesla (Signa—GE Healthcare, Avanto—Siemens Healthcare) or in a 3.0-Tesla (Skyra—Siemens Healthcare) scanner. A dedicated fistula protocol was considered when at least the following sequences were available: Axial T2 fast spin-echo (FSE) with small field of view (FOV), axial STIR (short tau inversion-recovery) with small FOV, Coronal T2 FSE, Coronal STIR, oriented orthogonal and parallel to anal canal long axis and axial and coronal 3D fat saturated T1 gradient echo before and after gadolinium administration.
Imaging evaluation was performed on a PACS workstation by two radiologists, with 2 and 20 years experience in abdominal imaging in order to confirm that appropriate images were obtained, that there was a definitive perianal fistula, and to record all the features analyzed in the study. Also, all MRI reports were reviewed to check any additional information that could have been missed but the ultimate decision regarding imaging features was based on the imaging review. Fistulas were defined as a tubular structure with an internal enteric opening, usually surrounded by a linear low intensity signal on T2-weighted images, representing fibrotic tissue. The other imaging features recorded were: fistula type according to Parks classification [6] (Table 1), mucosal internal opening position, distance from the anal verge, presence of signs suggesting acute inflammation, anal circumference involved by inflammation estimated in number of hours, associated rectal inflammation, and presence and volume of abscesses.
For fistula classification, when more than one communicating tract with the same mucosal opening was identified, it was designated under a special category named “multiple branched.” The internal fistula opening in clock hours was recorded both when it could be directly visualized and when it could be inferred from the course of the fistula track in the sphincter muscles [5].
Acute inflammation was defined as the presence of high T2 signal within a fistula tract and enhancement on the post-contrast images. High T2 signal is a well-defined criteria, and the presence of enhancement has been demonstrated to correlate with disease activity in recent studies [5, 7, 8]. Number of hours of anal circumference involved by inflammation was estimated by evaluating the proportion of anal canal exhibiting high signal intensity on axial STIR images similar to an approach previously described by Plumb et al. [9]. Rectal inflammation was considered present when rectal wall stratification and diffuse mucosal enhancement was seen. Abscess was defined as a fluid collection exhibiting high T2 signal and corresponding peripheral enhancement on T1 post-contrast images.
When more than one fistula was present in a patient and there was no evident communication between them, we only recorded the primary tract, defined as the larger/dominant one or the one associated with an abscess, as these usually require a more aggressive treatment approach.
Medical records were reviewed to assess if the patient had a diagnosis of Crohn’s disease by current clinical, endoscopic, radiologic, and pathological standard criteria either before or after the MR scan was performed. We separated our cohort in two different groups, one with a diagnosis of Crohn’s disease and another without Crohn’s disease. All available data were reviewed in order to assure that a patient assigned to the non-Crohn’s group did not have a diagnosis of an inflammatory bowel disease (Crohn’s or ulcerative colitis) at any point. Imaging features of both groups were compared using χ 2 and T student test for category and numeric variables.
Results:
A search of our electronic database for perianal fistulas resulted in a total of 207 consecutive MRI performed between 2012 and 2015. Accounting for the exclusion criteria and removing multiple exams from the same patient, 126 MRI scans from 126 patients were eligible for inclusion (Fig. 1).
Of 126 patients, 96 (76.2%) had a clinical diagnosis of Crohn’s disease. The mean age was 28.6 years for Crohn’s group and 42.4 years for non-Crohn’s group. This was significantly different between the two groups (p < 0.0001). Male patients predominated in both groups, accounting for 67.7% of patients with Crohn’s disease (65/96) and 60.0% of patients without Crohn’s (18/30).
Using the Parks classification, transphincteric fistula was the most common type for both groups (37/96 = 38.5% for Crohn’s and 15/30 = 50.0% for non-Crohn’s), followed by intersphincteric (34/96 = 35.4% for Crohn’s and 10/30 = 33.3% for non-Crohn’s), multiple branched (24/96 = 25.0% for Crohn’s and 5/30 = 16.7% for non-Crohn’s) and suprasphincteric (1/96 = 1.0% for Crohn’s and 0 cases for non-Crohn’s). This distribution was not significantly different between both groups (p = 0.62). Although we noted that multiple-branched fistulas were slightly more common in patients with Crohn’s disease than in patients without Crohn’s (24/96 = 25% vs. 5/30 = 16.7% for non-Crohn’s), this finding did not reach statistical significance (p = 0.34) (Figs. 2, 3).
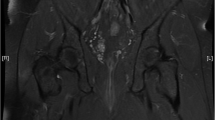
22-year-old male patient with Crohn’s disease. Axial STIR (A), axial T1 post-contrast images (B) and coronal T2-weighted images without fat saturation (C) demonstrating a multiple-branched fistula with transphincteric (white arrow) and suprasphincteric (black arrow) components and seton string. Axial T2-weighted image without fat saturation (D) shows diffuse rectal wall thickening (empty arrow)
Mean distance of the mucosal origin to the anal verge was 1.56 for Crohn’s and 1.75 for non-Crohn’s (p = 0.49). Imaging signs consistent with inflammation were identified in most patients in both groups, in 100% of Crohn’s patients and 96.7% (29/30) of the non-Crohn’s group. The number of “clock hours” of the anal circumference involved by inflammation represented a mean value of 3.3 h for the Crohn’s group and 2.4 h for the non-Crohn’s group (p = 0.05) (Figure 4).
An abscess was associated with the fistula in 32 cases of patients with Crohn’s disease (32/96 = 33.3%) and 9 cases (9/30 = 30.0%) of patients without Crohn’s (p = 0.73). The mean volume of the abscess was 1.71 and 1.50 cc3, respectively. Subcutaneous tissue inflammation was present in 79.2% (76/96) of patients with Crohn’s and 76.7% (23/30) of patients without Crohn’s (p = 0.77) (Tables 2, 3, 4).
Rectal inflammation was present in 30.2% (29/96) of cases with Crohn’s disease and in 6.7% (2/30) of cases without Crohn’s. This finding was significantly different between both groups (p = 0.009).
Discussion
Fistulas are defined as an abnormal communication between two epithelium-lined surfaces. In the case of a perianal fistula, the connection is between the mucosal layer of the anal canal and perianal skin [10].
A widely accepted theory for perianal fistulas is the “cryptoglandular hypothesis” in which an impaired drainage of the anal glands located within the intersphincteric space may initiate an infectious process, leading to a perianal abscess acutely and to a fistulous tract when incompletely drained [6]. Therefore, perianal abscess and fistulas are believed to be acute and chronic manifestations of the same disease process [4, 11, 12] and as many as 87% of patients with an acute abscess may subsequently develop a fistula.
Perianal fistulas may be caused by several inflammatory conditions and events; however, most cases are thought to be idiopathic. The association with Crohn’s disease is particularly important as perianal fistulas occur in 30–50% of CD patients at some stage during their lifetime [7]. A population-based study in 169 patients with Crohn’s disease performed in Olmsted County, Minnesota demonstrated a cumulative risk of at least one perianal fistula of 21% after 10 years [13]. In addition, perianal fistula may be the first presentation of inflammatory bowel disease. 45% of patients developed a perianal fistula before or at the time of the diagnosis of Crohn’s disease in the same study.
The symptoms and a physical examination may suggest the diagnosis of a perianal fistula; however, imaging evaluation plays an important role affecting the surgical treatment decision process and patient outcomes, particularly if an abscess or fistula is missed at the time of examination, as several studies have demonstrated [5, 14]. Several modalities have been used, such as fistulography, computed tomography, and anal endosonography, but the value of MRI has been well established and it is considered the gold standard imaging technique for perianal CD [15, 16].
Studies have demonstrated that combining an imaging modality (MRI or endoscopic ultrasound) with examination under anesthesia improves the accuracy of the initial assessment [17]. A previous study by Beets-Tan et al. [5] has demonstrated that MRI adds important information to that clinically obtained by the surgeon, and this is more evident in patients with complex fistulas and those related to Crohn’s disease.
The relationship of the primary tract to the sphincter complex is one of the main features assessed for treatment planning in order to avoid postoperative incontinence. The most well known classification for fistula-in-ano is the one proposed by Parks [6], which divides fistulas into four groups based on anatomy (Table 1). In his series, the most common fistula type was intersphincteric (45%), followed by transphincteric (30%), suprasphincteric (20%), and extrasphincteric (5%). In our series, the distribution was slightly different. Transphincteric was the most common type for both groups (and 38.5% for Crohn’s and 50.0% for non-Crohn’s), followed by intersphincteric (33.3% for non-Crohn’s and 35.4% for Crohn’s) and suprasphincteric (1/96 = 1.0% for Crohn’s and 0 case for non-Crohn’s). This is similar to that previously reported by Rosa et al. [18] in a study evaluating 844 patients surgically treated during a 30-year period, where 58.3% of cases were transphincteric fistula, being supra and extrasphincteric types, the most rare ones. We also observed that many patients presented with complex fistulas with multiple branches arising from the same mucosal origin. We created a special category, named multiple-branched fistulas, which included 25.0% of patients with Crohn’s disease and 16.7% of patients without Crohn’s disease. These more complicated fistulas seemed to be more common in patients with Crohn’s; however, this feature was not significantly different between both the groups (p = 0.62). These results are similar to those previously reported by Zbar et al. [19], in which a bifurcating fistula tract was more commonly seen in Crohn’s patients during endosonographic evaluation, presenting a high specificity for differentiating it from cryptogenic cases.
Clock hour position and anal verge distance of the mucosal original did not demonstrate any significant difference between both the groups. Most fistulas were in a low position (less than 2.0 cm above the anal verge) as was expected due to the anatomical location of anal glands and their implication on pathophysiology of perianal sepsis.
The presence of high T2signal within the fistula tract as well as post-contrast enhancement has been established to correlate with inflammatory process. These features were seen in most patients (100% of Crohn’s and 96.7% of non-Crohn’s). An explanation for such a high rate is that imaging studies are usually performed in the clinical setting of clinical disease activity or abnormal findings on physical examination, in which the presence of activity is very likely. One quantification system for inflammation was previously used by Plumb et al. [9]. described the number of hours of the anal circumference involved by inflammation. In our study, Crohn’s patients had a mean value of 3.3 h and non-Crohn’s patients 2.4 h. This almost reached statistical significance (p = 0.05).
Rectal inflammation was present in 30.2% of cases with Crohn’s disease and in 6.7% of cases without Crohn’s. This was the single finding that was significantly different between groups (p = 0.009). The colon is one of the most common locations of Crohn’s disease, so the association of perianal fistula and rectal inflammation is not surprising and likely relates to disease activity. The absence of rectal inflammation cannot be used to exclude Crohn’s due to the relapsing course of the disease; however, its presence can be highly suggestive of this condition. This may be useful when a young patient first presents with perianal fistula.
In our study, patients with perianal fistulas and Crohn’s disease were younger than those without Crohn’s disease. This was an expected finding since the onset of this multisystem disorder is most common in the second and third decades [20].
Our study has some limitations. All cases were treated in our institution, a tertiary academic center, where more complex cases are referred, leading to a potential bias and a high number of complicated fistulas, even in patients without Crohn’s disease. This may explain why no significant difference was seen in most imaging features between both groups. Also, a high proportion of our cases were associated with Crohn’s disease leading to a significantly smaller non-Crohn’s group and made it more difficult to obtain a statistical difference between some imaging findings. Several imaging scans with a non-dedicated fistula protocol that would only partially image the fistula (such as MRI enterography) were excluded, which was another source for bias.
Our conclusion is that although rectal inflammation presence might favor Crohn’s disease in a dedicated fistula MR scan, other imaging features commonly evaluated such as Park classification, mucosal opening, distance from anal verge, and activity signs cannot be used to distinguish both group, and physicians should not rely on them to suspect or exclude Crohn’s diagnosis in a patient first presenting with a perianal fistula.
References
Zanotti C, Martinez-Puente C, Pascual I, et al. (2007) An assessment of the incidence of fistula-in-ano in four countries of the European Union. Int J Colorectal Dis 22:1459–1462
Sainio P (1984) Fistula-in-ano in a defined population: incidence and epidemiological aspects. Ann Chir Gynaecol 73(4):219–224
Criado JM, del Salto LG, Rivas PF, et al. (2012) MR imaging evaluation of perianal fistulas: spectrum of imaging features. Radiographics 32:175–194
O’Malley RB, Al-Hawary MM, Kaza RK, et al. (2012) Rectal imaging: part 2, perianal fistula evaluation on Plevic MRI—what the radiologist needs to know. Am J Roentgenol 199:W43–W53
Beets-Tan RGH, Beets GL, van der Hoop AG, et al. (2001) Preoperative MR imaging of anal fistulas: does it really help the surgeon. Radiology 218:75–84
Parks AG, Gordon PH, Hardcastle JD (1976) A classification of Fistula-in-ano. Br J Surg 63:1–12
Szurowska E, Wypych J, Izycka-Swieszewska E (2007) Perianal fistulas in Crohn’s disease: MRI diagnosis and surgical planning. Abdom Imaging 32:705–718
Villa C, Pompili G, Franceschelli G, et al. (2012) Role of magnetic resonance imaging in evaluation of the activity of perianal Crohn’s disease. Eur J Radiol 81:616–622
Plumb AA, Halligan S, Bhatnagar G, Taylor SA (2015) Perianal sepsis in hematologic malignancy: MR imaging appearances and distinction from cryptoglandular infection in immunocompetent patients. Radiology 276:147–155
Vanbeckevoort D, Bielen D, Vanslembrouck R, Van Assche G (2014) Magnetic resonance imaging of perianal fistulas. Magn Reson Imaging Clin N Am 22:113–123
Halligan S, Stoker J (2006) State of the art: imaging of fistula in ano. Radiology 239(1):18–33
Sneider EB, Maykel JA (2013) Anal abscess and fistula. Gastroenterol Clin N Am 42:773–784
Schwartz DA, Loftus EV Jr, Tremaine WJ, et al. (2002) The natural history of fistulizing Crohn’s disease in Olmsted County. Minnesota. Gastroenterology 122:875–880
Morris J, Spencer JA, Ambrose NS (2000) MR imaging classification of perianal fistulas and its implications for patient management. Radiographics 20:623–635
Ong EMW, Ghazi LJ, Schwartz DA, Mortele KJ (2015) Guidelines for imaging of Crohn’s perianal fistulizing disease. Inflamm Bowel Dis 21(4):731–736
Gecse KB, Bemelman W, Kamm MA, et al. (2014) A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut 63:1381–1392
Schwartz DA, Ghazi LJ, Regueiro M, et al. (2015) Guidelines for the multidisciplinary management of Crohn’s perianal fistulas: summary statement. Inflamm Bowel Dis 21(4):723–730
Rosa G, Lolli P, Piccinelli D, Mazzola F, Bonomo S (2006) Fistula-in-ano: anatomoclinical aspects, surgical therapy and results in 844 patients. Tech Coloproctol 10:215–221
Zbar AP, Horesh N, Bucholtz V, et al. (2013) Are there specific endosonographic features in Crohn’s patients with perianal fistulae? J Crohn’s Colitis 7:490–496
Lichtenstein GR, Hanauer SB, Sandborn W (2009) J and the Practice Parameters Committee of the American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol 104:465–483
Acknowledgements
Aoife Kilcoyne would like to acknowledge the support of the Mac Erlaine Scholarship, from the Academic Radiology Research Trust, St. Vincent’s Radiology Group, Dublin, Ireland. Irai S Oliveira would like to acknowledge the support of the Instituto de Ensino e Pesquisa, from Hospital Sírio-Libanês, São Paulo, Brazil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Informed consent
This HIPAA compliant study was approved by Institutional Review Board (IRB) and a waiver of informed consented was obtained.
Rights and permissions
About this article
Cite this article
Oliveira, I.S., Kilcoyne, A., Price, M.C. et al. MRI features of perianal fistulas: is there a difference between Crohn’s and non-Crohn’s patients?. Abdom Radiol 42, 1162–1168 (2017). https://doi.org/10.1007/s00261-016-0989-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0989-z