Abstract
Iatrogenic and traumatic bile leaks are uncommon. However, given the overall increase in number of hepatobiliary surgeries and the paradigm shift toward nonoperative management of patients with liver trauma, they have become more prevalent in recent years. Imaging is essential to establishing early diagnosis and guiding treatment as the clinical signs and symptoms of bile leaks are nonspecific, and a delay in recognition of bile leaks portends a high morbidity and mortality rate. Findings suspicious for a bile leak at computed tomography or ultrasonography include free or contained peri- or intrahepatic low density fluid in the setting of recent trauma or hepatobiliary surgery. Hepatobiliary scintigraphy and magnetic resonance cholangiopancreatography (MRCP) with hepatobiliary contrast agents can be used to detect active or contained bile leak. MRCP with hepatobiliary contrast agents has the unique ability to reveal the exact location of bile leak, which often governs whether endoscopic management or surgical management is warranted. Percutaneous transhepatic cholangiography and fluoroscopy via an indwelling catheter that is placed either percutaneously or surgically are useful modalities to guide percutaneous transhepatic biliary drain placement which can provide biliary drainage and/or diversion in the setting of traumatic biliary injury. Surgical treatment of a bile duct injury with Roux-en-Y hepaticojejunostomy is warranted if definitive treatment cannot be accomplished through percutaneous or endoscopic means.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Bile duct injuries in the postoperative and posttraumatic settings are uncommon. Confounding the diagnosis of biliary tract injuries is the presence of nonbiliary fluid collections and the nonspecific clinical symptoms of the patients with bile leak. Bile duct injuries include leaks, transections, and strictures, which can lead to bilomas, biliary peritonitis, intra-abdominal abscess, cholangitis, biliary cirrhosis, portal hypertension, sepsis, and hepatic or multiple organ failure [1].
Several classification systems have been developed for iatrogenic bile duct injury. Before the advent of laparoscopy, biliary strictures were delineated by severity using the Bismuth classification, which was developed in the early 1980s [2, 3]. This classification system was intended to guide surgical repair and correlates with outcomes after treatment [3]. Secondly, the Strasberg classification from the mid-1990s is most widely used in clinical practice today [4]. It is an expansion of the Bismuth classification, and includes various types of injuries caused at laparoscopic cholecystectomy. Lastly, Stewart-Way is a more recent classification system that incorporates both the mechanism of bile duct injury as well as its anatomic location. The added utility of Stewart-Way is that this system can be used to help prevent iatrogenic bile duct injuries. This classification also differentiates between bile duct leaks and strictures, which is helpful in guiding preoperative evaluation and biliary reconstruction [1]. The use of these classifications is limited in daily clinical practice, where the most important distinction is the presence or absence of a complete bile duct transection. Additionally, none of the existing classifications take into account all therapeutic and prognostic implications [5].
Imaging plays a crucial role in the diagnosis of bile duct injury, as well as assessment of its location and severity. Imaging options are numerous and include ultrasonography (US), computed tomography (CT), magnetic resonance cholangiopancreatography (MRCP), hepatobiliary scintigraphy, percutaneous transhepatic cholangiography (PTC), and fluoroscopy with a contrast agent injected through a surgically or percutaneously placed biliary drainage catheter [6]. A noncontrast MRCP can be performed to assess the biliary tract, however, MRCP obtained with a hepatobiliary contrast agent has the unique ability to reveal the exact location of bile leak, which often governs whether endoscopic management or surgical management is warranted. A multimodality imaging approach to biliary injury is often necessary, as each modality has its own strengths and limitations.
Treatment options vary depending on the type of bile duct injury, but include endoscopic, percutaneous, and surgical interventions [6]. Optimal management of biliary injuries requires a multidisciplinary approach and is dependent on the type of injury, timing of injury detection, associated complications, condition of the patient, and local availability of an experienced hepatobiliary surgeon. Imaging is crucial for the initial diagnosis, assessment of injury extent, and preprocedural planning. Prompt recognition of bile duct injuries is crucial to avoid the potential high morbidity and mortality, impaired quality of life, and associated financial burden. Bile duct injury patients have increased lengths of stay, undergo more imaging and interventional procedures, and worse health-related quality of life scores when compared to similar patients who have undergone cholecystectomy or sustained blunt or penetrating trauma who do not have bile duct injury [5, 7–11].
In this article, the imaging features of iatrogenic and traumatic bile duct injury on multiple imaging modalities are reviewed and their treatments compared.
Initial bile duct injury patient management
Once a patient has been diagnosed with a biliary tract injury and has been stabilized with intravenous fluids, electrolyte and nutritional replenishment, and/or antibiotics, attention should be directed toward draining bilomas and abscesses, establishing biliary drainage, and obtaining complete cholangiographic characterization of the injury [2, 3, 12–14].
Iatrogenic bile leaks
Overview
There has been an increase in the number of hepatobiliary surgeries over the last few decades due to advancement in both open and laparoscopic surgical techniques. There has been a resultant increase in the number of biliary injuries, including bile leak, transection, stricture, and obstruction by surgical clips. These injuries can subsequently lead to the development of bilomas, biliary peritonitis, intra-abdominal abscesses, cholangitis, sepsis, biliary cirrhosis, portal hypertension, and hepatic or multiple organ failure. Open cholecystectomy, laparoscopic cholecystectomy, hepatic resection, hepatic transplantation, and liver biopsy are all associated with bile leaks [15, 16].
Laparoscopic cholecystectomy is the most frequently performed laparoscopic surgery worldwide, numbering over 750,000 annually in the United States alone [1]. The advantages of laparoscopic cholecystectomy over open cholecystectomy are reduced healing times, fewer wound infections, and less pain. The main disadvantage of the laparoscopic approach is the higher incidence of major bile duct injury with rates of 0.3%–0.72% compared to 0.1%–0.2% for open cholecystectomy [3, 8, 17–23]. The incidence of bile duct injury decreases with the experience of the surgeon. In the setting of laparoscopic cholecystectomy, the most frequent mechanisms of biliary leakage are attributed to slippage of the cystic duct ligature, a leak from gallbladder bed due to a deep dissection plane, a leak from an accessory bile duct such as Luschka’s ducts where there is a direct communication from the gallbladder to the right hepatic ductal system, or leak from an anomalous bile duct related to misidentification of biliary anatomy such as wrongly thinking that the common bile duct (CBD) is the cystic duct [24–26]. Delayed bile leaks are usually due to thermal or vascular injury during surgical dissection [27, 28]. Rarely, retained choledocholithiasis can result in suture breakdown by obstructing bile flow. Postoperative bile leaks are also seen in the setting of an intrahepatic position of the gallbladder, or if the gallbladder is friable due to chronic cholecystitis [7 (Fig. 1)].
A 16-year-old man after cholecystectomy presenting with abdominal pain. 2D MRCP A coronal B and axial C SSTSE images show a fluid collection in the gallbladder fossa (arrows) suspicious for a bile leak. D The patient was brought back for delayed images at 5 h, when coronal T1 fat-suppressed images showed the accumulation of excreted contrast into the peritoneal cavity, including posterior to the spleen (arrow), confirming a bile leak
Clinically significant bile leaks occur in as many as 2%–25% of patients after orthotopic liver transplantation or hepatic resection, particularly nonanatomic hepatic resections [29, 30]. After liver transplantation or hepaticojejunostomy (biliary-enteric anastomosis), biliary leaks can also occur due to injury from the removal or spontaneous migration of an external drainage tube. Posttransplant leaks can also occur at sites of biliary anastomosis [31]. Ablation of liver tumors has been associated with biliary necrosis, with subsequent biloma formation.
Anastomotic biliary strictures may complicate liver transplantation and Roux-en-Y hepaticojejunostomy. Less commonly, the strictures are the result of ischemia due to hepatic artery thrombosis or stenosis [32]. Major complications from stricture formation include cholangitis and secondary biliary cirrhosis [5, 6, 33].
The ability of surgeons to detect bile duct injuries intraoperatively is limited, as only ~25% of bile duct injuries are identified [1, 5]. Intraoperative cholangiography is not commonly performed, as it increases operative time, creates a risk of tear at the confluence of the cystic duct and CBD, and increases the cost of the procedure [34]. Thus, a surgeon must maintain a high index of suspicion for a bile duct injury postoperatively.
Clinical presentation
In the open cholecystectomy era, patients with bile duct injuries classically presented with jaundice, dilated bile ducts, and abdominal pain. In the laparoscopic era, bile duct injuries are often associated with a biliary fistula, are not jaundiced, and present with nonspecific vague clinical symptoms which can delay diagnosis and result in life-threatening illness. Conversely, patients with biliary strictures are often recognized earlier as they present more classically with jaundice, bile duct dilation, and abdominal pain [1, 35]. Biliary injuries that are not detected intraoperatively may manifest days, months, or years later [14, 36]. Intra-abdominal drains are no longer routinely placed after laparoscopic cholecystectomy because they increase both hospital stay and risk of infection. These drains did, however, allow for early identification of bile leaks [37]. Patients may present with signs or symptoms of bile leak or bile duct transection or ligation, including jaundice, biliary peritonitis, and cholangitis; however, diagnosis is frequently delayed due to nonspecific symptoms such as abdominal pain, malaise, nausea, and anorexia which may be attributed to other more frequent postoperative complications [14, 35, 36, 38, 39]. Late manifestations include recurrent cholangitis and secondary biliary cirrhosis due to strictures [14, 37].
Imaging findings and treatment
Imaging is crucial to establish a diagnosis of bile duct injury, delineate its extent, and help in preprocedural planning as incomplete cholangiographic characterization is associated with poor surgical outcomes [40]. Imaging options include US, CT, hepatobiliary scintigraphy, MRCP, ERCP, PTC, and fluoroscopy with contrast injected via an indwelling drain that was placed intraoperatively or percutaneously for a bile leak. A stepwise multimodality approach is usually warranted as each of these imaging options offers different advantages and limitations.
US
US is also considered a first-line imaging modality that can raise concern for a biliary injury. It has the ability to depict fluid collections, biliary duct dilation, and arterial injuries. However, US has a lower sensitivity for these findings and can lead to a diagnostic delay [35, 41].
CT
CT serves as a first-line imaging modality which can suggest bile leaks with a number of nonspecific imaging findings. CT can depict perihepatic or intrahepatic focal fluid collections, pericholecystic fluid, ascites, a collapsed gallbladder, biliary obstruction, and sequela of long-term bile duct obstruction such as lobar atrophy or biliary cirrhosis [35, 42]. Interestingly, bile ducts are usually not dilated after laparoscopic bile duct injury. Since many of these findings can be seen in the postoperative setting, a high index of suspicion must be maintained for any fluid collection that is encountered. In addition, hepatic arterial injuries are seen in patients with laparoscopic-related bile duct injury, as the right hepatic artery usually travels posterior to the CBD. In a study of 77,604 patients who underwent laparoscopic cholecystectomy, the incidence of vascular injury was 0.25% [43]. Three patterns of arterial injury predominate: transection, occlusion by clips, and thrombosis of the vessel. Combined biliary tract and hepatic artery injury can be complicated by bile duct necrosis, biliary stricture, biliary cirrhosis, and liver necrosis.
Thus, the integrity of the hepatic artery, especially the right hepatic artery, should be examined with Doppler US or CT angiography (CTA) prior to revision of an iatrogenic bile duct injury. Conventional angiography is only necessary if arterial occlusion is suspected by US or CTA. Arterial reconstruction should be pursued within 4 days of injury to circumvent hepatic necrosis, biliary-enteric anastomotic leakage, and delayed biliary stricture [44].
At our institution, patients undergo CT scanning using a 64-detector CT scanner (LightSpeed VCT; GE Medical Systems, Milwaukee, WI) with the following acquisition parameters: reconstruction thickness, 1.25 and 3.75 mm; noise index, 24; pitch, 1:0.984; gantry rotation time, 0.5 s. All patients with suspected bile leak and normal renal function receive a bolus of 100 mL of intravenous contrast (iopamidol, 370 mg iodine/mL, ISOVUE; Bracco Diagnostics, Monroe Township, NJ) at a rate of 3–5 mL/s with use of a power injector through an 18- or 20-gage cannula in an antecubital vein. CT examinations are acquired from the lung bases through the pubic symphysis after a 70-s delay (portal venous phase). In all cases, reformations in coronal and sagittal planes were provided (2.5 mm thickness × 2.5 mm intervals).
Hepatobiliary scintigraphy
Hepatobiliary scintigraphy demonstrates physiologic biliary excretion and has the ability to demonstrate active bile leaks, both free intraperitoneal as well as contained intrahepatic leaks. The progressive accumulation of radiotracer in the abdominal cavity that does not conform to the morphologic appearance of bowel is diagnostic of bile leak [45]. Cholescintigraphy is efficacious not only in detection of bile leaks, but also in determining the severity and rapidity of the leak with use of dynamic imaging. A substantial limitation of hepatobiliary scintigraphy has been its relatively poor anatomic definition and spatial resolution, which, in turn, necessitates a secondary diagnostic evaluation (such as MRCP or ERCP) to define the anatomy, localize the site of the leakage, and decide whether endoscopic or surgical management is appropriate. Another diagnostic pitfall can occur when trying to distinguish between activity from bowel and a contained bile leak [45].
To enhance diagnostic and anatomic accuracy in detection of biliary leaks, single-photon emission tomography–computed tomography (SPECT/CT) has been used in combination with dynamic and planar hepatobiliary scintigraphic imaging. In a study of patients with findings of bile leaks on planar hepatobiliary imaging, concurrent SPECT/CT imaging was found to provide additional useful information in 76%, which includes revealing the exact site and extent of bile collections and leaks, which in turn influenced immediate management of the patients [46]. In addition, in patients with suspected bile leaks, the diagnostic utility of hybrid SPECT/CT with scintigraphy is reported to be improved when compared to planar scintigraphic imaging alone, by providing not only anatomic detail, but also by decreasing the rate of false-positive diagnoses [47, 48].
MRCP
A noncontrast MRCP can be performed to assess the biliary tract (Table 1). MRCP with a hepatobiliary contrast agent is a powerful alternative to hepatobiliary scintigraphy in the dynamic evaluation of the biliary system as it has the unique ability to reveal the exact location of a bile leak [49]. There are two types of hepatobiliary-specific agents: hepatocyte-selective (mangafodipir trisodium) and combined (extracellular and hepatocyte-selective) agents (gadobenate dimeglumine and gadoxetic acid). Mangafodipir was removed from the US market in 2006, however, much of the experience with hepatobiliary-specific agents has been with this contrast agent and it shares delayed phase imaging characteristics with the other hepatobiliary-specific agents [50]. Gadolinium-based hepatobiliary contrast agents such as gadoxetate disodium (Eovist) have the ability to provide dynamic biliary imaging during the hepatocyte phase due to their excretion through both the kidneys (50%) and liver (50%). As a result, hepatobiliary contrast agents may be used to increase diagnostic accuracy in leak site localization when compared to traditional T2-weighted MRCP. These contrast agents shorten the T1 relaxation time of bile, resulting in high signal intensity of bile at T1-weighted imaging without sacrificing information about the biliary tract which is gleaned from the arterial, portal venous, and equilibrium phases or during heavily T2-weighted 2D and 3D images [51, 52]. The use of gadoxetate disodium for biliary applications is an off-label indication, since the United States Food and Drug Administration has not approved this agent for MRCP yet. Gadoxetate disodium has been associated with transient severe motion (TSM) in the arterial phase and efforts are being made to compensate for this limitation, for example, using multiple arterial phase acquisition [53].
In patients with normal liver function, the hepatobiliary phase is imaged approximately 20 min after the administration of gadoxetate disodium contrast, using fat-saturated 3D gradient-echo T1-weighted images. MR imaging enhanced with hepatobiliary contrast agents allows for improved characterization of biliary anatomy by providing higher signal-to-noise ratio in the bile duct than can be achieved with traditional T2-weighted MR imaging [54, 55]. By providing functional information as well as anatomic delineation of the biliary tract, hepatobiliary contrast agents may identify the exact site of a bile leak. This obviates the need for traditional biliary imaging modalities such as scintigraphy, which has less spatial resolution and anatomic detail than MRCP and ERCP. ERCP is somewhat limited in that it does not allow visualization of the biliary tract proximal to the site of injury or aberrant right hepatic ducts. Delayed imaging in the hepatobiliary phase may demonstrate direct evidence of bile leak with extravasation of contrast on T1W images within the liver, in the perihepatic region, or into the peritoneal cavity. Fat-saturated 3D gradient-echo T1-weighted images can be acquired with delays longer than 20 min if necessary to troubleshoot a suspected bile leak. A prospective study in 34 postlaparoscopic cholecystectomy patients reported a 95% sensitivity and 100% specificity of MR cholangiograms with hepatobiliary contrast agent mangafodipir trisodium in detecting bile leaks [15] (Figs. 2, 3, 4).
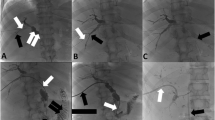
A 32-year-old woman status-postcholecystectomy presents with abdominal pain 1 month later and a bile leak. A Axial single-shot turbo spin echo (SSTSE) image shows a complex fluid collection in the gallbladder fossa (arrow). MRCP with gadoxetate disodium was performed. Delayed 20 min postcontrast coronal B, C, and axial D images show direct leakage of contrast from the cystic duct into the fluid collection (arrows). E and F Percutaneous transhepatic cholangiogram (PTC) was performed, which confirmed the leakage of contrast from the remnant cystic duct (arrows). CD, cystic duct; CBD, common bile duct; CHD, common hepatic duct
A 46-year-old woman with a history of hepaticojejunostomy presents with abdominal pain and hyperbilirubinemia. A Postcontrast coronal delayed phase image following the administration of a hepatobiliary contrast agent demonstrates obstruction of the left intrahepatic duct (dashed arrow). On axial B and coronal C delayed phase images of the right hepatic duct is shown to be patent (arrows). D 3D MRCP shows independent anastomoses of the right and left hepatic ducts (arrow). E The patient subsequently underwent drainage of the left system via percutaneous transhepatic cholangiography
One unusual potential complication of a bile leak is leakage of contrast into the pleural cavity which is diagnostic of thoracobiliary fistula [56]. Contrast pooling within a subhepatic, intrahepatic, or perihepatic fluid collection signifies a direct communication with the biliary tract and the presence of a biloma [57]. Finally, secondary signs of biliary injury on MR are similar to those on CT.
Disadvantages of hepatobiliary contrast-agent-enhanced MRCP include relative cost, diminished and variable filling of bile ducts if there is reduced hepatic function, and potential interference with T2-weighted images secondary to T2-shortening effect of concentrated contrast material within the biliary system [58]. The latter pitfall may be circumvented by acquiring conventional T2-weighted MRCP images prior to biliary excretion of contrast [54]. Lastly, there is the possibility that the exact site of biliary duct disruption may be obscured by a rapidly accumulating collection of hepatobiliary contrast-enhanced bile in the setting of brisk leakage [55].
ERCP
Endoscopic retrograde cholangiopancreatography (ERCP) is a relatively invasive procedure that has the dual capability of diagnosis and simultaneous treatment when nonoperative management of biliary injuries is pursued. ERCP allows for the placement of biliary stents and drainage catheters, which are standard treatment of CBD stenoses and leaks from the cystic duct stump or small peripheral ducts which require percutaneous drainage [59]. Biliary stent placement with or without sphincterotomy works by diminishing the pressure gradient between the bile duct and the duodenum, thus facilitating preferential bile flow into the duodenum, allowing for healing of the duct leak. The limitations of ERCP include its inability to assess the biliary tract proximal to a major duct transection or ligation and its limited utility after surgical biliary-enteric anastomosis [60]. Other unique pitfalls of ERCP in the postoperative setting include nonvisualization of transected or ligated ducts that are not connected to the central biliary tract, such as an aberrant right hepatic duct that may be the source of a leak. Another limitation is when there is proximity of a percutaneous drain to the leak site, which may divert contrast away from the leak, potentially producing false-negative results [49]. Finally, a substantial minority of patients develop pancreatitis after ERCP, and there is also the potential complication of procedural-related hemorrhage [52].
PTC
PTC is chosen when an intervention such as percutaneous transhepatic biliary drain (PTBD) placement is required to decompress an obstructed biliary system and control bile leakage. PTC is superior to ERCP for the evaluation of proximal bile duct injuries, common duct ligation, or transection. It is also superior for the evaluation of an aberrant right hepatic bile duct that may be ligated or transected [5, 19, 41, 60–62]. PTC is an invasive procedure with a major complication rate of 2%, and thus it is not used for routine diagnosis of bile duct injuries that may be diagnosed with noninvasive modalities [63].
Fluoroscopy
Fluoroscopy performed during the injection of contrast medium through an indwelling drain for bile drainage, placed either surgically or percutaneously, may opacify the bile ducts via the leakage site. This can facilitate PTBD placement, which can be difficult due to the lack of dilated bile ducts. This imaging modality should not be used in patients who are infected due to the risk of causing systemic sepsis, and it is most likely to be successful in the setting of a small biloma that has undergone sufficient drainage such that the biloma cavity has collapsed around the catheter [19, 62].
Treatment
The management of bile duct injuries requires a skilled multidisciplinary team including interventional radiology, gastroenterology, and hepatobiliary surgery; therefore, referral to a tertiary center or transplant/quaternary center is recommended. Percutaneous and endoscopic interventions provide definitive treatment for several types of bile duct injuries. They can also serve as adjuncts to definitive surgical repair, and may be the only treatment option in patients who are poor surgical candidates [6]. There is no role to our knowledge for exploratory laparotomy in the setting of suspected biliary injury, as it is associated with increased morbidity and the site of biliary injury can be easily overlooked [64].
Cystic duct leaks can be effectively managed in the vast majority of patients with CBD stenting for 4–8 weeks via ERCP, either with or without sphincterotomy. Success rates of this approach exceed 90%. Biliary injuries that involve damage to, but not transection of, the right sectoral bile duct can be managed nonoperatively with drainage and stenting via ERCP or PTC [37, 60, 65–67]. Fluid collections that are suspected to be bilomas should be drained promptly, as any delay can lead to serious complications including abscess, cholangitis, and sepsis [35].
Complete ductal ligation or transection, proximal duct injury and transection or ligation of an aberrant right hepatic bile duct, usually requires PTC with PTBD placement for biliary decompression and/or diversion [19, 41, 60–62]. Bile leaks from small peripheral ducts such as the ducts of Luschka can be definitively treated with percutaneous drainage and PTBD placement or endoscopic CBD stent placement to divert bile flow away from the leakage site [19]. Initial external drain placement for 2–4 weeks with concomitant antibiotic therapy should be done prior to internalization of the catheter. In patients with complete transection or ligation of the CBD where passage of a catheter is not feasible, or when there is a major bile leak, hepaticojejunostomy is usually necessary [37].
Biliary tract surgery is responsible for approximately 95% of biliary strictures [68]. The management of strictures is initially focused on reestablishing biliary drainage to relieve obstructive jaundice and/or cholangitis [19, 69]. The first-line treatment in these patients is endoscopic stent placement and balloon dilation, with success rates ranging between 27%–89% [70]. Percutaneous treatment of proximal bile duct strictures or those that develop after surgical reconstruction can be accomplished. However, there is no consensus to our knowledge as to the technical aspects of balloon dilation or the best use of stents [71]. Long-term ductal patency after percutaneous treatment alone is variable, ranging from 33% to 90% [70, 71]. Hepaticojejunostomy for postcholecystectomy benign bile duct strictures offers the best long-term results. However, recurrence of the stricture is a known complication after the procedure, with 68% recurring within 3 years and 80% recurring within 5 years [72].
Major complications can occur with percutaneous biliary procedures including sepsis, cholangitis, bile leakage, venous and arterial hemobilia, hemoperitoneum, subcapsular liver hematoma, pleural complications, and death. Of note, manipulation of bile duct catheters in patients with cholangitis should be avoided to reduce the risk of sepsis [73, 74].
Surgical treatment of a bile duct injury is warranted if definitive treatment cannot be accomplished through percutaneous or endoscopic means. Roux-en-Y hepaticojejunostomy is the treatment of choice for most major bile duct injuries, with long-term patency rates of more than 90% if performed by an experienced hepatobiliary surgeon [1, 5, 69, 75]. Surgical experience is crucial, as the long-term patency rate drops to only 17%–30% if the primary surgeon who caused the biliary injury performs the reconstruction. The morbidity and mortality rates of Roux-en-Y hepaticojejunostomy are reportedly 38%–47% and 2%–9%, respectively, in the hands of an experienced biliary surgeon and are higher in the hands of the primary surgeon [5, 8, 75]. Thus, the core components of successful biliary reconstruction are the eradication of intra-abdominal infection, optimization of the patient’s nutritional status, complete preoperative characterization of the bile duct in injury by cholangiography, and availability of an experienced hepatobiliary surgeon [76].
The most common complication of Roux-en-Y hepaticojejunostomy is anastomotic stricture, occurring 10%–19% of the time and usually within 2 years of the procedure [75]. If there is sepsis or organ failure, the possibility of performing a successful biliary reconstruction is compromised and surgical delay is warranted [37].
Blunt and penetrating trauma to the biliary tract
Overview
In the setting of hepatic trauma, biliary injury is seen with a frequency of 0.5%–21% depending on the criteria and methods used to detect bile leaks [77–81]. Since the mid-1980s, there has been a paradigm shift in the treatment of patients who have sustained hepatic trauma from definitive surgical treatment to nonoperative management of hemodynamically stable patients and damage control surgery in hemodynamically unstable patients [82–84]. Increased survival rates of these patients, due to improvements in transport to trauma centers and ICU care, has made bile leaks a more common complication [77, 80, 82, 85–87]. Data suggest that patients with posttraumatic bile leaks have longer hospitalizations, undergo more imaging examinations and therapeutic procedures, and incur higher hospital charges [86, 88, 89]. Conversely, early diagnosis of bile leaks after injury has been shown to result in a shorter hospital length of stay with imaging playing an integral role in decreasing morbidity and mortality [87] (Fig. 5).
A 22-year-old man following a gunshot wound. A Coronal CT demonstrates a bullet tract through the right chest passing through the liver (arrow). B Hepatobiliary scintigraphy scan 1 week later shows an intrahepatic bile leak (arrow) with patent cystic duct and CBD. C A follow-up CT performed 6 days later shows a hypodense subcapsular fluid collection (arrow). D The collection was drained via a percutaneous catheter
Biliary tract injuries are associated with multiple organ injuries, most commonly liver (91%), spleen (54%), and duodenum (54%) [90, 91]. Biliary injuries can be intrahepatic, extrahepatic, or a combination of both. It is unclear to our knowledge if the location of the bile leak is predictive of treatment outcome [81] (Fig. 6).
An 18-year-old man with a stab wound to the left upper quadrant. A Axial and B coronal CT images show a left hemothorax and small liver laceration with active extravasation of intravenous contrast into the left lobe of the liver (arrows). C–E The patient underwent resection of the left lateral lobe of the liver (arrows). Due to increasing abdominal distention and ascites, a hepatobiliary scintigraphy scan was performed E, which showed a small intrahepatic biloma (arrow)
Due to the protective effect of the liver, isolated gallbladder injury is rare, with a reported incidence in 2%–3% of blunt trauma patients undergoing laparotomy [90, 92]. Risk of gallbladder injury increases in the setting of blunt trauma in the presence of a distended preprandial gallbladder and in the presence of elevated serum alcohol, as the latter increases the sphincter of Oddi constriction resulting in gallbladder distention [92]. The most commonly reported cause of traumatic injury to the gallbladder is motor vehicle collision (Fig. 7).
A 31-year-old man with abdominal pain after a motor vehicle collision. A Axial CT images demonstrate a liver laceration extending from the capsule to the hilum (arrow), with surrounding free fluid seen on B axial and C coronal images (arrows). There was an associated splenic laceration (not shown). D Hepatobiliary scintigraphy scan showed a bile leak with free intraperitoneal spillage of radiotracer (arrows)
Injury to the extrahepatic biliary ducts frequently occurs at sites of anatomic fixation, such as the intrapancreatic portion of the CBD. Mechanisms of extrahepatic bile duct injury include acute deceleration and traumatic elevation of the liver with stretching and trauma of the CBD [37, 90]. Injuries to the intrahepatic bile ducts are seen in patients with parenchymal liver injury. CT imaging findings, including the American Association for the Surgery of Trauma (AAST) liver injury grade and location of the liver laceration, can be used to predict which patients are at risk for the development of bile leaks, whereas the presence of free fluid is a nonspecific finding. Specifically, bile leaks on hepatobiliary scintigraphy have been shown to have higher AAST grade injuries as well as more central lacerations [93]. A spectrum of AAST liver laceration grades is provided for review in Fig. 8.
Examples of AAST liver injury grading on CT. A A 17-year-old male with AAST grade I liver laceration after an MVC (arrow). B A 29-year-old man with multiple gunshot injuries and an AAST grade II liver laceration (arrow). C A 35-year-old man with a stab wound to the right flank and a grade III AAST liver laceration (arrow). D A 19-year-old man s/p GSW and AAST grade IV liver laceration (arrow). E A 24-year-old woman with AAST grade V liver laceration after her motor vehicle struck a tree (arrows)
Clinical presentation
Biliary complications, such as biloma and bile peritonitis, can present days to weeks after initial trauma, often with nonspecific progressive symptoms, including vague abdominal pain, malaise, nausea, vomiting, and anorexia. Rarely, patients will present with peritoneal signs, indicating an acute abdomen. These symptoms can all be masked or misinterpreted given the multiple organ injuries that are often present in blunt trauma patients. Since bile is sterile and is absorbed by the peritoneum, some patients may not experience any symptoms for weeks after initial trauma, until the bile becomes superinfected, at which time these patients may present with the systemic inflammatory response syndrome (SIRS) and respiratory distress [94]. Laboratory findings may demonstrate rising or persistently elevated serum bilirubin, and may be accompanied by jaundice.
Imaging findings
US
US plays a limited role in the initial evaluation of bile leak-related injury, especially as it relates to biliary injury, and is better suited for follow-up or assessment of known biliary injury complications such as bilomas. US has been advocated by some in the surgical literature as a quick and convenient imaging modality for routine follow-up assessment in nonoperative management of hepatic trauma to detect or confirm the absence of bile leak-related complications [45]. Possible findings in the setting of biliary injury may include gallbladder wall thickening, collapsed gallbladder in the setting of perforation, perihepatic and intrahepatic fluid collections, and ascites.
CT
Findings associated with posttraumatic biliary injury are subtle and often nonspecific on CT, which is routinely used as first-line imaging modality in the setting of trauma. CT findings of gallbladder injury may include collapsed gallbladder indicative of perforation, pericholecystic fluid, gallbladder wall thickening, and discontinuous gallbladder wall enhancement [90]. Displacement of the gallbladder from its fossa indicates avulsion, which can be complete or partial. Hyperdense layering fluid within the gallbladder lumen may represent intraluminal hemorrhage, while hyperdensity within the gallbladder wall is indicative of mural injury. In cases of liver laceration, injury extension to the gallbladder fossa should raise concern for gallbladder injury (Fig. 9). Delayed imaging obtained as part of the trauma CT may be helpful in the setting of suspected gallbladder injury, potentially revealing increasing amounts of hyperdense intraluminal or extraluminal fluid or contrast (Fig. 10). However, caution should be taken to differentiate these findings from imaging pitfalls such as vicarious contrast excretion or hyperdense bile [7, 89].
A 36-year-old man status-poststab wound to the right upper abdomen. A Sagittal CT image of the abdomen and pelvis reveal an AAST grade 3 liver laceration extending to the gallbladder wall (arrow). B Coronal CT image demonstrates dependent hyperattenuating material within the gallbladder lumen (arrow), concerning for hemorrhage. At surgery, the patient underwent cholecystectomy for laceration of the posterior wall of the gallbladder with intraluminal clot
A 31-year-old man who sustained a roll-over motor vehicle collision. Sagittal CT images of the abdomen and pelvis in the A portal venous and B 5-min delayed phase demonstrate a focus of hyperattenuating material (arrows) within the gallbladder lumen which increases in size on delayed phase imaging, raising concern for active intraluminal hemorrhage
CT findings associated with biliary duct injury are even less specific. Liver lacerations, focal perihepatic or intrahepatic fluid collections, and ascites may be the only indicators of bile duct injury. Therefore, a high level of suspicion is required in the presence of these findings. Suspicion should increase when there are other organ injuries, particularly the spleen and duodenum. In these cases, follow-up imaging of the biliary tract with hepatobiliary scintigraphy or MRCP with hepatobiliary agents plays an integral role in the setting of potential biliary leaks as there may be delayed complications of liver injury. For example, progressive growth of a well-circumscribed, low-attenuation perihepatic, or intraparenchymal fluid collection on CT suggests a biloma. Similarly, persistent or increasing low-attenuation intraperitoneal fluid in the setting of recent trauma is concerning for bile leakage. Finally, additional findings of peritoneal thickening and hyper-enhancement are suggestive of bile peritonitis [93] (Fig. 11).
A 22-year-old man status-postgunshot wound to the abdomen. A Axial and B coronal CT images of the abdomen and pelvis show a large laceration involving the right lobe of the liver (arrows) with hemorrhage tracking into the pelvis (arrow). C Hepatobiliary scintigraphy scan performed 5 days later shows a large photopenic defect (arrow) with D multiple intrahepatic bilomas (arrows)
The CT scan protocol used at our institution in the setting of blunt and penetrating trauma is as previously described with two caveats. Per our institution’s protocol, patients who have sustained blunt trauma do not receive either oral or rectal contrast. Delayed images through the abdomen and pelvis were acquired at 5–10 min in select cases when an abnormality was detected on the portal venous phase by a radiologist who reviewed the images in real-time at the CT scanner while the patient is still on the table.
Hepatobiliary scintigraphy
As mentioned previously hepatobiliary scintigraphy provides both anatomic and physiologic information regarding bile leaks and can be used to distinguish between both free intraperitoneal and contained intrahepatic leaks. Its main limitation is that it lacks the spatial resolution needed for the accurate identification of the exact sites of bile leaks, information which governs treatment decisions; thus evaluation with additional modalities such as MRCP and ERCP is needed (Fig. 12).
A 19-year-old man with a gunshot wound to the right upper quadrant. A Axial CT shows a laceration traversing the liver (arrow). B Coronal reformat shows the bullet tract (arrow). C, D A hepatobiliary scintigraphy scan performed 5 days later shows a contained bile leak along the bare area of the liver (arrows)
MRCP
MR cholangiopancreatography (MRCP) with hepatocellular contrast agents provides exquisite anatomic detail as well as functional information and has the added advantage of potentially revealing the exact site of bile leak and permitting safe and accurate distinction between fluid of biliary and nonbiliary origin. MRCP with hepatocyte-selective agents can be performed as the initial imaging examination, as it provides the diagnosis and can guide subsequent management, whether endoscopic, percutaneous, or surgical [15] (Fig. 13).
A 69-year-old man with abdominal pain 3 weeks after laparoscopic cholecystectomy. A Axial and B sagittal CT images show a fluid collection in the gallbladder fossa with a small amount of air (arrows). Bile leak was suspected clinically and MRCP was performed with gadobenate dimeglumine. C Coronal T2 fat-suppressed images show a thickened proximal duodenum (arrow). D Radial 2D MRCP images confirm a fluid collection in the gallbladder fossa (arrow), and E 3D MRCP images show a defect in the duodenal wall directly communicating with the fluid collection (arrow). Duodenal perforations are extremely rare complications of laparoscopic cholecystectomy, but should be suspected by the presence of air 3 weeks postoperatively. Such injuries require intervention and should not be mistaken for bile leak
ERCP
ERCP has both diagnostic and therapeutic capabilities. The exact location of biliary disruption may be identified with ERCP. Subsequently, treatment with stenting with or without sphincterotomy may be utilized when nonoperative management is desired. Biliary stenting is especially useful in the setting of injury to the major branches of the extrahepatic bile ducts such as the CBD or the common hepatic duct, as sphincterotomy alone is unlikely to result in healing [81]. The stent may bypass the site of injury, allowing for healing, as well as prevent development of posttraumatic stricture formation at the site of injury [86].
PTC, fluoroscopy, and PTBD
PTC and fluoroscopy via an indwelling catheter, placed either percutaneously or surgically, are useful modalities to guide PTBD placement which can provide biliary drainage and/or diversion in the setting of traumatic biliary injury. As mentioned previously, PTC has the advantage of demonstrating the proximal biliary tract, CBD, and aberrant right hepatic duct [5, 19, 41, 59, 61, 62] which is a distinct advantage over ERCP.
There is a lack of consensus, to our knowledge, both in the literature and practice for an algorithmic approach to the diagnosis of posttraumatic bile leaks. Decisions are often based on the extent of the biliary injury, associated organ injuries, and local expertise [7, 81]. Hemodynamically stable patients who have sustained blunt trauma often undergo CT scanning at the time of admission to delineate their injuries and triage their management. If there is a low suspicion for a bile leak and anatomic detail of the biliary tract is not necessary, hepatobiliary scintigraphy is appropriate for excluding the presence of a bile leak. At our institution, our surgery colleagues routinely obtain hepatobiliary scintigraphy in these cases 3–4 days after traumatic liver injury. Conversely, if there is a high clinical suspicion for biliary injury such as rising serum albumin levels, increasing ascites, enlarging perihepatic fluid collections, or central liver lacerations, MRCP with hepatobiliary contrast material is obtained to provide both anatomic and functional information that guides treatment. Clearly, a multimodality approach is often warranted to care for these complex patients.
Treatment
The traditional management of bile duct injuries was hepaticojejunostomy, but surgery can be difficult to perform if there are adhesions, inflammation, damaged liver parenchyma, or if the patient is a poor surgical candidate [81]. The literature has shown promising results with endoscopic management which suggest that ERCP should be considered as first-line therapy for traumatic bile leaks [81, 95]. The goal of endoscopic treatment is to decrease the pressure gradient within the biliary tract to allow for healing. Several studies have demonstrated the efficacy of plastic stent placement; however, there is debate as to whether concomitant sphincterotomy is also necessary [79, 81, 86, 96, 97]. It is unlikely that sphincterotomy alone would allow a major leak to heal. Another consideration with sphincterotomy is that it carries a short-term risk of pancreatitis and a long-term risk of stricture, especially in young patients [76]. The optimal duration of biliary stenting remains to be elucidated, however, it varies from 3–16 weeks in prior reports [77, 81]. The success rate of endoscopic treatment of biliary injuries ranges from 90% to 100% in patients with incomplete circumferential injury, regardless of whether it was due to iatrogenic or traumatic injury [77, 86, 96, 97]. Importantly, the severity and site of biliary injury does not preclude endotherapy as a management option for patients [81]. In the case of complete circumferential transection of a bile duct, Roux-en-Y hepaticojejunostomy is usually warranted.
Conclusions
Although biliary injuries are a relatively rare entity, they have become more prevalent due to an overall increased number of hepatobiliary surgeries and a trend toward nonoperative management of patients with blunt hepatic trauma. The clinical signs and symptoms of biliary injury are nonspecific, thus imaging plays a critical role in their detection and treatment triage. At CT or US, free or contained peri-or intrahepatic low density fluid in the setting of recent hepatobiliary surgery or trauma should raise suspicion for biliary injury and not just assumed to be the more commonly encountered postoperative or posttraumatic collections, seromas, and hematomas. Hepatobiliary scintigraphy and MRCP with hepatobiliary contrast agents can be used to detect active or contained bile leak. MRCP with hepatobiliary contrast agents has the added advantage of revealing the exact location of bile leak given its exquisite anatomic detail, which in turn can affect the choice of treatment. ERCP can play both a diagnostic and concomitant therapeutic role in some cases; however, its success depends on the location and extent of biliary disruption, which are better evaluated on imaging. PTC and fluoroscopy via an indwelling catheter placed endoscopically or surgically allow for PTBD placement, which can provide decompression and/or diversion of bile leaks. Surgical treatment of a bile duct injury with Roux-en-Y hepaticojejunostomy is warranted if definitive treatment cannot be accomplished through percutaneous or endoscopic means. The successful management of bile duct injury depends on their prompt recognition, characterization of the type and extent of injury, control of inflammation/infection, and the availability of a hepatobiliary surgeon.
References
Stewart L (2014) Iatrogenic biliary injuries. Surg Clin North Am 94:297–310
Bismuth H (1982) Postoperative strictures of bile ducts. In: Blumgart LH (ed) The biliary tract, 5th edn. New York: Churchill-Livingstone, pp 209–218
Bismuth H, Majno PE (2001) Biliary strictures: classification based on the principles of surgical treatment. World J Surg 25:1241–1244
Strasberg SM, Hertl M, Soper NJ (1995) An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 180:101–125
Lau WY, Lai EC, Lau SH (2010) Management of bile duct injury after laparoscopic cholecystectomy; a review. ANZ J Surg 80:75–81
Thompson CM, Saad NE, Quazi RR, et al. (2013) Management of bile duct injuries: role of the interventional radiologist. RadioGraphics 33:117–134
Melamud K, LeBedis CA, Anderson SW, et al. (2014) Biliary imaging: multimodality approach to imaging of biliary injuries and their complications. RadioGraphics 34:613–623
Flum DR, Cheadle A, Prela C, et al. (2003) Bile duct injury during cholecystectomy and survival in Medicare beneficiaries. JAMA 290:2168–2173
de Reuver PR, Rauws EA, Bruno MJ, et al. (2007) Survival in bile duct injury patients after laparoscopic cholecystectomy: a multidisciplinary approach of gastroenterologists, radiologists and surgeons. Surgery 142:1–9
Moore DE, Feurer ID, Holzman MD, et al. (2004) Long-term detrimental effect of bile duct injury on health-related quality of life. Arch Surg 139:476–481
Melton GB, Lillemoe KD, Cameron JL, et al. (2002) Major bile duct injuries associated with laparoscopic cholecystectomy: effect of surgical repair on quality of life. Ann Surg 235:888–896
Heyland DK, Novak F, Drover JW, et al. (2001) Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 286:944–953
Kudsk KA (2006) Immunonutrition in surgery and critical care. Ann Rev Nutr 26:463–479
Connor S, Garden OJ (2006) Bile duct injury in the era of laparoscopic cholecystectomy. Br J Surg 93:158–168
Aduna M, Larena JA, Martin D, et al. (2005) Bile duct leaks after laparoscopic cholecystectomy: value of contrast-enhanced MRCP. Abdom Imaging 30:480–487
Zimmitti G, Roses RE, Andreou A, et al. (2013) Greater complexity of liver surgery is not associated with an increased incidence of liver-related complications except for bile leak: an experience with 2,628 consecutive resections. J Gastrointest Surg 17:57–64 (discussion p 64–55)
MacFayden BV Jr, Vecchio R, Ricardo AE, et al. (1998) Bile duct injury during cholecystectomy: the United States experience. Surg Endosc 12:315–321
Nuzzo G, Giuliante F, Giovannini I, et al. (2008) Advantages of multidisciplinary management of bile duct injuries occurring during cholecystectomy. Am J Surg 195:763–769
Saad N, Darcy M (2008) Iatrogenic bile duct injury during laparoscopic cholecystectomy. Tech Vasc Interv Radiol 11:102–110
Roslyn JJ, Binns GS, Hughes EF, et al. (1993) Open cholecystectomy. A contemporary analysis of 42,474 patients. Ann Surg 218:129–137
Deizel DJ, Millikan KW, Economou SG, et al. (1993) Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg 165:9–14
Vecchio R, MacFayden BV, Latteri S (1998) Laparoscopic cholecystectomy: an analysis on 114,005 cases of United States series. Int Surg 83:215–219
Tantia O, Jain M, Khanna S, et al. (2008) Iatrogenic biliary injury: 13,305 cholecystectomies experienced by a single surgical team over more than 13 years. Surg Endosc 22:1077–1086
Kapoor V, Baron RL, Peterson MS (2004) Bile leaks after surgery. Am J Roentgenol 182:451–458
Hoeffel C, Azizi L, Lewin M, et al. (2006) Normal and pathologic features of the postoperative biliary tract at 3D MR cholangiopancreatography and MR imaging. RadioGraphics 26:1603–1620
Schnelldorfer T, Sarr MG, Adams DB (2012) What is the duct of Luschka? A systematic review. J Gastrointest Surg 16:656–662
Davidoff AM, Pappas TN, Murray EA, et al. (1992) Mechanisms of major biliary injury during laparoscopic cholecystectomy. Ann Surg 215:196–202
Richardson MC, Bell G, Fullarton GM (1996) Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: an audit of 5913 cases. West of Scotland Laparoscopic Cholecystectomy Audit Group. Br J Surg 83:1356–1360
Ernst O, Sergent G, Mizrahi D, et al. (1999) Biliary leaks: treatment by means of percutaneous transhepatic biliary drainage. Radiology 211:345–348
Tewani SK, Turner BG, Chuttani R, et al. (2013) Location of bile leak predicts the success of ERCP performed for postoperative bile leaks. Gastrointest Endosc 77:601–608
Valek V, Kala Z, Kysela P (2005) Biliary tree and cholecyst: post surgery imaging. Eur J Radiol 53:433–440
Caiado AHM, Blasbalg R, Marcelino AS, et al. (2007) Complications of liver transplantation: multimodality imaging approach. RadioGraphics 27:1401–1417
Lillemoe KD, Melton GB, Cameron JL, et al. (2000) Postoperative bile duct strictures: management and outcome in the 1990s. Ann Surg 232:430–441
Fletcher DR, Hobbs MS, Tan P, et al. (1999) Complications of cholecystectomy: risks of the laparoscopic approach and protective effects of operative cholangiography: a population-based study. Ann Surg 229:449–457
Lee CM, Stewart L, Way LW (2000) Postcholecystectomy abdominal bile collections. Arch Surg 135:538–544
Slater K, Strong RW, Wall DR, et al. (2002) Iatrogenic bile duct injury: the scourge of laparoscopic cholecystectomy. ANZ J Surg 72:83–88
Pioche M, Ponchon T (2013) Management of bile duct leaks. J Visc Surg 150:S33–S38
Bauer TW, Morris JB, Lowenstein A, et al. (1998) The consequences of a major bile duct injury during laparoscopic cholecystectomy. J Gastrointest Surg 2:61–66
Vachhani PG, Copelan A, Remer EM, et al. (2015) Iatrogenic hepatopancreaticobiliary injuries: a review. Semin Intervent Radiol 32:182–194
Stewart L, Way LW (1995) Bile duct injuries during laparoscopic cholecystectomy: factors that influence results of treatment. Arch Surg 130:1123–1128
Koffron A, Ferrario M, Parsons W, et al. (2001) Failed primary management of iatrogenic biliary injury: incidence and significance of concomitant hepatic arterial disruption. Surgery 130:722–728
Chapman WC, Abecassis M, Jarnagin W, et al. (2003) Bile duct injuries 12 years after the introduction of laparoscopic cholecystectomy. J Gastrointest Surg 7:412–416
Deizel DJ, Millikan KW, Economou SG, et al. (1993) Complications of laparoscopic cholecystectomy: a national survey of 4292 hospitals and an analysis of 77,604 cases. Am J Surg 165:9–14
Li J, Frilling A, Nadalin S, et al. (2008) Management of concomitant hepatic artery injury in patients with iatrogenic major bile duct injury after laparoscopic cholecystectomy. Br J Surg 95:460–465
Chiu WC, Wong-You-Cheong JJ, Rodriguez A, et al. (2005) Ultrasonography for interval assessment in the nonoperative management of hepatic trauma. Am Surg 71:841–846
Singh AK, Nachiappan AC, Verma HA, et al. (2010) Postoperative imaging in liver transplantation: what radiologists should know. Radiographics 30:339–351
Arun S, Santhosh S, Sood A, et al. (2013) Added value of SPECT/CT over planar Tc-99m mebrofenin hepatobiliary scintigraphy in the evaluation of bile leaks. Nucl Med Commun 34:459–466
Sharma P, Kumar R, Das KJ, et al. (2012) Detection and localization of post-operative and post-traumatic bile leak: hybrid SPECT-CT with 99mTc-Mebrofenin. Abdom Imaging 37:803–811
Khalid TR, Casillas VJ, Montalvo BM, et al. (2001) Using MR cholangiopancreatography to evaluate iatrogenic bile duct injury. AJR 177:1347–1352
Seale MK, Catalano OA, Saini S, e al. (2009) Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics 29:1725–1748
Kantarci M, Pirimoglu B, Karabulut N, et al. (2013) Non-invasive detection of biliary leaks using Gd-EOB-DTPA-enhanced MR cholangiography: comparison with T2-weighted MR cholangiography. Eur Radiol 23:2713–2722
Mungai F, Berti V, Colagrande S (2013) Bile leak after elective laparoscopic cholecystectomy: role of MR imaging. J Radiol Case Rep 7:25–32
Pietryga JA, Burke MB, Marin D, et al. (2014) Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology 271:426–434
Yeh BM, Liu PS, Soto JA, et al. (2009) MR imaging and CT of the biliary tract. Radiographics 29:1669–1688
Hyodo T, Kumano S, Kushihata F, et al. (2012) CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol 85:887–896
Lee NK, Kim S, Lee JW, et al. (2009) Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics 29:1707–1724
Brunaud L, Sebbag H, Bresler L, et al. (2000) Left hepatic duct injury and thoracobiliary fistula after abdominal blunt trauma. Hepatogastroenterology 47:1227–1229
Gupta RT, Brady CM, Lotz J, et al. (2010) Dynamic MR imaging of the biliary system using hepatocyte-specific contrast agents. AJR 195:405–413
Weber A, Feussner H, Winkelmann F, et al. (2009) Long-term outcome of endoscopic therapy in patients with bile duct injury after cholecystectomy. J Gastroenterol Hepatol 24:762–769
Perini RF, Uflacker R, Cunningham JT, et al. (2005) Isolated right segmental hepatic duct injury following laparoscopic cholecystectomy. Cardiovasc Intervent Radiol 28:185–195
Pomerantz BJ (2009) Biliary tract interventions. Tech Vasc Interv Radiol 12:162–170
Covey AM, Brown KT (2008) Percutaneous transhepatic biliary drainage. Tech Vasc Interv Radiol 11:14–20
Saad WF, Wallace MJ, Wojak JC, et al. (2010) Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage and percutaneous cholecystostomy. J Vasc Interv Radiol 21:789–795
Stewart L (2002) Treatment strategies for bile duct injury and benign biliary stricture. In: Poston G, Blumgart L (eds) Hepatobiliary and pancreatic surgery, 1st edn. London: Martin Dunitz, pp 315–329
Mazer LM, Tapper EB, Sarmiento JM (2011) Non-operative management of right posterior sectoral duct injury following laparoscopic cholecystectomy. J Gastrointest Surg 15:1237–1242
Perera MT, Monaco A, Silva MA, et al. (2011) Laparoscopic posterior sectoral bile duct injury: the emerging role of nonoperative management with improved long-term results after delayed diagnosis. Surg Endosc 25:2684–2691
Li J, Frilling A, Nadalin S, et al. (2010) Surgical management of segmental and sectoral bile duct injury after laparoscopic cholecystectomy: a challenging situation. J Gastrointest Surg 14:344–351
Venbrux AC, Osterman FA Jr (2008) Percutaneous management of benign biliary strictures. Tech Vasc Interv Radiol 11:21–42
Misra S, Melton GB, Geschwind JF, et al. (2004) Percutaneous management of bile duct strictures and injuries associated with laparoscopic cholecystectomy: a decade of experience. J Am Coll Surg 198:218–226
Laasch HU, Martin DF (2002) Management of benign biliary strictures. Cardiovasc Intervent Radiol 25:457–466
Saad WE (2008) Percutaneous management of postoperative anastomotic biliary strictures. Tech Vasc Intervent Radiol 11:143–153
Pitt HA (2001) Surgical therapy of iatrogenic lesions of biliary tract. World J Surg 25:1360–1365
Winick AB, Waybill PN, Venbrux AC (2001) Complications of percutaneous transhepatic biliary interventions. Tech Vasc Interv Radiol 4:200–206
Saad WE, Wallace MJ, Wojak JC, et al. (2010) Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage and percutaneous cholecystectomy. J Vasc Intervent Radiol 21:789–795
Walsh RM, Henderson JM, Vogt DP, et al. (2007) Long-term outcome of biliary reconstruction from bile duct injuries from laparoscopic cholecystectomies. Surgery 142:450–456
Stewart L, Way LW (2009) Laparoscopic bile duct injuries: timing of surgical repair does not influence success rate—a multivariate analysis of factors influencing surgical outcomes. HPB (Oxford) 11:516–522
Singh V, Narasimhan KL, Verma GR, et al. (2007) Endoscopic management of traumatic hepatobiliary injuries. J Gastroenterol Hepatol 22:1205–1209
Pachter HL, Knudson MM, Esrig B, et al. (1996) Status of nonoperative management of blunt hepatic injuries in 1995: a multicenter experience with 404 patients. J Trauma 40:31–38
Sugiyama M, Atomi Y, Matsuoka T, et al. (2000) Endoscopic biliary stenting for treatment of persistent biliary fistula after blunt hepatic injury. Gastrointest Endosc 51:42–44
Asenio JA, Demetriades D, Chahwan S, et al. (2000) Approach to the management of complex hepatic injuries. J Trauma 48:66–69
Spinn MP, Patel MK, Cotton BA, et al. (2013) Successful endoscopic therapy of traumatic bile leaks. Case Rep Gastroenterol 7:56–62
Croce MA, Fabian TC, Menke PG, et al. (1995) Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients: results of a prospective trial. Ann Surg 221:744–753
Baghdanian AA, Baghdanian AH, Khalid M, et al. (2016) Damage control surgery: use of diagnostic CT after life-saving laparotomy. Emerg Radiol. Epub ahead of print
Stein DM, Scalea TM (2006) Nonoperative management of spleen and liver injuries. J Intensive Care Med 21:296–304
Cogbill TH, Moore EE, Jurkovich GJ, et al. (1988) Severe hepatic trauma: a multicenter experience with 1,335 liver injuries. J Trauma 28:1422–1438
Bridges A, Wilcox CM, Varadarajulu S (2007) Endoscopic management of traumatic bile leaks. Gastrointest Endosc 65:1081–1085
Wahl WL, Brandt MM, Hemmilla MR (2005) Diagnosis and management of bile leaks after blunt liver injury. Surgery 138:742–747 (discussion 747–748)
Fleming KW, Lucey BC, Soto JA, et al. (2006) Posttraumatic bile leaks: role of diagnostic imaging and impact on patient outcome. Emerg Radiol 12:103–107
Vassiliu P, Toutouzas KG, Velmahos GC (2004) A prospective study of posttraumatic biliary and pancreatic fistuli. The role of expectant management. Injury 35:223–227
Gupta A, Stuhlfaut JW, Fleming KW, et al. (2004) Blunt trauma of the pancreas and biliary tract: a multimodality imaging approach to diagnosis. Radiographics 24:1381–1395
Chen X, Talner LB, Jurkovich GJ (2001) Gallbladder avulsion due to blunt trauma. Am J Roentgenol 177:822
Wittenberg A, Minotti AJ (2005) CT diagnosis of traumatic gallbladder injury. Am J Roentgenol 185:1573–1574
LeBedis CA, Anderson SW, Mercier G, et al. (2015) The utility of CT for predicting bile leaks in hepatic trauma. Emerg Radiol 22:101–107
Franklin GA, Richardson JD, Brown AL, et al. (2007) Prevention of bile peritonitis by laparoscopic evacuation and lavage after nonoperative treatment of liver injuries. Am Surg 73:611–616 (discussion 616–617)
Davids PH, Ringers J, Rauws EA, et al. (1993) Bile duct injury after laparoscopic cholecystectomy: the value of endoscopic retrograde cholangiopancreatography. Gut 34:1250–1254
Lubezky N, Konikoff FM, Rosin D, et al. (2006) Endoscopic sphincterotomy and temporary internal stenting for bile leaks following complex hepatic trauma. Br J Surg 93:78–81
Sharma BC, Mishra BC, Kumar R, et al. (2009) Endoscopic management of bile leaks after blunt abdominal trauma. J Gastroenterol Hepatol 24:757–761
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflicts of interest.
Disclosures
The authors have nothing to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
LeBedis, C.A., Bates, D.D.B. & Soto, J.A. Iatrogenic, blunt, and penetrating trauma to the biliary tract. Abdom Radiol 42, 28–45 (2017). https://doi.org/10.1007/s00261-016-0856-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0856-y