Abstract
Purpose
To evaluate the qualitative and quantitative benefit of multiple arterial phase acquisitions for the depiction of hypervascularity in FNH explored MR imaging using an extracellular contrast agent.
Methods
Between 2007 and 2014, all patients who underwent MR imaging for the exploration of FNH were included. The protocol included a single or a triple arterial phase (“single” and “triple” group, respectively). Arterial phases were visually divided into four types: (1) angiographic, (2) early, (3) late, and (4) portal. Signal intensity on arterial phase images was visually recorded as intense, moderate, or low for each lesion. Lesion-to-liver contrast (LLC) and relative lesion enhancement (RE) were calculated and compared between the two groups using the Mann–Whitney test.
Results
Thirty-five women were included (mean 45-year old, range 20–66), with 50 FNH (mean size 30 mm). Single and triple groups included 20 patients (30 FNH) and 15 patients (20 FNH), respectively. Signal intensity was intense in all lesions in the triple group and in 22/30 (73%) in the single group (p = 0.041). Intense signals were more frequently found in the early arterial phase (p < 0.001). RE was not significantly different (1.78 ± 0.84 vs. 1.98 ± 1.81 p = 0.430, in the single and triple groups, respectively) but LLC was significantly higher in the triple group (0.32 ± 0.10 vs. 0.22 ± 0.10, p = 0.005). LLC was significantly higher in the first two arterial phases in the triple group (p < 0.001).
Conclusion
Acquisition of three arterial phases improves the visualization of hypervascularity of FNH, as lesions show high visual signal intensity and contrast. Optimal visualization is obtained in the early arterial phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Focal nodular hyperplasias (FNH) are benign hepatocellular lesions that do not require surveillance or treatment when they are asymptomatic [1, 2]. In most cases, they are discovered incidentally in young women who are asymptomatic or with minor nonspecific symptoms. Thus the diagnosis should be made with noninvasive techniques anytime possible, and biopsy should only be performed to confirm a doubtful diagnosis.
MR imaging is the reference technique for the noninvasive diagnosis of FNH [3, 4]. The diagnosis is based on the association of several features, including homogeneity, signal intensity similar to the surrounding liver on both T1- and T2-weighted images, the presence of a central scar, and marked homogeneous contrast enhancement on arterial phase images without washout in portal venous images [3, 4]. The final diagnosis cannot be reached when intense arterial enhancement is missing. Isointense images during the arterial phase using extracellular contrast agents were found in the study by Mortele et al. and Ba-Ssalamah et al. in 5% [5] and up to 12% [6] of FNH, respectively. FNH are always hypervascular, thus an absence of marked arterial contrast uptake is due to ineffective sampling of peak enhancement by imaging techniques.
To overcome these limitations and improve sampling time, parallel imaging using phased array coils has been shown to provide faster image acquisition, enabling reconstruction of multiple arterial phases. Various arterial multiphase acquisition techniques have been developed in the past decade and mainly been evaluated in hepatocellular carcinoma (HCC) [7–9]. Conventional gradient echo sequences are accelerated with auto-calibrating reconstruction for cartesian sampling (ARC) or sensitivity encoding (SENSE) techniques. Temporal resolution is increased by decreasing the spatial resolution, increasing slice thickness, and decreasing matrix size. Images are obtained by linear and independent filling of the Fourier plan for each phase, regardless of acceleration. More recently, techniques using a complex filling of the Fourier plan [10], or combined dynamic contrast-enhanced liver MRI and MR angiography using interleaved variable density sampling have been described [11]. Hope et al. analyzed arterial enhancement of a series of FNH with these techniques [12] and showed that multiphase acquisitions were better using a hepatobiliary contrast agent (gadoxetic acid). However, there are no existing studies on arterial enhancement of FNH during multiphasic acquisitions with an extracellular contrast agent. Indeed, although multiphasic acquisitions with gadoxetic acid are effective by compensating the shorter peak enhancement of vessels with increased temporal sampling, it is unclear whether or not these results can be obtained with extracellular contrast agents.
Thus the purpose of this study was to evaluate the qualitative and quantitative benefit of multiple arterial phase acquisitions for the depiction of hypervascularity in FNH explored MR imaging using an extracellular contrast agent.
Materials and methods
Patient selection
This single-center retrospective study was approved by our local institutional review board and informed consent was waived. Between 2007 and 2014, all patients who underwent liver MR imaging in our center were identified in our imaging database. Patients with a suspected or proven diagnosis of FNH were identified by searching with the terms “focal nodular hyperplasia” or “FNH” in MR imaging reports. Another search was performed in the pathological database to identify patients with resected FNH who could have been missed or misdiagnosed on preoperative MR imaging. Clinico-biological data were extracted from the medical charts of each selected patient.
Lesion selection and diagnostic reference
The final diagnosis of FNH was based on the histological analysis of a biopsy or typical follow-up imaging features showing stability of lesions for at least 12 months. A lesion was considered to be FNH when the five following criteria were present: (1) lesion homogeneity, (2) iso or slight hypointensity on T1-weighted images, (3) iso or slight hyperintensity on T2-weighted images, (4) marked and homogeneous contrast enhancement during the arterial phase without washout on portal venous images, and (5) the presence of a central scar showing T2-weighted hyperintensity, and T1-weighted hypointensity, with contrast enhancement on delayed phase images. When all criteria were not fulfilled, the lesion was explored by contrast-enhanced ultrasound. When typical early enhancement with a centrifugal spoke wheel appearance was present, the lesion was considered to be FNH, if it was 35 mm in diameter or less (12–13). Biopsy and pathological analysis was performed in all other cases. Only lesions larger than 1 cm were selected to obtain reliable qualitative and quantitative analyses. In the presence of several FNHs, the three largest lesions were analyzed.
A total of 69 patients were selected. Thirty-four were excluded because the examination was performed on a 3.0 T system (n = 19), the MR exam was incomplete (N = 3), the lesions were smaller than 10 mm (n = 7), or because patients had less than 12 months of follow-up (n = 5) (Fig. 1). 3.0 T examinations were excluded to avoid signal analysis bias mainly due to the higher contrast-to-noise ratio at 3.0 T than at 1.5 T. The final population included 35 women mean age 45-year old (range 20–66).
MR protocols
All MR imaging examinations were performed on a 1.5 T clinical system (General Electric, Signa HDXT) with a 16-channel-phased array body coil. Table 1 shows the different imaging protocols. Patients underwent either a fixed delay-single arterial phase acquisition (“single” group), or a protocol including a fixed delay-triple arterial phase acquisition (“triple” group). Single- and triple-phase acquisitions corresponded to two different periods in time: single-phase acquisitions were performed between 2007 and 2011, and triple-phase acquisitions between 2011 and 2014.
Both MR protocols included the following sequences: fat-suppressed respiratory-gating T2-weighted fast spin echo (FSE), T1-weighted with in and out phase reconstructions, diffusion-weighted (b = 200 s2/mm, and b = 600 s2/mm), and dynamic contrast-enhanced 3D T1 GRE. After an unenhanced acquisition, a dose of 0.2 mL/kg of extracellular contrast agent (Dotarem, Guerbet, Aulnay-sous-Bois, France) was injected at a rate of 2 mL/s followed by an injection of a saline flush (20 mL at 2 mL/s). Arterial phase acquisitions began 20 s after beginning an injection without bolus tracking in both groups. The arterial phase was obtained in a single acquisition during a 24–29-s breath-hold in the single group. In the triple group, the arterial phase was performed using triple-phase dynamic imaging with linear sampling of the Fourier k-space as fast as 8 s for each phase. This dynamic acquisition provides three sets of images in a 24-s breath-hold. Portal venous phase and delayed phase images were then obtained in both groups at 70 s and 5 min, respectively. All images were acquired in the axial plane.
Image analysis
A consensus review of all MRI examinations was retrospectively performed by two abdominal radiologists (_ and _) with 5 and 25 years of experience, respectively, in the field of liver imaging. They were aware of the diagnosis of FNH. A study coordinator (_) who did not participate in the reading sessions reviewed the diagnostic criteria with both readers and checked all contrast-enhanced ultrasound examinations (CEUS) results performed to validate the diagnosis of FNH (n = 3 patients and 4 lesions in the single group, and n = 3 patients and 6 lesions in the triple group).
Image quality and definition of arterial phases
A semiquantitative evaluation of image quality was performed with the following score: (1) marked respiratory artifacts, no interpretation possible; (2) minimal respiratory artifacts, acceptable quality, and interpretation possible; (3) excellent or very good image quality with no respiratory artifacts.
Arterial phases were defined according to vascular and liver enhancement as: angiographic when contrast medium was visible in the aorta and hepatic artery, with no portal venous enhancement; early arterial when mild portal enhancement was seen with no liver enhancement, late arterial when there was both portal venous and liver enhancement with no enhancement of the hepatic veins; and portal when hepatic veins showed enhancement (Fig. 2).
Illustration of the four different types of arterial phase. A The angiographic phase corresponded to the presence of contrast medium visible in the aorta and the hepatic artery with no portal venous enhancement; B early arterial corresponded to mild portal enhancement with no liver enhancement; C late arterial corresponded to both portal venous and liver enhancement with no enhancement of the hepatic veins; D and portal corresponded to enhancement of the hepatic veins
Qualitative analysis of the lesions
T1 and T2-weighted signal intensity was assessed visually as hypo-, iso-, or hyperintense compared to the surrounding liver. Similarly, signal intensity on arterial phase images was recorded as intense, moderate, or low. Signal intensity was considered to be low when the lesion was similar to that of the surrounding liver resulting in poor visualization of the FNH. The signal was considered to be intense when it was similar to that of the aorta. It was considered to be moderate in all other cases. Size (measured as the largest diameter on the axial plane on the best visual sequence of the lesion) and the presence of a central scar, defined as a central hyperintense T2-weighted or hypointense T1-weighted image with contrast enhancement on delayed phase images, were also noted.
Quantitative analysis of the lesions
Lesion and liver signal intensities (SI) were measured in each patient on precontrast and arterial phase contrast-enhanced 3D T1 GRE sequences (all arterial sets). Values were obtained by placing the largest round or oval region of interest (ROI) (at least 20 mm2) on each lesion, avoiding the central scar when present. When the lesion was small or not visible the ROI was placed by copy-pasting it from another sequence. ROIs were also placed in the surrounding liver, excluding vessels, bile ducts, and artifacts.
The lesion-to-liver contrast (LLC), the relative enhancement of lesions (REFNH), were calculated by the following formulas:
where SIFNH and SILIVER corresponding to the signal intensity of the FNH and the liver on arterial phase images, respectively, and SIART and SIUN corresponding to the signal intensity of lesions on arterial phase images, and on unenhanced images, respectively. These formulas were chosen because they do not require the measurement of the noise, which can be rather difficult when using parallel imaging, as the noise may vary within the field of view [13, 14].
Statistical analysis
Data were expressed as means and standard deviations or medians and interquartile ranges (IQR) for quantitative data, and frequencies and percentages for categorical data. Continuous values were analyzed and compared using the nonparametric Mann–Whitney test. Categorical data were analyzed and compared using the Fisher exact test or the χ 2 test. p values were considered statistically significant when p < 0.05. Statistical analyses were performed using SPSS for Windows (version 20.0, SPSS Inc). The graphics data were performed using GraphPad v5.0 software (Prism Inc., US).
Results
Twenty patients were included in the single group, mean age 47-year old (range 20–66), and 15 were included in the triple group, mean age 42-year old (range 20–66, p = 0.292) (Table 2). Most MRI were performed to characterize focal liver lesions detected on ultrasound either incidentally (n = 20) or due to abdominal pain (n = 2), dyspepsia (n = 1), cholestasis (n = 2), acute pancreatitis (n = 1), anemia (n = 1), or hematuria (n = 1). Other indications for MRI were exploration of extrahepatic cancer (n = 4), monitoring of chronic hepatitis C virus infection (n = 1), alcohol-related chronic liver disease (n = 1), or Crohn’s disease (n = 1). None of the patients had cirrhosis or steatosis.
Fifty lesions were analyzed, 30 in the single group and 20 in the triple group. There was only one FNH in 65% (13/30) of patients in the single group and in 55% (11/20) of patients in the triple group (p = 0.550). Four patients had two lesions in the single group; three patients had three lesions or more. One patient had two FNH in the triple group, two patients had three lesions or more.
The diagnosis of FNH was made by imaging in 49/50 lesions (98%) and pathological examination of the resected specimen in the remaining lesion (2%). Thirteen out of thirty (43%) and 4/20 (20%) lesions were evaluated by both MRI and CEUS in the single and triple groups. respectively, while the others were evaluated by MRI alone.
The median diameter of lesions was 30 mm (range 11–160). FNH in the triple group were a median 24 mm (range 12–160 mm), and lesions in the single group were a median 20 mm (range 11–76 mm, p = 0.215, Mann–Whitney test). Seventy-two percent (36/50) of FNH had a central scar, 67% (20/30) in the single group, and 80% (16/20) in the triple group with no significant difference between the two groups (p = 0.24). All lesions larger than 28 mm had a central scar.
Image analysis
Image quality and artifacts
Table 3 shows the distribution of image quality and artifacts for the two groups. Briefly, image quality was rated as excellent and acceptable in 55% and 45% of MRI in the single group and in 73% and 27% in the triple group, respectively. All MRI could be interpreted. There was no statistically significant difference between the two groups (p = 0.226).
Only two MR examinations in the single group showed minor respiratory artifacts that did not prevent interpretation of small lesions. None of the images in the triple group had respiratory artifacts. There was no significant difference between the two groups (p = 0.319).
Qualitative analysis of arterial phases and lesions
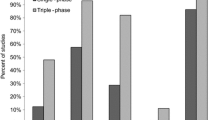
Table 3 shows the distribution of the different arterial phases in the two groups of patients. The arterial phase was classified as angiographic, early arterial, late arterial, and portal in 10% (2/20), 60% (12/20), 25% (5/20), and 5% (1/20) of the patients in the single group, respectively. In the triple group, images were classified as angiographic, early arterial, late arterial, and portal phase in 87% (13/15), 73% (11/15), 80% (12/15), and 60% (9/15) of the patients, respectively. Figure 3 shows the distribution of the phases in the two groups.
Visual assessment of FNH signal intensity in the arterial phase was considered intense in all lesions in the triple group, in at least on one of the three acquisitions and in 22/30 (73%) of the lesions in the single group (p = 0.041). Signal intensity was found to be low in the arterial phase in three lesions in the single group; these lesions were only visualized by correlation with other sequences, and a final diagnosis could not be made by MRI (Fig. 4). A comparison of the different types of arterial phases shows that an intense signal was more frequently observed on images in the early arterial phase (81%, 29/36) compared to that obtained at the angiographic phase (42%, 8/19), the late arterial phase (64%, 18/28), or the portal venous phase (0%, 0/17) (overall p < 0.001, vs. angiographic p = 0.006, vs. late p = 0.0003, and vs. portal p < 0.001) (Fig. 5).
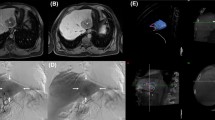
FNH in a 48-year-old female with malignant melanoma explored with a single arterial phase. The lesion was located in segment 4, and appeared slightly hyperintense on T2-w images (arrow in A) and hypointense on T1-w images (B). After contrast medium injection, the lesion showed mild contrast enhancement on arterial phase images (angiographic type, C) and was isointense on portal venous phase images (D). The central scar was visible on T2-w images, with contrast uptake on delayed phase images (short arrow in D). Diagnosis was confirmed by CEUS showing a typical spoke wheel appearance
Typical FNH in an asymptomatic 33-year-old female explored with a triple arterial phase acquisition. The lesion was homogeneous and showed a slight signal hyperintensity on T2-w images (A) and signal isointense on T1-w images. After contrast medium injection a triple arterial phase acquisition was performed (C–E). Images were classified as angiographic (C), early arterial (D), and late arterial (E). The lesion showed higher signal intensity and a higher lesion-to-liver contrast on the angiographic and the early arterial phase images. A central scar was clearly depicted showing T2-w signal hyperintensity and contrast uptake (arrows in A and E)
Quantitative analysis of arterial phases and lesions
Table 3 summarizes quantitative data. When considering the “best” arterial phase (highest RE) in the triple group, the RE was not significantly different between the two groups [median (IQR) 1.90 (1.01–2.36) vs. 1.38 (1.07–1.73) in the single and triple groups, respectively, p = 0.43, Fig. 6]. There was no significant difference between the different RE obtained at different arterial phases (p = 0.078, and p = 0.743 for the single and the triple group, respectively, Fig. 6). In the triple group, the best RE was obtained in 35% (7/20) of the patients on an angiographic phase, in 30% (6/20) on an early arterial phase, and in 35% (7/20) on a late arterial phase.
Boxplots of the relative enhancement (A), and lesion-to-liver contrast (B) of the lesions in the single and the triple groups. For the triple group, the highest value obtained between the three different acquisitions were plotted, for each lesion. The relative enhancement was not significantly different between the two groups, while the contrast was higher in the “triple” group. Boxes represent the 25th and 75th percentiles, whiskers the 10th and 90th percentiles, and dots the outliers. Boxplots of the relative enhancement (C) and lesion-to-liver contrast (D) of the lesions in the single and the triple groups for each type of arterial phase. The relative enhancement was not different between the different phases for both groups (p = 0.078, and p = 0.743, respectively). Regarding the lesion contrast, it was not significantly different for the single group, but was significantly higher for the first two phases in the triple group. Boxes represent the 25th and 75th percentiles, whiskers the 10th and 90th percentiles, and dots the outliers
The LLC was significantly higher in the triple group [median (IQR) 0.22 (0.16–0.30) vs. 0.31 (0.22–0.40), p = 0.005, Fig. 6]. There was no significant difference between the different LLCs in the different types of arterial phases in the single group (p = 0.072), but the LLC was significantly higher in the first two arterial phases in the triple group (p < 0.001, Fig. 6). In the single group, the best LLC was visible in an early arterial phase in 22 lesions (73 %), and in a late arterial phase in the remaining eight lesions (27%). In the triple group, the best LLC was obtained in 45% (9/20) of the lesions on an angiographic phase, in 35% (7/20) in an early arterial phase and in 20% (4/20) in a late arterial phase (p = 0.0002). There was no significant correlation between RE or LLC and the size of the FNHs (p = 0.80 and p = 0.25 respectively).
Discussion
The results of the present study show that multiple arterial phases more effectively depict intense arterial enhancement, i.e., hypervascularity, in FNHs than a single arterial phase. All FNHs explored with by triple-phase MRI showed marked signal intensity in at least one of the three acquisitions, while almost 10% of those explored by a single phase had low arterial enhancement preventing a definite diagnosis with MR imaging. In addition, lesion-to-liver contrast was significantly higher in the triple group. Finally, the best phase for optimal visualization of FNH was the early arterial phase, which was most frequently obtained by triple-phase acquisition.
As reported in the literature, arterial enhancement is a key finding for the noninvasive diagnosis of FNH [1, 2]. Thus, when it is missing the diagnosis cannot be confirmed [5, 6]. In the present study, certain FNHs in the single-phase group had low arterial enhancement, while this was never observed for FNHs in the triple-phase group because intense enhancement was found on at least one arterial phase. These results are similar to those in the only published study on this topic by Hope et al. [12]. It is important to remember that FNH are always hypervascular. Thus failure to visualize arterial enhancement must be because peak enhancement is missed. Other imaging techniques with better temporal resolution, such as contrast-enhanced ultrasound can be performed. However, typical CEUS features may be missing, especially in large lesions [15, 16]. This is why for most teams CEUS remains a second-line examination that is more suitable for the diagnosis of atypical or small FNH.
It is important to determine the best arterial phase to visualize the lesions. This should correspond to the phase with the greatest lesion enhancement and minimal liver enhancement to obtain the highest lesion-to-liver contrast [17]. A dominant arterial phase has been described as the optimal phase for the analysis of hypervascular liver lesions and corresponds to the delay between opacification of hepatic arterial vessels and that of hepatic veins [18]. However, since then multiple sub-hepatic arterial dominant phases have been described [19]. In the present study, high visual intensity of lesions was more frequently observed on early and late arterial phases, and the lesion-to-liver contrast was quantitatively higher on angiographic and early arterial phases. The combination of these two results confirms that the early arterial phase is the best as previously reported by Hope et al. [12].
Numerous studies have evaluated the detectability of hypervascular lesions, mainly HCC. In a descriptive study including various liver lesions explored with single and double arterial phases, like in our study, Low et al. have shown that the optimal phase was the early arterial phase [20]. However, there are no studies to date comparing the arterial enhancement of different hypervascular lesions. Maximum enhancement of HCC has been shown to occur at a later arterial phase, which is defined as the opacification of portal veins and the absence or minimal enhancement of hepatic veins [7, 17, 21]. This shows that the optimal phase is probably different depending on the lesion of interest, but in all cases these results show the usefulness of multiple arterial phase acquisition because the type of lesion has not always been identified before MR imaging is performed.
An important point is the frequent use of an empirical fixed delay for the acquisition of a single arterial phase, which is usually between 15 and 20 s [12, 20]. This delay does not take into account variables such as the size of the patient or his/her cardiac function. This can lead to great variability in the delay of enhancement of the liver and hypervascular lesions. Multiple arterial phase acquisitions overcome this limitation [7, 9, 17, 20, 22]. The injection test and automatic detection of bolus could also be used, the latter having been showed superior to the former [18, 23, 24]; however, certain studies have shown the limitations of this technique alone. Indeed, Kanematsu et al. reported that up to 27% of arterial phases were considered to be too early or too late, despite the use of an injection test [21]. A combination of multiple arterial phases and an injection test could be an interesting option. However, Takahashi et al. reported the same detection rate for HCC with and without an injection test combined with triple arterial phase and extracellular contrast agents [8], suggesting that the added value of multiphase acquisitions is probably greater than that of the injection test.
Our study has several limitations besides its retrospective design. First, histological evidence was not obtained in most FNHs and a selection bias cannot be excluded. However, histological proof is not usually obtained for all FNH in clinical practice. It has now been clearly established that histological evidence is not required for most FNH when typical features are found with either MRI [3, 4] or CEUS [4, 15, 16]. Second, patients did not serve as their own controls. For this, they would have to have undergone MRI with both acquisition protocols and two contrast medium injections. This is not justifiable for benign lesions. Also, arterial phase acquisitions were performed at a fixed empirical delay, and no injection test was performed. However, the study population included young patients with no history of cardiac dysfunction, thus limiting the influence of hemodynamic function of the patients on the results. Finally, we did not study inter-observer variability as the analyses were performed in consensus.
In conclusion, the acquisition of three arterial phases improves visualization of the hypervascularity of FNH with lesions showing high signal intensity and a higher lesion-to-liver contrast. The optimal phase is the early arterial phase. Thus, we recommend the systematic use of multiple arterial phase acquisition for the exploration of FNH with MR imaging.
References
Vilgrain V (2006) Focal nodular hyperplasia. Eur J Radiol 58:236–245
Ronot M, Vilgrain V (2014) Imaging of benign hepatocellular lesions: current concepts and recent updates. Clin Res Hepatol Gastroenterol. doi:10.1016/j.clinre.2014.01.014
Hussain SM, Terkivatan T, Zondervan PE, et al. (2004) Focal nodular hyperplasia: findings at state-of-the-art MR imaging, US, CT, and pathologic analysis. Radiographics 24:3–17 (discussion 18–19)
Soussan M, Aubé C, Bahrami S, et al. (2010) Incidental focal solid liver lesions: diagnostic performance of contrast-enhanced ultrasound and MR imaging. Eur Radiol 20:1715–1725
Mortele KJ, Praet M, Van Vlierberghe H, et al. (2002) Focal nodular hyperplasia of the liver: detection and characterization with plain and dynamic-enhanced MRI. Abdom Imaging 27:700–707
Ba-Ssalamah A, Schima W, Schmook MT, et al. (2002) Atypical focal nodular hyperplasia of the liver: imaging features of nonspecific and liver-specific MR contrast agents. AJR Am J Roentgenol 179:1447–1456
Mori K, Yoshioka H, Takahashi N, et al. (2005) Triple arterial phase dynamic MRI with sensitivity encoding for hypervascular hepatocellular carcinoma: comparison of the diagnostic accuracy among the early, middle, late, and whole triple arterial phase imaging. AJR Am J Roentgenol 184:63–69
Takahashi N, Yoshioka H, Yamaguchi M, Saida Y, Itai Y (2003) Accelerated dynamic MR imaging with a parallel imaging technique for hypervascular hepatocellular carcinomas: usefulness of a test bolus in examination and subtraction imaging. J Magn Reson Imaging 18:80–89
Yoshioka H, Takahashi N, Yamaguchi M, et al. (2002) Double arterial phase dynamic MRI with sensitivity encoding (SENSE) for hypervascular hepatocellular carcinomas. J Magn Reson Imaging 16:259–266
Budjan J, Ong M, Riffel P, et al. (2014) CAIPIRINHA-Dixon-TWIST (CDT)-volume-interpolated breath-hold examination (VIBE) for dynamic liver imaging: comparison of gadoterate meglumine, gadobutrol and gadoxetic acid. Eur J Radiol 83:2007–2012
Salmani Rahimi M, Korosec FR, Wang K, et al. (2015) Combined dynamic contrast-enhanced liver MRI and MRA using interleaved variable density sampling. Magn Reson Med 73:973–983
Hope TA, Saranathan M, Petkovska I, et al. (2013) Improvement of gadoxetate arterial phase capture with a high spatio-temporal resolution multiphase three-dimensional SPGR-dixon sequence. J Magn Reson Imaging 38:938–945
Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO (2007) Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging 26:375–385
Heverhagen JT (2007) Noise measurement and estimation in MR imaging experiments. Radiology 245:638–639
Bertin C, Egels S, Wagner M, et al. (2014) Contrast-enhanced ultrasound of focal nodular hyperplasia: a matter of size. Eur Radiol 24:2561–2571
Roche V, Pigneur F, Tselikas L, et al. (2014) Differentiation of focal nodular hyperplasia from hepatocellular adenomas with low-mecanical-index contrast-enhanced sonography (CEUS): effect of size on diagnostic confidence. Eur Radiol. doi:10.1007/s00330-014-3363-y
Murakami T, Kim T, Takamura M, et al. (2001) Hypervascular hepatocellular carcinoma: detection with double arterial phase multi-detector row helical CT. Radiology 218:763–767
Earls JP, Rofsky NM, DeCorato DR, Krinsky GA, Weinreb JC (1997) Hepatic arterial-phase dynamic gadolinium-enhanced MR imaging: optimization with a test examination and a power injector. Radiology 202:268–273
Goncalves Neto JA, Altun E, Vaidean G, et al. (2009) Early contrast enhancement of the liver: exact description of subphases using MRI. Magn Reson Imaging 27:792–800
Low RN, Bayram E, Panchal NJ, Estkowski L (2010) High-resolution double arterial phase hepatic MRI using adaptive 2D centric view ordering: initial clinical experience. AJR Am J Roentgenol 194:947–956
Kanematsu M, Goshima S, Kondo H, et al. (2006) Double hepatic arterial phase MRI of the liver with switching of reversed centric and centric K-space reordering. AJR Am J Roentgenol 187:464–472
Hong HS, Kim HS, Kim MJ, et al. (2008) Single breath-hold multiarterial dynamic MRI of the liver at 3T using a 3D fat-suppressed keyhole technique. J Magn Reson Imaging 28:396–402
Van Beers BE, Materne R, Lacrosse M, et al. (1999) MR imaging of hypervascular liver tumors: timing optimization during the arterial phase. J Magn Reson Imaging 9:562–567
Hussain HK, Londy FJ, Francis IR, et al. (2003) Hepatic arterial phase MR imaging with automated bolus-detection three-dimensional fast gradient-recalled-echo sequence: comparison with test-bolus method. Radiology 226:558–566
Acknowledgments
Authors would like to thank Bastien Perez for his technical advice in the conduction of this study.
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Caroline Rousseau, Maxime Ronot, Valérie Vilgrain, and Marc Zins declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Rousseau, C., Ronot, M., Vilgrain, V. et al. Optimal visualization of focal nodular hyperplasia: quantitative and qualitative evaluation of single and multiphasic arterial phase acquisition at 1.5 T MR imaging. Abdom Radiol 41, 990–1000 (2016). https://doi.org/10.1007/s00261-015-0630-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0630-6