Abstract
Purpose
To compare frequency and inter-reader agreement for LI-RADS v2014 major features at CT vs. MRI in pathology-proven cases of hepatocellular carcinoma.
Methods
Pathology reports and imaging studies from patients having undergone liver transplant or hepatectomy for hepatocellular carcinoma were reviewed. Size, location, washout, and capsule appearance for each lesion were recorded by two radiologists. Cohen’s kappa and intraclass correlation coefficients (ICC) were calculated.
Results
One hundred and thirty-four patients with 184 tumors were reviewed. Seventy-seven percentage of lesions were imaged by CT and 23% by MRI. No lesions were evaluated with both modalities. Mean lesion diameter was 2.6 ± 1.3 cm (ICC = 0.92). Arterial phase hyperenhancement was seen in 86% of lesions (κ = 0.75). Washout was seen in 82% of studies (κ = 0.61). Arterial phase hyperenhancement and washout were seen equally at CT and MRI (p = 1.00 and 0.46, respectively). Capsule was infrequently observed (27%) but was seen more commonly at MRI (44%) than at CT (17%) with p = 0.002 and (κ = 0.56). Forty-seven percent of lesions with at least one prior study met LI-RADS criteria for threshold growth. The rates of LI-RADS categories 3, 4, and 5 were 9%, 37%, and 54%, respectively. More 1–2 cm LI-RADS 5 lesions were seen at MRI (43%) than at CT (8%), p = 0.01.
Conclusion
A combined LI-RADS 4/5 group was 91% sensitive for hepatocellular carcinoma. Arterial enhancement and washout were seen more frequently than capsule, the sole finding seen more frequently at MRI than at CT. Inter-reader reliability was substantial for arterial hyperenhancement and washout but moderate for capsule. Capsule remains an important finding in small arterially enhancing lesions (1–2 cm) which require a second major criterion to upgrade to a LI-RADS 5 lesion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Rapid advancements in percutaneous, medical, and surgical therapy, including liver transplantation, rely on accurate noninvasive diagnosis and staging of hepatocellular carcinoma (HCC). While ultrasound may be used in screening or surveillance of HCC, contrast-enhanced CT and MRI are recommended for final noninvasive diagnosis by many societies including the American Association for the Study of Liver Diseases (AASLD), the Organ Procurement and Transplantation Network (OPTN), and others [1–6].
The Liver Imaging Reporting and Data System (LI-RADS) provides standardized reporting of imaging features in patients at risk for HCC. The goal of LI-RADS is to allow for consistent application of terminology, to reduce variability in reporting, to improve communication with referring physicians, and to provide data for quality assurance and research purposes [7]. For OPTN listing, LI-RADS 5 or OPTN 5 lesions are considered diagnostic of HCC without the need for histologic confirmation [1, 2]. This necessitates accurate diagnosis of HCC to minimize false positives and negatives because many patients with cirrhosis and imaging features consistent with HCC receive MELD exception points and thereby improve their standing on liver transplant waiting lists [8–11]. Existing literature suggests that using the characteristic enhancement patterns on which the LI-RADS criteria are structured, contrast-enhanced CT and MRI are moderately sensitive for small HCCs between 1 and 2 cm and are highly sensitive for larger HCCs measuring greater than 2 cm [12–17].
Retrospective studies have shown that careful application of more stringent diagnostic criteria to smaller lesions may reduce the rate of false positives at liver transplant [18]. Other studies have shown high inter-observer reliability in the overall grading of lesions using LI-RADS and other criteria [19]. Reported inter-observer reliability for individual features is variable, with high agreement in arterial phase hyperenhancement at MRI but poor agreement on washout or capsule appearance [20]. The inter-reader agreement for major imaging features in HCCs has not yet been compared at CT vs. MRI, and the frequency of LI-RADS features and categories assigned to HCCs at CT vs. MRI is not yet known.
The primary aim of this project was to estimate the frequency of observation and compare inter-reader agreement for LI-RADS v2014 major features at CT vs. MRI in pathology-proven hepatocellular carcinoma. The secondary purpose was to estimate and compare the frequency of LI-RADS categorization at CT vs. MRI. Both goals would increase understanding of the impact that choice of imaging modality has on final LI-RADS category.
Methods
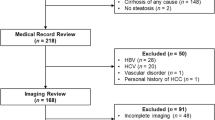
This study was reviewed by the local institutional review board, and informed consent was waived for this retrospective study. Pathology reports from liver specimens (explants and partial hepatectomies) of 605 sequential patients with cirrhosis between December 2008 and October 2013 were reviewed to identify specimens with at least one focus of histologically proven hepatocellular carcinoma. Lesions which were completely necrotic and without residual findings to confirm a diagnosis of HCC at pathology were excluded. Patients having undergone localized therapy (transarterial chemoembolization, wedge resection, thermal or ethanol ablation) prior to transplant or resection without available pre-treatment imaging were also excluded, as were lesions evaluated with gadoxetate disodium enhanced MRI (n = 8) or those with suboptimal arterial phase imaging (n = 4).Finally, lesions smaller than 1 cm at pathology were excluded because these lesions were felt to be too small to reliably identify at imaging, particularly given the inevitable delay between imaging and surgery. If multiple remote pre-treatment examinations were available, the two most recent pre-treatment studies were evaluated for determination of threshold growth.
Image acquisition
For CT studies performed at our institution, quad-phase technique was used with noncontrast imaging of the abdomen followed by late arterial phase, portovenous phase, and 3–5 min delayed phase imaging following the intravenous administration of iodinated contrast (iohexol, GE Healthcare, Milwaukee, WI). Patients undergoing MRI were imaged at 1.5 or 3.0 Tesla. Typical sequences included coronal T2 SSFSE, axial T2 with fat saturation, axial in and out of phase, axial diffusion-weighted imaging (DWI) with B = 500, precontrast axial T1 with fat saturation, and multiphase post-contrast T1 with fat saturation imaging of the abdomen following the administration of IV contrast.
Image interpretation
Each lesion described in the pathology report was correlated with the most recent pre-treatment multiphase imaging examination, CT or MRI. No lesions were evaluated at both CT and MRI. Studies were independently reviewed by two board-certified abdominal imagers who had access to full imaging datasets as well as the lesion size and segmental location as noted in the pathology report. In cases where identifying lesions of the correct size and location was difficult, or the two readers disagreed on lesion location, lesions were excluded from further analysis. Each reader measured the size of each lesion on the most conspicuous imaging phase and threshold growth compared to prior imaging (if available) was determined. Each lesion was then assessed by both readers in terms of arterial phase hyperenhancement, portal venous and delayed phase enhancement characteristics as well as the presence or absence of washout and capsule appearance. Mirroring the LI-RADS lexicon, washout was defined as a temporal reduction in enhancement relative to liver from an earlier to later phase and capsule appearance was defined as a peripheral rim of smooth hyperenhancement in the portal venous or delayed phase that is unequivocally thicker or more conspicuous than the rims of surrounding background nodules. In cases of disagreement between the two readers, images were re-reviewed and a consensus categorization and grading was performed. Each lesion was then assigned a LI-RADS category based on the v2014 guidelines [7]. Readers were blinded to the radiology report, although knew that all lesions were histologically confirmed as HCC.
Statistical analysis
Interval data are presented as an average and standard deviation. Inter-observer agreement of categorical data was evaluated using Cohen’s kappa statistic. A Fisher’s exact test was used to compare the proportion of lesions demonstrating arterial phase hyperenhancement, washout, and capsule appearance as well as rates of LI-RADS category between MRI and CT. Excel 12.1.0 (Microsoft, Redmond, WA) and R 3.0.2 (The R Foundation for Statistical Computing, Vienna Austria) were used for statistical analysis. A p value of <0.05 was used to determine significance for all tests.
Results
There were a total of 134 patients with 184 hepatocellular carcinomas with pre-therapy imaging who were included in this study. 108 patients were males and 26 were females, with a mean age of 58.2 ± 8.1 years. The average time between the most recent pre-treatment prior imaging study and surgery, either hepatectomy or transplant, was 12.7 ± 8.7 months. Mean lesion size was 2.6 ± 1.3 cm with ICC of 0.92 and 95% CI [0.88, 0.95]. Overall, 63 (34%) lesions measured ≥1 and <2 cm, while 121 (66%) were ≥2 cm. Because patients may have had multiple lesions and different lesions may have been noted at different times during serial follow-up imaging while awaiting transplant, a total of 108 unique CT and 33 unique MRI studies (141 total studies) were reviewed. By lesion, 141 (77%) were imaged by multiphase CT, and 43 (23%) lesions by MRI. Many of the CT and MRI studies reviewed were performed at outside institutions because our institution is a tertiary referral center for liver transplant. 79/141 (56%) studies reviewed were external, while 62/141 (44%) were performed at our institution. Additionally, CT studies spanned a time period during which a transition occurred where a delayed phase was commonly acquired. As a result, only 71/141 (50%) of CT studies included a delayed phase. CT studies without a delayed phase were not included in the washout, capsule, or LI-RADS analysis unless otherwise explicitly stated.
Major criteria
Table 1 shows the number of lesions that demonstrated each LI-RADS major criterion. Figure 1 shows the examples of each major criterion.
Examples of LI-RADS major criteria for HCC. A A large peripheral HCC in a 48-year-old man shows arterial enhancement. B HCC (white arrow) in a 65-year-old man demonstrates washout on delayed phase imaging. C Capsule appearance in addition to washout on delayed phase images is seen in a 58-year-old woman with cirrhosis and HCC. D Graphic representation of threshold growth defined as greater than 50% increase in diameter in 6 months or greater than 100% increase in 1 year
Arterial enhancement was the most commonly observed major criterion, seen in 159 (86%) of lesions, and was seen slightly more frequently at CT vs. MRI (87% vs. 86%, p = 1.00). Between the two raters, there was agreement on arterial phase characteristics in 156 (95%) cases (κ = 0.75).
Washout appearance was the second most frequently seen major criterion, seen in 94 (82%) lesions, and seen similarly at MRI and CT with delay (79% vs. 85%, p = 0.46). Agreement between readers on the presence or absence of washout occurred in 89% of cases (κ = 0.61).
Capsule appearance was seen in 31 (27%) lesions and was more commonly observed at MRI (44%) than at CT (17%), p = 0.002. Capsule appearance was most discrepant between the two readers, with 21 (18%) disagreements (κ = 0.56), with 13 (18%) disagreements at CT and 8 (19%) disagreements at MRI. Figure 2 shows four examples of cases in which there was disagreement between our readers on the presence or absence of capsule appearance. Subgroup analysis performed on CT studies with a delayed phase vs. those without a delayed phase revealed that washout was seen in 60/71 (85%) of studies with a delay compared to 37/70 (53%) of studies without a delay (p = 0.0001). Capsule was seen in 12/71 (17%) of CT studies with a delayed phase and in 7/70 (10%) of studies without a delayed phase (p = 0.32).
Discrepancy of capsule appearance assessment between readers. Axial contrast-enhanced MRI images in the delayed phase from a 57-year-old man (A) and a 66-year-old woman (B). Axial contrast-enhanced CT images also in the delayed phase from a 64-year-old man (C) and a 68-year-old man (D). All patients had pathologically proven hepatocellular carcinomas, and there was disagreement between readers on the presence of capsule appearance. In each case, the ultimate determination affected the LI-RADS category for the lesion. This effect was most pronounced for lesions between 1 and 2 cm, because arterially enhancing lesions ≥2 cm with washout qualify as LI-RADS 5 without additional major criteria
Of the 99 lesions with a relevant prior, 47 (47%) lesions demonstrated threshold interval growth (new lesion ≥1 cm, greater than 100% diameter increase in 12 months, or 50% in 6 months), with a similar distribution at both CT and MRI (49% and 42% respectively, p = 0.64). Figure 3 shows a LI-RADS 5 lesion demonstrating all 4 major criteria, arterial hyperenhancement, delayed phase washout, capsule appearance, and threshold growth at both CT and MRI.
Comparison of MRI and CT imaging of a LI-RADS 5 lesion in a 67-year-old man with cirrhosis and hepatocellular carcinoma. Arterially enhancing (A, B) segment 5/6 lesion which demonstrates washout and capsule appearance (C, D) was initially imaged at MRI (A, C). The lesion was imaged 4 months later at CT (B, D) re-demonstrating the same features in addition to a diameter increase meeting criteria for threshold growth
LI-RADS categorization
Figure 4 and Table 2 detail the distribution of consensus LI-RADS grading. ICC for LI-RADS category between individual readers was 0.59 with 95% CI of [0.30, 0.95]. There were 10 (9%) LI-RADS 3 lesions, 42 (37%) LI-RADS 4 lesions, and 61 (54%) LI-RADS 5 lesions. At CT with delay, the rates of LI-RADS 3, 4, and 5 lesions were 10%, 38%, and 52%, respectively. At MRI, these rates were 7%, 35%, and 56% (1 lesion was categorized LI-RADS 2 by MRI), respectively. At CT, 8% (n = 2) of 1–2 cm lesions were categorized LI-RADS 5, and at MRI, 43% (n = 6) were categorized LI-RADS 5 (p = 0.01). The presence or absence of capsule appearance changed the LI-RADS category (either between LI-RADS 3/4 or LI-RADS 4/5) in 7/8 (88%) small lesions where readers disagreed on capsule. In contrast, only 3/25 (12%) disagreements in capsule appearance resulted in change of category (all LI-RADS 4/5) of larger lesions (p = 0.0002).
Discussion
We evaluated 184 pathologically proven HCCs in 134 patients who underwent liver transplantation or partial hepatectomy at our institution between December 2008 and October 2013. The most commonly visualized major feature was arterial phase hyperenhancement followed by washout appearance. Inter-observer reliability for arterial hyperenhancement and washout were substantial. Capsule appearance was rarely seen. Inter-observer reliability for overall LI-RADS category was moderate.
Mirroring results of prior reports comparing the rates of portal venous and delayed phase washout at both CT and MRI [21, 22], there was a significant increase in the rate of HCCs demonstrating washout on CTs with a delayed phase compared to CTs without a delayed phase The rate of washout seen at CT with a delayed phase and MRI was similar. These results reaffirm added value in delayed phase imaging despite the increased radiation dose resulting from an additional CT acquisition.
Only 54% of lesions were categorized as LI-RADS 5. A lesion categorized LI-RADS 5 was modestly sensitive (50%–59%) for HCC. This is an expected result because the LI-RADS categories were designed to have high specificity (not tested in this study) at the cost of sensitivity. Creating a composite category of LI-RADS 4 (probably HCC) and LI-RADS 5 (definitely HCC) would provide 90%–91% sensitivity. Further study including non HCC lesions such as other liver tumors and benign nodules will be needed to determine the specificity of this combined category.
Capsule appearance was the least commonly seen major feature and had only moderate inter-observer reliability. Nonetheless, capsule is an important characteristic, particularly in arterially enhancing lesions between 1 and 2 cm. In lesions of this size, both capsule and washout appearance are required in order to characterize a lesion as LI-RADS 5. Because of the increased incidence of capsule appearance on MRI compared to CT, more lesions between 1 and 2 cm were categorized as LI-RADS 5 on MRI. This is an important finding as MRI may up-categorize observations affecting transplant listing using the Milan or UCSF criteria [23, 24], though a single lesion measuring less than 2 cm will not influence a patient’s standing on the transplant list as it does not qualify as T2 disease [25].
Analysis of inter-reader variability between CT and MRI showed an unexpectedly low Cohen’s kappa statistic for arterial phase hyperenhancement at MRI (κ = 0.22). Because the calculation of Cohen’s kappa involves removing agreement by chance, the result is dependent on the prevalence of the finding being compared as well as the sample size being examined. In the case of arterial phase enhancement at MRI, the vast majority of cases (86%) demonstrated arterial phase hyperenhancement, and therefore, a nonenhancing lesion was rare, increasing the expected rate of agreement by chance and artificially decreasing kappa the smaller MRI group.
There were a number of limitations of this single-center, retrospective study. As a tertiary referral center for liver transplant, 56% of the studies reviewed were from other institutions, resulting in heterogeneous technique and image quality. Accordingly, we were unable to evaluate the presence of ancillary features such as T2 signal intensity and DWI in patients imaged using MRI.
It is important to note that the absolute time between imaging and pathology appears lengthy (12.9 ± 8.9 months). This reflects the length of the transplant waiting list in our region. Patients with an imaging diagnosis of HCC often received local therapy such as TACE or ablation. Because imaging after treatment was excluded from this study, only the pre-therapy imaging could be reviewed. Because a majority of patients underwent localized therapy prior to transplantation, we were forced to limit analysis to those lesions that were proven to be HCC on pathology. This prevented the detection of false positive lesions and specificity was not calculated. Lastly, patients are typically referred to our institution at the initial diagnosis of HCC, so prior studies were not always available which prevented evaluation of threshold growth in some cases.
In conclusion, we have evaluated the sensitivity and reproducibility of LI-RADS in a large series of pathologically proven HCCs. Arterial phase hyperenhancement and washout appearance are the most common major criteria and demonstrate the most robust inter-reader variability. Rates of observed arterial hyperenhancement and capsule were similar at both CT and MRI. While infrequently seen overall, capsule appearance is more common at MRI and may impact LI-RADS categorization primarily in lesions between 1 and 2 cm.
References
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53(3):1020–1022. doi:10.1002/hep.24199
Wald C, Russo MW, Heimbach JK, et al. (2013) New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology 266(2):376–382. doi:10.1148/radiol.12121698
Practice guidelines for management of hepatocellular carcinoma (2009) 2009. Korean J Hepatol 15(3):391–423. doi:10.3350/kjhep.2009.15.3.391
European Association for the Study of the Liver (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56(4):908–943. doi:10.1016/j.jhep.2011.12.001
Omata M, Lesmana LA, Tateishi R, et al. (2010) Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatology international 4(2):439–474. doi:10.1007/s12072-010-9165-7
Kudo M, Izumi N, Kokudo N, et al. (2011) Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Digest Dis 29(3):339–364. doi:10.1159/000327577
Quality and safety resources: liver imaging–reporting and data system. Am Coll Radiol. http://www.acr.org/Quality-Safety/Resources/LIRADS. Accessed 16 Sep 2015
Freeman RB Jr, Wiesner RH, Harper A, et al. (2002) The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transplant 8(9):851–858. doi:10.1053/jlts.2002.35927
Freeman RB Jr, Gish RG, Harper A, et al. (2006) Model for end-stage liver disease (MELD) exception guidelines: results and recommendations from the MELD Exception Study Group and Conference (MESSAGE) for the approval of patients who need liver transplantation with diseases not considered by the standard MELD formula. Liver Transplant 12(12 Suppl 3):S128–S136. doi:10.1002/lt.20979
Sharma P, Balan V, Hernandez JL, et al. (2004) Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transplant 10(1):36–41. doi:10.1002/lt.20012
Ioannou GN, Perkins JD, Carithers RL Jr (2008) Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology 134(5):1342–1351. doi:10.1053/j.gastro.2008.02.013
Burrel M, Llovet JM, Ayuso C, et al. (2003) MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology 38(4):1034–1042. doi:10.1053/jhep.2003.50409
Rode A, Bancel B, Douek P, et al. (2001) Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr 25(3):327–336
Kim YK, Kim CS, Chung GH, et al. (2006) Comparison of gadobenate dimeglumine-enhanced dynamic MRI and 16-MDCT for the detection of hepatocellular carcinoma. AJR Am J Roentgenol 186(1):149–157. doi:10.2214/ajr.186.4_supplement.a149
Krinsky GA, Lee VS, Theise ND, et al. (2001) Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology 219(2):445–454. doi:10.1148/radiology.219.2.r01ma40445
Sangiovanni A, Manini MA, Iavarone M, et al. (2010) The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 59(5):638–644. doi:10.1136/gut.2009.187286
Lim JH, Kim CK, Lee WJ, et al. (2000) Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic livers: accuracy of helical CT in transplant patients. AJR Am J Roentgenol 175(3):693–698. doi:10.2214/ajr.175.3.1750693
Fowler KJ, Karimova EJ, Arauz AR, et al. (2013) Validation of organ procurement and transplant network (OPTN)/united network for organ sharing (UNOS) criteria for imaging diagnosis of hepatocellular carcinoma. Transplantation 95(12):1506–1511. doi:10.1097/TP.0b013e31828eeab2
Petruzzi N, Mitchell D, Guglielmo F, et al. (2013) Hepatocellular carcinoma likelihood on MRI exams: evaluation of a standardized categorization system. Acad Radiol 20(6):694–698. doi:10.1016/j.acra.2013.01.016
Davenport MS, Khalatbari S, Liu PS, et al. (2014) Repeatability of diagnostic features and scoring systems for hepatocellular carcinoma by using MR imaging. Radiology 272(1):132–142. doi:10.1148/radiol.14131963
Furlan A, Marin D, Vanzulli A, et al. (2011) Hepatocellular carcinoma in cirrhotic patients at multidetector CT: hepatic venous phase versus delayed phase for the detection of tumour washout. Br J Radiol 84(1001):403–412. doi:10.1259/bjr/18329080
Cereser L, Furlan A, Bagatto D, et al. (2010) Comparison of portal venous and delayed phases of gadolinium-enhanced magnetic resonance imaging study of cirrhotic liver for the detection of contrast washout of hypervascular hepatocellular carcinoma. J Comput Assist Tomogr 34(5):706–711. doi:10.1097/RCT.0b013e3181e1a88e
Mazzaferro V, Regalia E, Doci R, et al. (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. New Engl J Med 334(11):693–699. doi:10.1056/nejm199603143341104
Yao FY, Ferrell L, Bass NM, et al. (2001) Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33(6):1394–1403. doi:10.1053/jhep.2001.24563
Edge SBD, Compton CC, Fritz AG, Greene FL, Trotti A (2010) AJCC cancer staging handbook, 7th edn. Chicago: American Joint Committee on Cancer
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Study performed with approval from UCSF Committee on Human Research (IRB) approval.
Rights and permissions
About this article
Cite this article
Ehman, E.C., Behr, S.C., Umetsu, S.E. et al. Rate of observation and inter-observer agreement for LI-RADS major features at CT and MRI in 184 pathology proven hepatocellular carcinomas. Abdom Radiol 41, 963–969 (2016). https://doi.org/10.1007/s00261-015-0623-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0623-5