Abstract
Purpose
Splenomegaly and splenic siderosis are well-known findings in beta-thalassemia major. We explored the relation between splenic size, splenic and hepatic siderosis in transfusion-dependent beta-thalassemic patients, assessed by MR imaging.
Materials and methods
Abdominal MR imaging studies of 47 consecutive thalassemic patients and 10 healthy subjects, used as controls, were retrospectively reviewed. The signal intensity ratios of spleen and liver to the right paraspinous muscle (S/M, L/M, respectively) were calculated on T1, intermediate, and T2*-weighted gradient-echo sequences, splenic volume was estimated on axial images and serum ferritin levels were recorded.
Results
Decreased S/M on all MR sequences was displayed in 36 patients. Six patients presented with normal S/M on all MR sequences and 5 patients displayed splenic hypointensity only on T2* sequence. No correlation between S/M and L/M was found whereas both L/M and S/M correlated with serum ferritin (P < 0.03). Splenic volume correlated to L/M (P < 0.05) but not to S/M values.
Conclusion
In transfusion-dependent patients with beta-thalassemia, iron deposition in spleen cannot be predicted by the degree of hepatic siderosis, whereas splenomegaly relates to liver, but not splenic, iron overload. MR imaging can be a valuable tool in elucidating iron kinetics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
MRI features of spleen in beta-thalassemia major
Beta-thalassemia major is a hereditary hemolytic anemia that is characterized by deficient synthesis of beta chains of hemoglobin [1] and is treated by systematic red blood transfusions that eventually lead to increase of total iron stores. The most severe complications of iron overload are myocardiopathy and liver disease, whereas endocrine abnormalities, including diabetes mellitus, hypogonadism, and short stature are common complications that lead to poor quality of life and long-term morbidity [1, 2]. Chelation therapy has eliminated the toxic effects of iron and increased life expectancy of thalassemic patients; however, the monitoring of patients and evaluation of the effects of chelation therapy are based on regular and accurate estimation of iron stores [2, 3].
During the last decade, MR imaging has emerged as a noninvasive alternative to liver biopsy for quantification of liver iron overload, although there is no consensus between authors in regard to the optimal MR imaging technique [4–11]. MR imaging can also provide evidence of iron overload in other than liver tissues, which are difficult to access with biopsy, such as myocardium [12, 13], pituitary [14], pancreas [15], adrenals [16], and abdominal lymph nodes [17]. At our institution, we employ MR imaging for indirect quantification of hepatic and cardiac iron overload in a routine basis since 2000.
Decreased signal intensity (SI) of the spleen, most apparent on T2 spin echo (SE) and T2* gradient echo (GRE) sequences is a typical finding in beta-thalassemia and other pathologic conditions that require multiple red blood cell transfusions and is due to iron deposition within the reticuloendothelial cells (RES) of spleen [18–20]. However, the relation between splenic siderosis with hepatic iron overload has not been adequately documented in medical literature. Furthermore, in some of the transfusion-dependent thalassemic patients who referred to our hospital for MR evaluation of hepatic and myocardial iron overload, there was normal SI of the spleen coexisting with hypointense liver and pancreas. This pattern of iron distribution is unexpected for transfusional iron overload, and, on the contrary, is considered compatible with primary hemochromatosis [21, 22].
The goals of our study are, first, to describe the MR imaging findings of spleen, in terms of SI and size, in a series of 66 transfusion-dependent patients with beta-thalassemia major and, second, to investigate the relation between splenic size and siderosis and hepatic siderosis, as expressed by respective SI ratios, on T1, T2*, and intermediate-weighted MR imaging sequences.
Materials and methods
Study group
Abdominal MR imaging studies and medical records of 66 consecutive patients with beta-thalassemia major, aged 14–42 years old (23 men and 38 women, mean age 26.8 ± 6 years, median age 30.2 years) were retrospectively reviewed. The study protocol was approved by the board for retrospective studies of our institution. All MR imaging studies were performed at a single institution between May 2000 and January 2003 as part of the diagnostic work-up for the estimation of hepatic iron overload in beta-thalassemic patients. All patients received systematic red blood cell transfusions at 2–3 week intervals and subcutaneous chelation with desferrioxamine (30–50 mg/day). Serum ferritin levels, estimated 1–3 weeks before or after the MR examination, had been recorded to the patient archive of our MR Unit and ranged from 250 to 6520 ng/mL (normal values 20–200 ng/mL).
MR imaging studies of five nonthalassemic patients with no splenic or splenic-related pathology were included as the control group. The control and study groups did not present substantial differences in regard to age and sex.
MR imaging
Technique
All examinations were performed on a 1.5T MR imaging system (Vision Plus, Siemens, Erlangen, Germany). Body coil was used for both excitation and signal detection. The MR imaging technique comprised of a T1-weighted in-phase GRE sequence (120/4/90), an intermediate-weighted in-phase GRE sequence (PD) (120/4/20), and a T2*-weighted GRE sequence (120/15/20). All sequences were obtained in the axial plane, with slice thickness 10 mm, image matrix 256 × 192, and FOV 400 mm. This MR imaging protocol is routinely employed in our institution for the noninvasive estimation of liver iron overload in patients with thalassemia and is based on the technique proposed by Gandon et al. [9] from the University of Rennes, France.
Image interpretation
All MR imaging studies were retrospectively reviewed for the assessment of splenic size and SI by two experienced abdominal radiologists at the computer console and the results were recorded by consensus. The size of the spleen in both patients and healthy subjects was measured by using a technique previously described in a study for measuring normal spleens in 10 mm collimated unenhanced CT images [23]. The maximal width (W), determined as the largest diameter in any transverse section, the maximal thickness (T m), defined as the largest distance between the inner and outer borders of spleen perpendicular to the maximal width plane, the thickness at hilum (T h), determined as the distance between the inner and outer borders of spleen perpendicular to the splenic width through the hilum as well as the splenic length (L), assessed by the number of consecutive transverse sections through the spleen were measured. The splenic volume (Vol) was calculated using the formula: Vol = 30 + 0.58 (W × L × T h) [23]. The spleen was considered enlarged, if the determined splenic volume exceeded the respective mean + 2 standard deviation (mean + 2SD) value, as determined by normative data for the respective sex [24]. Estimation of splenic size was performed on the T1-weighted GRE images, which had the best spatial resolution.
Splenic SI in both patients and healthy subjects was quantitatively assessed on each of the respective sequences as the average of two SI measurements obtained at circular regions of interest (ROIs) that measured 1–2 cm2 and were located at the periphery of the posterior segment of splenic parenchyma. The SI of liver was calculated as the average measurements of two similar ROIs located in the right liver lobe, away from vascular structures. All measurements were performed on the same slice. Subsequently, the SI ratios of spleen and liver to the right paraspinous muscle (S/M and L/M ratios, respectively) were calculated on the above MR sequences.
The data were statistically analyzed using the Statistical Package for the Social Sciences (SPSS 8.0 for windows, Chicago, Illinois, USA). Linear regression analysis (Pearson’s r) was employed to correlate L/M ratios, S/M ratios, splenic size, and ferritin levels. T-test was employed to compare the S/M values with the presence of splenomegaly. Statistical significance was set at 0.05.
Results
Nineteen patients (19/66, 28.7%) were splenectomized. Our study group included the remaining 47 patients, where spleen was present (25 male, mean age 24.2 ± 4.7 years, age range 12–32 years). None of the patients presented with focal areas of signal abnormality indicative of focal lesions in the spleen.
Of the 47 nonsplenectomized patients, 36 presented with splenic hypointensity on all MR imaging sequences (S/M less than mean value − 2SD of normal controls in the respective sequences), 6 patients presented with SIs within the range of normal controls in all MR imaging sequences and 5 patients showed normal S/M on T1 and PD sequence and mild hypointensity (less than mean value − 2SD of normal controls) on T2* sequence. According to the SI of the spleen, patients were categorized into two groups: group 1 (Figs. 1, 2) that comprised of the 36 patients with splenic hypointensity on all MR sequences and group 2 (Fig. 3) that comprised of the total number of patients with normal signal in at least T1 and PD sequences (total number 11) (Table 1).
A T1-weighted (TR/TE/FA: 120/4/90), B intermediate (PD)-weighted (120/4/20), and C T2*-weighted (120/15/20) MR images of a 20-year-old man with beta-thalassemia and severe iron overload (serum ferritin 4700 ng/mL). Both liver and spleen are markedly hypointense with signal intensities equal to background noise in all MR imaging sequences. There is also excessive splenomegaly (spleen volume 1596 cm3). (Spleen to muscles SI ratios: 0.1 on T1, 0.2 on intermediate, and 0.1 on T2*-weighted sequences, respectively. Liver to muscle signal intensity ratios: 0.1 on T1, 0.08 on intermediate, and 0.1 on T2*-weighted sequences, respectively).
A T1-weighted (TR/TE/FA: 120/4/90), B intermediate (PD)-weighted (120/4/20), and C T2*-weighted (120/15/20) MR images of a 28-year-old woman with beta-thalassemia and mild iron overload (serum ferritin 680 ng/mL). The spleen volume is within normal limits (310 cm3) but is hypointense in all MR imaging sequences; the signal drop is more prominent on the T2*-weighted sequence. The liver has normal signal on the T1- and intermediate-weighted sequences and is hypointense only on the T2*-weighted sequence. (Spleen to muscles SI ratios: 0.7 on T1, 0.8 on intermediate, and 0.4 on T2*-weighted sequences, respectively. Liver to muscle signal intensity ratios: 1.4 on T1, 1.2 on intermediate, and 1 on T2*-weighted sequences, respectively).
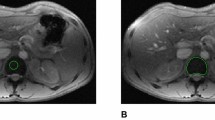
A T1-weighted (TR/TE/FA: 120/4/90), B intermediate (PD)-weighted (120/4/20), and C T2*-weighted (120/15/20) MR images of a 21-year-old man with beta-thalassemia and moderate iron overload (serum ferritin 1540 ng/mL). The spleen has increased volume (682.5 cm3) but normal SI in all MR imaging sequences, whereas the liver is hypointense. (Spleen to muscles SI ratios: 1.1 on T1, 1.4 on intermediate, and on 1.8 on T2*-weighted sequences, respectively. Liver to muscle signal intensity ratios: 0.2 on T1, 0.1 on intermediate, and 0.1 on T2*-weighted sequences, respectively).
No correlation between S/M and L/M was found in any sequence. S/M correlated negatively with mean ferritin values in all sequences (Pearson’s correlation, r = −0.545, P ∼ 0.00 on T1, r = −0.308, P = 0.01 on PD and r = −0.282, P = 0.03 in T2* MR sequences, respectively, Fig. 4). Serum ferritin correlated also with L/M (Pearson’s correlation, r = −0.4, P = 0.001 on T1, r = −0.4, P ∼ 0 on PD, and r = −0.2, P = 0.03 in T2*, respectively).
In regard to the splenic size, the mean splenic volume of the study group was 689.8 ± 468 cm3 (min. 113.5 cm3, max. 2234 cm3) and the mean length (L), T h, and W were 4 ± 3.1, 8.6 ± 6, and 10.2 ± 7.3 cm, respectively. Splenomegaly (splenic volume exceeding mean value + 2SD of the reported normative data for men and women) was present in 35 patients, predominantly male (22 male, 13 female, x 2 = 5.144, P = 0.02).
Normal controls presented with mean splenic volume 267 ± 63 cm3 and mean splenic S/M 1 ± 0.1 on T1, 1.4 ± 0.2 on PD, and 1.6 ± 0.1 on T2* sequence.
The mean splenic volume of group 1 (707 cm3) was found to be increased compared to that of group 2 (684 cm3), but the difference was not statistically significant (t = 0.144, P = 0.886) (Table 1). Splenomegaly was also more frequent in group 1 patients (9/11 patients, 83.3%) compared to group 2 patients (26/36 patients, 71%) but that did not reach statistical significance (Fisher’s exact test, x 2 = 0.536, P = 0.699). Splenic volume of the whole study group correlated negatively with L/M on T1 and PD GRE sequences (Pearson’s correlation, r = −0.359, P = 0.01 on T1 and r = −0.295, P = 0.05 on PD GRE sequence, respectively, Fig. 5) but not with L/M values on T2* GRE sequence or with S/M values on any GRE MR imaging sequence.
Discussion
Beta-thalassemia major is a severe dyserythropoietic, hypochromic, and microcytic anemia that requires systematic red blood cell transfusions. Ineffective erythropoiesis leads to tissue hypoxia that, if not adequately treated with blood transfusions, stimulates increased iron absorption from the intestine and incites bone marrow hyperplasia as well as extramedullary hemopoiesis in the spleen, liver, and adjacent to thoracic vertebrae [1, 2]. Therefore, in beta-thalassemia iron enters the body by two pathways: first, destruction of senescent native and transfused red blood cells—that is the main source of iron overload—and, second, increased absorption of iron from the intestine [25]. Iron derived from phagocytosis of red blood cells accumulates initially in the RES cells of the spleen, liver, and bone marrow and after their saturation redistributes in the parenchymal cells with high oxygen demands, such as hepatocytes, pancreatic cells, myocardium, and anterior lobe of the pituitary gland [25].
Since the early implementation of MR imaging there was a striking reduction in SI of the iron-overloaded liver and spleen due to the strong paramagnetic properties of intracellular ferritin and hemosiderin [26, 27]. Many subsequent studies have documented close correlations between liver iron concentration and hepatic T2 relaxation times or SI ratios [4–10]. Currently, MR imaging is employed with increasing frequency for the noninvasive quantification of hepatic and cardiac iron overload. Decreased SI of the spleen on T2 SE or T2* GRE sequences has been associated either with transfusional iron overload of various etiologies, such as hemolytic anemia, myelodysplastic syndromes and malignancies or rhabdomyolysis [21, 22]. In rhabdomyolysis, myoglobin, an iron-containing protein is released in circulation and subsequently metabolized in liver and spleen [21]. Gomori et al. [18] found a close correlation between iron concentration and T2 relaxation time of splenic parenchyma in patients with beta-thalassemia, whereas Oie et al. [19] refer that the most sensitive sequence for the detection of iron overload in the liver and spleen is T2* GRE sequence and found a moderate correlation between serum ferritin and S/M ratios on T2*-weighted sequence, but not between S/M and liver iron concentration.
In the current study, no correlation was documented between L/M and S/M in any imaging sequence, a moderate correlation between S/M and serum ferritin was found, similar to the findings by Ooi et al. In beta-thalassemia, iron deposits in the RES cells of spleen and bone marrow but as regards liver, iron accumulates both in RES cells and in hepatocytes. Moreover, there are differences between the RES cells of liver and spleen in regard to iron accumulation. It has been shown that after superparamagnetic iron oxide (SPIO) administration, magnetic susceptibility effects on T2*-weighted images depend on intracellular SPIO cluster size [28, 29]. In the spleen the T2* effect was decreased as compared with that in the liver, because of smaller cluster size in the spleen [28, 29]. Recent studies have also reported that the degree of hepatic iron overload, as assessed by MR imaging, relates to iron deposition in the adrenals and abdominal lymph nodes [16, 17]; however, other studies have documented that iron deposition in myocardium and pituitary cannot be predicted by the degree of hepatic siderosis [12, 30]. These observations may reflect diversity in iron uptake and excretion between the various tissues.
It was noteworthy that in 11/47 (23%) of our transfusion-dependent thalassemic patients the spleen exhibited normal SI in at least two sequences, that is compatible with no or very mild siderosis. Iron deposition in the parenchymal cells of liver with sparing of the spleen has not been previously described in transfusion-dependent patients, where splenic siderosis is expected to predominate. On the other hand, hepatic siderosis with normal splenic SI is considered typical for primary hemochromatosis [22], and has also been sporadically described in dyserythropoietic conditions that do not require red blood cell transfusions [20]. There are scarce descriptions of patients with primary hemochromatosis who displayed splenic hypointensity on MR imaging, the cause of which remains uncertain [21, 27]. MR imaging can be a valuable tool in the assessment of iron distribution in various human tissues and the evaluation of chelation regimens. Differences in genotypes may account for different patterns of iron distribution between patients with beta-thalassemia. Most recent studies report the presence of hemochromatosis gene mutations in beta-thalassemia carriers [31, 32].
Extramedullary hemopoiesis is a well-known complication in beta-thalassemia and other pathologic conditions associated with ineffective erythropoiesis [1, 33]. Extramedullary hemopoiesis may involve the spleen as microscopic diffuse infiltration and cause splenomegaly, or, rarely, present as mass-like lesions, that may be hyperintense on T2-weighted sequence and contrast to the remaining hypointense splenic parenchyma [33, 34]. A drawback of our study is that we lack biopsies of the spleen, that might explain this unexpected splenic hypointensity, therefore diffuse extramedullary hemopoiesis cannot be ruled out in these thalassemic patients with normal splenic signal.
Splenomegaly is a common complication of beta-thalassemia major that is commonly seen in patients with poor compliance to chelation therapy [35] and aggravates red blood cell destruction leading to increased transfusion demands and further total iron overload. Studies measuring splenic size in thalassemic patients have been based so far on clinical assessment [35] and ultrasound [36] and report increased splenic dimensions. Splenic size was found to depend on blood transfusion cycles decreasing up to the 10th posttransfusion day and reaching its maximum size again prior to the next blood transfusion, in relevance to blood volume sequestrated in this organ [35]. Splenic size is an important parameter in deciding spleen elimination therapy, since partial or total splenectomy is indicated in patients with excessive red blood cell consumption and splenomegaly [37]. In our study 74% of the patients were found to have increased splenic size. Splenic size correlated with L/M and serum ferritin values, conforming to the results of previous clinical studies that refer to an increased frequency of splenomegaly in thalassemic patients with severe iron overload [35, 36]. Nevertheless, splenomegaly did not correlate with the degree of splenic siderosis, as expressed by S/M ratios. MR imaging can provide quantification of splenic siderosis and measurement of splenic size in the same study, which is important in determining the transfusion requirements and need for splenectomy.
In conclusion, spleen siderosis may not follow the degree of liver iron overload, in transfusion-dependent patients with beta-thalassemia major. Although the spleen is usually hypointense due to iron deposition in RES cells, occasionally, the spleen may display normal SI in the presence of hypointense liver, similar to the pattern of iron distribution seen in primary hemochromatosis. On the other hand, splenomegaly was found to correlate with the degree of liver iron overload. MR imaging due to its capability to assess iron overload and volume of each individual solid viscera, during a single MR study of the upper abdomen, may be of value to elucidate iron kinetics, to determine the transfusion requirements and need for splenectomy, and to contribute in the evaluation of the effect of new chelating agents.
References
Weatherall DJ, Clegg JB, Na-Nakorn S, et al. (1969) The pattern of disordered haemoglobin synthesis in homozygous and heterozygous b-thalassemia. Br J Haematol 16:251–267
Kattamis C, Metaxotou-Mavromati A, Ladis V, et al. (1982) The clinical phenotype of beta and delta beta thalassemias in Greece. Eur J Pediatr 139:135–138
Gabutti V, Piga A (1996) Results of long term iron chelating therapy. Acta Haematol 95:26–36
Gandon Y, Guyader D, Heautot JF, et al. (1994) Hemochromatosis: diagnosis and quantification of liver iron with gradient echo MR imaging. Radiology 193:533–538
Ernst O, Sergent G, Bonvarlet P, et al. (1997) Hepatic iron overload: diagnosis and quantification with MR imaging. AJR 168:1205–1208
Papakonstantinou OG, Maris TG, Kostaridou V, et al. (1995) Assessment of liver iron overload by T2-quantitative magnetic resonance imaging: correlation with serum ferritin concentration and histologic grading of siderosis. Magn Reson Imaging 13:967–977
Fenzi A, Bortolazzi M, Marzola P (2003) Comparison between signal-to-noise ratio, liver-to-muscle ratio and 1/T2 for the noninvasive assessment of liver iron content by MRI. J Magn Reson Imaging 17:589–592
Alustiza JM, Artetxe J, Castiella A, et al. (2004) Gipuzkoa hepatic iron concentration by MRI study group MR quantification of hepatic iron concentration. Radiology 230:479–484
Gandon Y, Olivie D, Guyader D, et al. (2004) Non-invasive assessment of hepatic iron stores by MRI. Lancet 363:357–362
Jensen PD (2004) Evaluation of iron overload. Br J Haematol 124:697–711
St Pierre TG, Clark PR, Chua-Anusorn W, et al. (2005) Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105:855–861
Anderson LJ, Westwood MA, Holden S, et al. (2004) Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol 127:348–355
Westwood M, Anderson LJ, Firmin DN, et al. (2003) A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging 8:33–39
Argyropoulou MI, Metafratzi Z, Kiortsis DN, et al. (2000) T2 relaxation rate as an index of pituitary iron overload in patients with beta-thalassemia major. Am J Roentgenol 75:1567–1569
Midiri M, Lo Casto A, Sparacia G, et al. (1999) MR imaging of pancreatic changes in patients with transfusion dependent beta-thalassemia major. AJR 173:187–192
Drakonaki E, Papakonstantinou O, Maris T, et al. (2005) Adrenal glands in beta-thalassemia major: magnetic resonance (MR) imaging features and correlation with iron stores. Eur Radiol 15:2462–2468
Papakonstantinou O, Maris TG, Kostaridou S, et al. (2005) Abdominal lymphadenopathy in beta-thalassemia: MRI features and correlation with liver iron overload and posttransfusion chronic hepatitis C. AJR 185:219–224
Gomori JM, Grossman RI, Drott HR (1988) MR relaxation times and iron content of thalassemic spleens: an in virto study. AJR 150:567–569
Ooi GC, Khong PL, Chan GC, et al. (2004) Magnetic resonance screening of iron status in transfusion-dependent beta-thalassemia patients. Br J Haematol 124:385–390
Yoon DY, Choi BI, Han JK, et al. (1994) MR findings of secondary hemochromatosis: transfusional versus erythropoetic. J Comput Assist Tomogr 18:416–419
Siegelman ES, Mitchell DG, Rubin R, et al. (1991) Parenchymal versus reticuloendothelial iron overload in the liver: distinction with MR imaging. Radiology 179:361–366
Siegelman ES, Mitchell DG, Semelka RC (1996) Abdominal iron deposition: metabolism, MR findings and clinical importance. Radiology 199:13–22
Prassopoulos P, Daskalogiannaki M, Raissaki M, et al. (1997) Determination of normal splenic volume on computed tomography in relation to age, gender and body habitus. Eur Radiol 7:246–248
Geraghty EM, Boone JM, McGahan JP, et al. (2004) Normal organ volume assessment from abdominal CT. Abdom Imaging 29:482–490
Cazzola M, Borgna-Pignatti C, de Stefano P, et al. (1983) Internal distribution of excess iron and sources of serum ferritin in patients with thalassemia. Scand J Haematol 30:289–296
Gomori JM, Horev G, Tamary H, et al. (1991) Hepatic iron overload: quantitative MR imaging. Radiology 179:367–369
Johnston DL, Rice L, Vick EW, et al. (1989) Assessment of tissue iron overload by nuclear magnetic resonance imaging. Am J Med 87:40–47
Tanimoto A, Oshio K, Suematsu M, et al. (2001) Relaxation effects of clustered particles. J Magn Reson Imaging 14:72–77
Tanimoto A, Pouliquen D, Kreft BP, et al. (1994) Effects of spatial distribution on proton relaxation enhancement by particulate iron oxide. J Magn Reson Imaging 4:653–657
Argyropoulou MI, Kiortsis DN, Efremidis SC (2003) MRI of the liver and the pituitary gland in patients with beta-thalassemia major: does hepatic siderosis predict pituitary iron deposition? Eur Radiol 13:12–16
Martins R, Picanco I, Fonseca A, et al. (2004) The role of HFE mutations on iron metabolism in beta-thalassemia carriers. J Hum Genet 49:651–655
Melis MA, Cau M, Deidda F, et al. (2002) H63D mutation in the HFE gene increases iron overload in beta-thalassemia carriers. Haematologica 87:242–245
Katz DS (1999) Splenic extramedullary hematopoiesis: large focal lesion in a patient with thalassemia. AJR 173:1715–1716
Gabata T, Kadoya M, Mori A, et al. (2000) MR imaging of focal extramedullary hematopoiesis in polycythemia vera: case report. Abdom Imaging 25:514–516
Karpathios T, Antypas A, Dimitiriou P, et al. (1982) Spleen size changes in children with homozygous beta-thalassaemia in relation to blood transfusion. Scand J Haematol 28:220–226
Tassiopoulos T, Rombos Y, Konstantopoulos K, et al. (1995) Spleen size in beta-thalassaemia heterozygotes. Haematologia (Budap) 26:205–209
Piomelli S (1995) The management of patients with Cooley’s anemia: transfusions and splenectomy. Semin Hematol 32:262–268
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Papakonstantinou, O., Drakonaki, E.E., Maris, T. et al. MR imaging of spleen in beta-thalassemia major. Abdom Imaging 40, 2777–2782 (2015). https://doi.org/10.1007/s00261-015-0461-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0461-5