Abstract
Purpose
Computed tomographic (CT) fluoroscopy-guided percutaneous cryoablation is an effective therapeutic method used to treat focal renal masses. The purpose of this study is to quantify the radiation dose to the patient and interventional radiologist during percutaneous cryoablation of renal masses using CT fluoroscopic guidance.
Methods
Over a 1-year period, the CT fluoroscopy time during percutaneous cryoablation of renal masses was recorded in 41 patients. The level of complexity of each procedure was designated as simple, intermediate, or complex. Patient organ radiation doses were estimated using an anthropomorphic model. Dose to the interventional radiologist was estimated using ion chamber survey meters.
Results
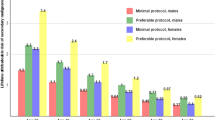
The average CT fluoroscopy time for technically simple cases was 47 s, 126 s for intermediate cases, and 264 s for complex cases. The relative risk of hematologic stomach and liver malignancy in patients undergoing this procedure was 1.003–1.074. The lifetime attributable risk of cancer ranged from 2 to 58, with the highest risk in younger patients for developing leukemia. The estimated radiation dose to the interventionalist without lead shielding was 390 mR (3.9 mGy) per year of cases.
Conclusions
The radiation risk to the patient during CT fluoroscopy-guided percutaneous renal mass cryoablation is, as expected, related to procedure complexity. Quantification of patient organ radiation dose was estimated using an anthropomorphic model. This information, along with the associated relative risk of malignancy, may assist in evaluating risks of the procedure, particularly in younger patients. The radiation dose to the interventionist is low regardless of procedure complexity, but highlights the importance of lead shielding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Computed tomographic (CT) fluoroscopy is well suited for the rapid targeting, localization, and placement of needles and probes during percutaneous interventional procedures [1]. Percutaneous thermal ablation has demonstrated an increasingly prominent role as a minimally invasive treatment for renal tumors [2–4]. However, concerns regarding radiation exposure to both the patient and radiologist during CT fluoroscopy-guided procedures have been raised [5, 6]. In recent years, clinicians have become increasingly conscious of cumulative radiation dose to their patients [7]. Several recent studies have focused on estimation of effective dose (ED) and skin dose during CT-guided cryoablation [8, 9]. However, different techniques utilized during CT fluoroscopy-guided tumor ablation may variably affect the estimated radiation dose and risk of cancer induction for radiosensitive organs.
A variety of techniques may be utilized by the interventionalist performing the CT-guided fluoroscopic procedure, including alterations in patient positioning, CT gantry angulation, intravenous contrast material administration, multi-probe ablation, and hydrodissection of adjacent structures [1, 10]. The techniques described above may all result in increased fluoroscopy time and radiation dose required to perform tumor ablation. While mean dose-length products (DLP) have been reported for various types of percutaneous interventions, including for thermal ablation, the resulting ED and skin dose calculations serve only as estimates [10]. Furthermore, the dose to the operator has not been quantified. Therefore, the purpose of this study was to measure the radiation dose to the patient and operator during CT fluoroscopy-guided percutaneous cryoablation of renal masses, taking into account differing patient positioning and fluoroscopy protocols, and to use these measurements to estimate the patient’s risk of developing radiation-induced cancer.
Materials and methods
Patient population
The Institutional Review Board at our institution approved this retrospective study, and informed consent was waived. The study population included 41 renal percutaneous cryoablation procedures in 39 patients performed during the calendar year 2009. The mean age was 65 years, and the range 22–86 years, with a standard deviation of 14 years. Twenty-seven of the 39 patients were male (69 %) and 12 were female. The average BMI (body mass index) of the patients was 29.1. Demographic information and procedural details, including the number of renal masses treated, number of probes used, and use of optional techniques such as intravenous contrast material and hydrodissection were obtained retrospectively from medical records.
Percutaneous cryoablation technique
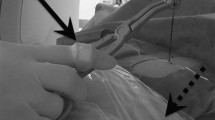
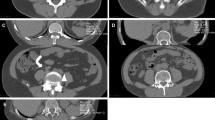
All procedures were performed or supervised by an attending radiologist. Patients were positioned at the discretion of the operator based on characteristics of the renal mass such as the size of the lesion, the location within the kidney and the location of adjacent organs. Moderate sedation was administered. A General Electric LightSpeed Xtra (GE Healthcare, Milwaukee, WI) 80 cm wide-bore 16-slice MDCT scanner was used for both the phantom experiment and the clinical procedures. A limited non-contrast CT scan through the kidneys was routinely performed for planning purposes. After administration of local anesthesia, CT fluoroscopic guidance was utilized for probe insertion (Perc24, Endocare Inc, Irvine, A). The kVp was typically set to 120, while the mAs was set to 50–100 at the discretion of the operator, according to the size of the patient. The number of probes utilized was also at the operator’s discretion. Administration of intravenous contrast material with an additional limited diagnostic CT through the kidneys was performed at the discretion of the operator when visualization of the lesion was difficult on the preliminary non-contrast CT. When adjacent organs such as bowel were in excessively close proximity to the lesion, hydrodissection was performed by introducing an 18-gage needle interposed between the kidney and the organ using CT fluoroscopic guidance followed by infusion of 100–300 mL of saline until an adequate buffer zone was achieved. A 10 min freeze −8 min thaw −10 min freeze cycle was conducted. After removal of the probes, a repeat non-contrast diagnostic CT scan through the region of the kidneys was performed to assess for complications such as hematoma or pneumothorax and for assessment of the cryoablation zone.
Phantom
An adult male anthropomorphic phantom (Model 702-D, CIRS, Norfork, VA) was used to quantify organ-specific radiation dose. This commercially available phantom has been validated for organ dosimetry. It consists of material with densities intended to mimic soft tissue, cortical bone, and bone marrow, and is divided into 39 axial slabs, each 25 mm thick. Each slab contains numerous holes measuring 5 mm in diameter, which are used to hold detectors for organ dose measurements. Major organs are represented by assignable, anatomically located holes, and each hole is optimized for precise, specific organ dosimetry [11].
Phantom scanning technique
The phantom was subjected to a simulated CT fluoroscopy-guided percutaneous cryoablation procedure based on usual clinical percutaneous cryoablation technique as described above. Both prone and lateral decubitus positions were analyzed. As per usual clinical practice, the phantom was exposed to two limited non-contrast CT scans through the kidneys to mirror the pre-procedure and post-procedure scans. It was then exposed to 60 s of continuous fluoroscopy in both positions, to simulate insertion and positioning of cryoablation probes. In clinical practice, CT gantry tilting may be performed on occasion to facilitate fluoroscopic guidance. Therefore, the effect of gantry tilting to 24.5° (the maximum allowed by the scanner) was assessed. The CT parameters used in the phantom simulation were considered to be within the limits of the standard of care (Table 1).
The phantom was exposed to each fluoroscopy protocol three times to obtain the average and standard deviation for each organ dose. Organ doses were divided by the exposure time to obtain dose rates for individual organs. Twenty detector calibration factors for each scan were created and stored in a laptop computer by using AutoSense PC Software (Model TN-RD-45; Thomson-Nielsen, Ottawa, Ontario, Canada).
Organ dose measurements
One medical physicist and members of his staff performed the radiation component of this study. Both physicists had 8 years of experience in dosimetry determination with metal oxide semiconductor field effect transistor (MOSFET) detectors. MOSFET detectors (Model TN1002R-D, Best Medical Canada) were used to obtain point doses for organs within the phantom. Since CT fluoroscopy is a targeted technique, specific organs were chosen to accurately reflect dose deposition. Two radiologists, who have a combined 11 years of experience performing cryoablation procedures, determined the appropriate locations for dosimeter placement. Multiple detectors were assigned to single organs spanning multiple contiguous axial slices (n = 12). Multiple detectors were also placed within the same slice to ensure accurate distribution within a given organ. MOSFET detectors were distributed among the following components of the phantom: liver, spleen, pancreas, bilateral kidneys, bilateral adrenal glands, bone marrow of the lumbar spine, and skin surface.
Effective dose determination
The dose values from the three 60-s fluoroscopy exposures to the phantom were averaged for each individual organ. The ED was then calculated according to the International Commission on Radiological Protection (ICRP) Report 103 as the sum of the measured absorbed organ doses multiplied by the applicable tissue weighting factors [12]. The uncertainty in ED was estimated by quadrature summation of the standard deviation of the organ dose measurements.
Determination of lifetime attributable and relative risk for radiation-induced cancer
The average dose for individual organs was used to calculate the lifetime attributable risk (LAR) for radiation-induced cancer. Organ-specific LAR values have been tabulated for 0.1 Gy of exposure based on age and gender [7]. Estimation of the LAR of radiation-induced stomach cancer, liver cancer, and leukemia was calculated for each of the phantom positions in patients exposed at varying ages. These tissues were selected as the most radiation sensitive in the fluoroscopic field of the procedure. The ages for the risk calculations were selected based on the demographics of the patient population. LAR was calculated using the dose rate for each organ multiplied by the clinical fluoroscopic exposure time. These exposure durations were based on average times from the study population as well as the complexity of the procedure. The level of complexity of each cryoablation procedure was designated as either: (a) simple: single renal mass requiring 1–2 probes; (b) intermediate: if hydrodissection was performed or IV contrast material was administered in addition to meeting criteria for a simple procedure, or a single renal mass requiring 3–4 probes; and (c) complex: single mass requiring 5 or more probes, or ablation of more than 1 renal lesion. These classifications were felt to be clinically relevant and necessary to create distinct risk stratification groups. The LAR was calculated by multiplying the ratio of each estimated absorbed dose to 0.1 Gy by the tabulated value for each organ measured [7].
The average dose for each individual organ was also used to calculate relative risk (RR) values. RR values were estimated according to the following formula:
for which LR is the unexposed baseline lifetime risk of cancer incidence per 100,000.
Dose to the radiologist
When performing these procedures, radiologists typically occupy one of two positions near the scanner. Position 1 is 38.1 cm from the gantry and 30.5 cm from the table. Position 2 is 101.6 cm from the gantry and 61 cm from the table. An assistant or trainee typically occupies position 2. During 1 min of CT fluoroscopy, the ion chamber detectors (Fluke Biomedical, Model: Victoreen 451P) were held 116.8 cm from the floor in these two positions.
Results
Forty-one CT fluoroscopy-guided percutaneous cryoablation procedures were performed on 39 patients over the course of 1 year. The complex case category had the largest standard deviation in procedure time (Table 2). One patient in the complex case category had two unilateral lesions, which were treated with one probe each. This patient required the least amount of CT fluoroscopy time, which was substantially less time than the other five complicated cases.
Organ dose measurements
In the prone position during the pre-procedure scan, the highest organ dose was measured in the skin. In the decubitus position during the pre-procedure scan, the highest dose was measured in the pancreas and stomach. In the prone position during fluoroscopy, the highest doses were recorded in the skin, kidneys, pancreas, and liver. With the CT gantry tilted, there was no significant difference in dose recordings in the specified organs compared to no gantry angulation. In the left lateral decubitus position, the highest doses were recorded in the skin, liver, and pancreas. Compared to the prone position, there was a significant decrease in dose to the dependent left kidney with left lateral decubitus positioning.
Effective dose measurements
Overall, the prone patient position during fluoroscopy had the highest ED, followed by the prone position using a tilted gantry, and then by the left lateral decubitus position (Table 3).
Lifetime attributable risk (LAR) and relative risk for radiation-induced leukemia, liver, and stomach cancer
For leukemia, LAR ranged from five excess cases per 100,000 patients in 60-year-old women undergoing a simple procedure in the decubitus position, to 58 excess cases in 30-, 40-, and 50-year-old men undergoing the procedure in the prone position with the CT gantry in a tilted position. For liver cancer, LAR ranged from one excess case in 60-year-old women undergoing a simple procedure in the prone position with a tilted gantry, to 21 excess cases in 30-year-old men undergoing a complex procedure in the decubitus position. For stomach cancer, LAR ranged from three excess cases in 60-year-old men undergoing the procedure in the lateral decubitus position, to 28 excess cases in 30-year-old women undergoing the procedure in the prone position (Table 4).
The highest relative risk of 1.074 was seen for the incidence of leukemia in 30-year-old women undergoing the procedure in the prone position with a gantry tilted (Table 5). The associated estimated LAR for women at this age was 43 excess cases per 100,000 patients (Table 4).
Dose to the radiologist
The dose to the radiologist was estimated in the absence of shielding garments to allow for more accurate measurement. If the same radiologist performed all 41 percutaneous cryoablation procedures at the reported CT fluoroscopic times, the estimated radiation dose at a distance of 38.1 cm from the bore of the scanner is estimated to be 5.03 mR (0.0503 mGy) per min of CT fluoroscopy time, or 390 mR (3.9 mGy) over 41 procedures (which took place over one calendar year at our institution). The dose to an assistant standing behind the primary radiologist is estimated to be 1.14 mR (0.0114 mGy) per min or 88 mR (0.88 mGy) over 41 procedures.
Discussion
We believe this to be the first report that quantifies absorbed organ radiation doses during CT fluoroscopy-guided cryoablation procedures of solid renal tumors. The organs directly in the field of fluoroscopic view (liver, spleen, kidneys, pancreas, and adrenals) have the overall highest absorbed doses, as expected. In the left lateral decubitus position, the liver had the highest overall absorbed dose, which was higher than the absorbed dose in the prone position. There is likely a small amount of absorption of photons by the CT table, which decreases the dose for dependent organs and increases the dose for nondependent organs. Furthermore, for certain positions, such as lateral decubitus, there is an added effect of certain organs being closer to the x-ray tube.
Patient effective dose measurements
Overall, the prone position during CT fluoroscopy had the highest overall ED, followed by prone position using a tilted gantry and the left lateral decubitus position. We did not use the DLP or CTDI to estimate ED, because CT fluoroscopy is an anatomically focused procedure that does not have a z-axis measurement.
Lifetime risks of radiation
For more complex cryoablation procedures, the longer CT fluoroscopy time resulted in a greater estimated lifetime attributable and relative risk of developing radiation-induced cancer in radiosensitive organs. We estimate that the lifetime excess risk of developing leukemia using the tilted gantry technique is significantly higher than the excess risk of cancer induction for other techniques and types of cancer. The excess risk of leukemia using a tilted gantry is two times higher than the neutral gantry position for any patient age, gender, and level of procedural complexity. Younger women who undergo complex procedures have a higher risk. After a single complex procedure using a tilted gantry, the estimated lifetime excess relative risk was 6.7 and 7.4 % in 60-year-old and 30-year-old females, respectively. We suspect that the combination of a tilted gantry and prone patient position may expose the medullary hematopoietic tissue of the lumbar spine to more incident radiation compared to the neutral gantry position. For comparison, the lifetime excess relative risk for breast or lung cancer in young women aged 15–25 years undergoing a single CT angiographic examination of the heart is 5.5 %, which is a high excessive risk in this susceptible population [13]. The mechanism of the tilted gantry technique’s potential of inducing leukemia should be investigated further, perhaps in a small animal model.
Our estimates of lifetime excess relative risk of cancer for solid tumors of the stomach and liver, while the patient is in the prone or lateral decubitus position and with the tilted CT gantry range from 1.0 to 2.0 % for simple and intermediate complexity procedures. Although the radiation-related risk is small, the use of this technology to treat renal tumors, along with the other inherent risks of this procedure, should be weighed against the risks posed by alternative procedures (e.g., general anesthesia and surgical tumor resection).
As expected, the lifetime excess relative risk during more technically complex procedures is higher. This is especially true in younger women, for whom the lifetime excess risk for stomach cancer in a 30-year-old woman is as high as 3.9 % for a single complex cryoablation procedure. The potential for cancer induction in younger patients is more substantial, especially young patients with hereditary conditions such as Von Hippel-Lindau syndrome, who may have multiple solid renal tumors and could undergo numerous complex cryoablation procedures. Over time, the cumulative radiation dose from diagnostic CT examinations also must be considered in this patient population. For all age groups, the risk of radiation-induced cancer would be expected to increase if multiple follow-up multi-detector CT studies were performed. Although this cumulative effect has been established to increase breast cancer risk [14], it likely applies to the radiation-induced malignancies examined in this study as well.
Study limitations
Our estimates of radiation risk are based on compilations by the biological effects of ionizing radiation (BEIR) committee, and should be interpreted with caution for several reasons as stated in the BEIR VII report and earlier phantom model studies [7, 13]. Another limitation of our particular phantom model measurement of dose and therefore estimation of risk is inherent to the MOSFET sensors. The sensors are points in space used to estimate a dose within a volume of tissue; therefore, they are subject to angulation. The tilted CT gantry protocol may cause more direct exposure to the sensor compared to the neutral gantry protocol. We attempted to minimize this potential limitation by performing each CT fluoroscopic protocol three times.
Because this was a phantom study, our results provide an estimate of organ doses and EDs during CT fluoroscopy-guided cryoablation of solid renal tumors. The actual doses for any individual will vary from patient-to-patient, depending on tube current setting, z-axis coverage, patient body habitus, and the complexity of the procedure. These calculations are based on a GE 16-slice large bore scanner, and may not be generalizable to other scanners. The large standard deviation of total CT fluoroscopy time during the complex procedures highlights the fact that the duration of a given patient’s procedure is highly variable, and results in variability in estimated organ dose that is most likely more significant than the variability due to differences in CT scanners.
Interventional radiologists utilizing CT fluoroscopy during cryoablation procedures typically wear radiation protective garments, while they move and work in a scatter field that is non-isotropic in both space and time. One needs to interpret the dose to the radiologist estimated in this study with this understanding [15].
Practical applications
Our study quantifies ED and specific organ doses for CT fluoroscopy-guided cryoablation of solid renal tumors. Our data can be useful to the practicing interventional radiologist and referring physician in assessment of the potential risk for cancer induction, and discussing these risks with the patient. When performing the procedure, the interventional radiologist should be cognizant of the organs receiving the highest radiation dose for a given patient position. In light of the ED measurements presented in this study, the interventional radiologist may want to consider performing the procedure in the decubitus position, if possible. Although we noted a higher lifetime excess risk of leukemia when using a tilted gantry technique compared to the risk of solid organ tumors in the decubitus position or with neutral gantry techniques, further investigation is needed before changes in procedural protocol related to gantry angulation can be recommended.
As there is no known minimum threshold for carcinogenesis, minimizing patient radiation dose in diagnostic and interventional radiology is very important in order to minimize stochastic radiation risks [16]. The radiation-related risk to the patient during CT fluoroscopy-guided percutaneous renal cryoablation is determined by the complexity of the procedure and should be considered in the context of the cumulative dose from follow-up diagnostic CT scans and possible repeat CT-guided procedures. Physicians and the public are becoming more concerned about the cumulative dose from imaging studies. Younger patients, in particular, are more susceptible. The risks and benefits of percutaneous CT fluoroscopy-guided renal cryoablation should be carefully considered and weighed against those of alternative therapeutic options. The latency period for radiation-induced leukemia after radiation exposure ranges from 5 to 20 years; for solid tumors, the latency period ranges from 10 to 30 years [17–19]. Thus, use of alternative therapies should be considered for younger patients. Additionally, judicious use of fluoroscopy time and utilizing techniques to reduce radiation dose seem prudent, especially in younger patients. The calculated interventional radiologist’s dose per year of procedures was low, 390 mR (3.9 mGy), far lower than the occupational limit of 5000 mR (50 mSv) for whole body dose per year. When lead shielding is worn, it is likely that the dose is miniscule. For reference, a study by Ho et al. found that the median yearly body dose to actively practicing vascular surgeons was 0.20 mSv under a lead apron [20]. Judicious use of fluoroscopy time and increased distance from the CT gantry will further minimize the radiation dose to the interventional radiologist.
References
Daly B, Krebs TL, Wong-You-Cheong JJ, Wang SS (1999) Percutaneous abdominal and pelvic interventional procedures using CT fluoroscopy guidance. Am J Roentgenol 173(3):637–644
Allen BC, Remer EM (2010) Percutaneous cryoablation of renal tumors: patient selection, technique, and postprocedural imaging. Radiographics 30(4):887–900
Georgiades CS, Hong K, Bizzel C, Geschwind JF, Rodriguez R (2008) Safety and efficacy of CT-guided percutaneous cryoablation for renal cell carcinoma. J Vasc Interv Radiol 19(9):1302–1310
Mazaris EM, Varkarakis IM, Solomon SB (2008) Percutaneous renal cryoablation: current status. Future Oncol 4(2):257–269
Silverman SG, Tuncali K, Adams DF, et al. (1999) CT fluoroscopy-guided abdominal interventions: techniques, results, and radiation exposure. Radiology 212(3):673–681
Paulson E, Sheafor DH, Enterline DS, McAdams HP, Yoshizumi TT (2001) CT fluoroscopy–guided interventional procedures: techniques and radiation dose to radiologists. Radiology 220(1):161–167
National Research Council (U.S.) (2006) Committee to assess health risks from exposure to low level of ionizing radiation. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2, vol. xvi. Washington D.C.: National Academies Press, p 406
Leng S, Christner JA, Carlson SK, et al. (2011) Radiation dose levels for interventional CT procedures. Am J Roentgenol 197(1):W97–W103
Park BK, Morrison PR, Tatli S, et al. (2012) Estimated effective dose of CT-guided percutaneous cryoablation of liver tumors. Eur J Radiol 81(8):1702–1706
Arellano RS, Gervais DA, Mueller PR (2011) CT-guided drainage of abdominal abscesses: hydrodissection to create access routes for percutaneous drainage. Am J Roentgenol 196(1):189–191
Adult male phantom model 702-D handling instructions (package insert) (2002) Norfolk, VA: CIRS, p 200X
Radiological Protection in Biomedical Research (1991) A report of Committee 3 adopted by the International Commission on Radiological Protection. Ann ICRP 22(3):1–28 (v–xxiv)
Hurwitz LM, Reiman RE, Yoshizumi TT, et al. (2007) Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: implications for cancer induction. Radiology 245(3):742–750
Little MP, Boice JD (1999) Comparison of breast cancer incidence in the Massachusetts tuberculosis fluoroscopy cohort and in the Japanese atomic bomb survivors. Radiat Res 151(2):218–224
Balter S (2001) Interventional fluoroscopy: physics, technology, and safety. New York: Wiley, p 308
Huda W, Slone RM (1995) Review of radiologic physics, vol. xv. Baltimore: Williams & Wilkins, p 286
Wolbarst AB (2005) Physics of radiology, vol. xv, 2nd edn. Madison: Medical Physics Pub, p 647
Hall EJ, Giaccia AJ (2012) Radiobiology for the radiologist, 7th edn. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins
Curry TS, et al. (1990) Christensen’s physics of diagnostic radiology, vol. xi, 4th edn. Philadelphia: Lea & Febiger, p 522
Ho P, et al. (2007) Ionizing radiation absorption of vascular surgeons during endovascular procedures. J Vasc Surg 46(3):455–459
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stewart, J.K., Looney, C.B., Anderson-Evans, C.D. et al. Percutaneous cryoablation of renal masses under CT fluoroscopy: radiation doses to the patient and interventionalist. Abdom Imaging 40, 2606–2612 (2015). https://doi.org/10.1007/s00261-015-0456-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0456-2