Abstract
Purpose: The aim of this study was to investigate the diagnostic performance of contrast-enhanced CT (CECT) findings for bowel ischemia and necrosis in closed-loop small-bowel obstruction (CL-SBO). Materials and Methods: Thirty-five patients with CL-SBO confirmed by laparotomy (n = 34) or multiplanar reconstruction of thin slice CT images (n = 1) were included. Based on the surgical and clinical findings, these patients were classified into three groups: necrosis group (n = 16), ischemia without necrosis group (n = 11), and no-ischemia group (n = 8). Two blinded radiologists retrospectively reviewed CECT including multiplanar reconstruction images and evaluated 12 CT findings. The sensitivity and specificity of each finding were compared among the three groups, and logistic regression analysis was performed. Results: High attenuation of the bowel wall, intraperitoneal air, reduced enhancement of the mesenteric arteries, and small-bowel feces signs showed high specificities of 100%, 100%, 89%, and 89% but low sensitivities of 31%, 25%, 44%, and 31%, respectively, for the prediction of bowel necrosis in CL-SBO. According to multivariate logistic regression analysis, reduced bowel-wall enhancement, reduced enhancement of the mesenteric veins, and a lack of engorgement of the mesenteric veins were significant for predicting bowel ischemia or necrosis (P < 0.05). Conclusions: Reduced enhancements of bowel wall and mesenteric veins were good indicators of bowel ischemia or necrosis. On the contrary, engorgement of the mesenteric veins was a predictor of a viable bowel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Small-bowel obstruction (SBO) is responsible for approximately 15% of cases of acute abdominal pain [1]. In particular, SBO with strangulation or bowel ischemia has a high mortality rate if the correct diagnosis and subsequent laparotomy are delayed [2–4]. However, the imaging diagnosis of bowel ischemia remains a considerable challenge [5–10]. Although CT may help determine whether immediate surgery is needed [11–15], CT findings are not exactly equivalent to the surgical findings [16]. A different detection rate of bowel ischemia in SBO using CT (63–100%) [5–10] and the various CT signs suggestive of ischemia has been described previously [6, 7, 11, 12, 17–22]. It is difficult to precisely predict bowel ischemia or necrosis by solely diagnosable finding for bowel ischemia.
Closed-loop small-bowel obstruction (CL-SBO) is the most common cause of bowel ischemia (59–84%) [5, 7, 12] and is considered a surgical emergency. Various axial CT signs are suggestive of CL-SBO, such as a U- or C-shaped configuration of the bowel loop, a radial distribution of mesenteric vessels converging toward the torsion site, a fusiform tapering of the loops (beak signs), and whirl signs [2, 17]. In addition, commonly used multidetector-row CT (MDCT) can be used for easy detection of a closed loop by tracing the thin sectional imaging [23]. MDCT with intravenous contrast enhancement facilitates the estimation of vascular compromise and ischemia [24, 25].
The aim of this study was to investigate the diagnostic performance of the contrast-enhanced CT (CECT) findings for bowel ischemia and necrosis in CL-SBO to predict the need for bowel resection or the feasibility of conservative treatment, including late adhesiolysis.
Materials and methods
Patients
Informed consent was waived by the institutional review board for this retrospective study. Our study was compliant with the Health Insurance Portability and Accountability Act. We reviewed 336 consecutive patients with clinical episodes and CT findings of acute SBO between November 2006 and October 2010. Fifty-one of these patients presenting with CL-SBO on MDCT scans or at laparotomy were included in the study. Sixteen patients were excluded because of one of the following reasons: CECT scans were unavailable (n = 14), a closed loop of the small bowel was too short to evaluate on CT images (n = 1), or sudden clinical deterioration was observed between the CT scan and laparotomy during conservative management which indicated a remarkable change of the bowel condition (n = 1).
Therefore, 35 patients (19 females and 16 males, age range 34–90 years, mean 66.5 years) with CL-SBO confirmed by laparotomy (n = 34) or multiplanar reconstruction (MPR) of thin slice CT images (n = 1) were included in the study. CL-SBO on MDCT was confirmed by two radiologists by tracing the dilated closed loop (single and isolated segment of small bowel) and proximal loop and the collapsed distal loop on thin sectional imaging. The causes of CL-SBO were postoperative adhesions (n = 32) or internal hernias (n = 3). Of the 34 patients who underwent laparotomy, 32 patients (94%) were operated on within 1–34 h (median 4.3 h) after the CT scan. Two patients who had presented with no clinical change until the surgery were operated on more than 2 days after the CT scan.
Surgical findings
In 34 patients who underwent laparotomy, the small bowel viability was evaluated by inspection during surgery (color and peristalsis of the bowel wall and pulsation of the mesenteric arteries) and by histological examination of the surgical specimen. Based on the surgical and histological findings, patients were classified into three groups (Table 1): 16 patients with evidence of bowel necrosis requiring small bowel resection (necrosis group), 11 patients with impaired bowel circulation requiring simple adhesiolysis without the need for bowel resection (ischemia group), and 8 patients without impaired bowel circulation (n = 7) or who was managed conservatively (n = 1) (no-ischemia group).
CT technique
CT examinations were performed with 64-slice MDCT systems (Aquilion 64, Toshiba Medical Systems, Tokyo, Japan) (n = 26) or 4-slice MDCT systems (Somatom Plus 4 Volume Zoom, Siemens Medical Systems, Erlangen, Germany) (n = 9). CT scans were obtained from the diaphragm to the symphysis pubis, with a tube potential of 120 kV and an automatically adjusted tube current (200–300 mAs) for each CT system. After precontrast CT scans were obtained, 2 or 3 phase contrast-enhanced scans were performed using 100–150 mL intravenous contrast material (iohexol, Omnipaque 300; Daiichi Sankyo, Tokyo, Japan, or iopamidol, Oiparomin 300; Fuji Pharma, Tokyo, Japan) at an injection rate of 3–4 mL/s. Image acquisition delay times were fixed at 40 and 80 s after the bolus injection of intravenous contrast material in order to simplify the scanning procedure. In three cases, additional 180 s delayed images were obtained. No oral or rectal contrast material was administered to any patient.
Image review
Throughout this study, CT images were reviewed independently by two experienced radiologists (KN and HI) who were blinded to the clinical data and pathohistological results. All images were interpreted on a picture archiving and communication system (PACS) viewer. In all cases, MPR images orthogonal to the axis of the closed loop and other optional imaging planes were made by reviewers’ own operation on an imaging workstation (Aquarius, TeraRecon, Tokyo, Japan) from the source 1-mm-thick axial sections.
Twelve CT findings that have been reported to be valuable for the diagnosis of bowel ischemia associated with SBO [2, 5, 6, 9, 11, 12] were evaluated: (1) bowel-wall thickening, considered as thicker than 2 mm in a distended loop, however, partly subjective evaluation due to the difficulty of accurately measuring the thickness of the wall; (2) target (halo) signs which indicate submucosal edema; (3) high attenuation of the bowel wall, compared with the attenuation of adjacent bowel-wall segments on the precontrast CT scan; (4) reduced bowel-wall contrast enhancement, subjectively evaluated by comparing with other bowel-wall segments 40 and 80 s after the contrast material injection; (5) mesenteric edema, defined as hazy fluid attenuation in the mesentery of the closed loop; (6) whirl signs, a swirl of mesenteric fat attenuation and vessels with adjacent rotated bowel loops; (7) reduced enhancement of the mesenteric arteries; (8) veins, evaluated by tracing arteries and veins on optional MPR obtained 40 and 80 s after the contrast material injection; (9) engorgement of the mesenteric veins, defined as dilatation of mesenteric veins around the site of obstruction compared with those distant from the obstruction site; (10) small bowel feces signs, defined as the presence of gas bubbles and debris within the lumen of the closed loop; (11) a large amount of ascites; and (12) intestinal pneumatosis or intraperitoneal air.
Statistical analysis
The frequency of each CT finding was calculated. Interobserver agreement was assessed using κ statistics with the following scale: fair agreement, 0.21–0.40; moderate agreement, 0.41–0.60; substantial agreement, 0.61–0.80; and almost perfect agreement, 0.81–1.0 [26]. Discordant findings between the two reviewers were resolved in conference.
Because bowel ischemia and necrosis were thought to be sequential entities dependent on the degree and duration of circulatory impairment of the bowel, several CT findings suggesting bowel necrosis were expected to overlap with those suggesting bowel ischemia. Hence, the sensitivity and specificity of each CT finding for bowel necrosis and for bowel ischemia or necrosis were calculated. The Mann–Whitney U test was used for group comparisons.
Logistic regression analysis was performed to assess which CT finding was an independent predictor of bowel ischemia or necrosis in CL-SBO. CT findings with a P value < 0.2 in the univariate analysis were incorporated into the multivariate analysis.
All statistical analyses were performed using StatMate IV software (Atoms, Tokyo, Japan). Statistically significant differences were defined as having P values less than 0.05.
Results
Interobserver agreement and diagnostic value of CT findings (Table 2)
In 11 CT findings, the κ values showed substantial to almost perfect agreement. The frequency of reduced bowel-wall enhancement (13 of 16, 81%) and reduced enhancement of the mesenteric veins (14 of 16, 88%) were significantly higher, and engorgement of the mesenteric veins (1 of 16, 6%) were significantly lower in the necrosis group than the no-ischemia group (P < 0.005). Reduced enhancement of the mesenteric veins was observed in 14 of 16 patients (88%) in the necrosis group, which was significantly higher than the ischemia group (5 of 11, 46%, P < 0.05). High attenuation of the bowel wall, intestinal pneumatosis, or intraperitoneal air, reduced enhancement of the mesenteric arteries, and small bowel feces signs showed high specificities of 100% (19 of 19), 100% (19 of 19), 89% (17 of 19), and 89% (17 of 19) but low sensitivities of 31% (5 of 16), 25% (4 of 16), 44% (7 of 16), and 31% (5 of 16), respectively, for the prediction of bowel necrosis in CL-SBO.
Logistic regression analysis (Table 3)
Reduced bowel-wall enhancement, whirl signs, reduced enhancement of the mesenteric veins, engorgement of the mesenteric veins, and a large amount of ascites showed a P value < 0.2 for the prediction of bowel ischemia or necrosis according to the univariate analysis. Therefore, these factors were incorporated into the multivariate analysis. For the prediction of bowel necrosis, the following factors were incorporated into the multivariate analysis: reduced bowel-wall enhancement, reduced enhancement of the mesenteric arteries, reduced enhancement of the mesenteric veins, engorgement of the mesenteric veins, and small bowel feces signs.
Multicollinearity due to high correlations was present among reduced bowel-wall enhancement, reduced enhancement of the mesenteric veins, and engorgement of the mesenteric veins. Hence, 3 patterns of analyses that incorporated one of the 3 findings separately were performed to exclude the multicollinearity effect.
According to the multivariate analysis, reduced enhancement of the mesenteric veins and a lack of engorgement of the mesenteric veins were significant for predicting bowel ischemia or necrosis (P < 0.05). Reduced bowel-wall enhancement and reduced enhancement of the mesenteric veins were significant factors for predicting bowel necrosis (P < 0.05).
Discussion
CL-SBO is a form of mechanical obstruction in which two or more points along the course of the bowel are obstructed at a single constrictive lesion [5, 17] and represents the most common cause of strangulation due to circulatory impairment caused by involvement of the mesentery [2, 4, 5, 17]. Fifty-nine to eighty-four percent of CL-SBO cases had bowel ischemia or necrosis [5, 7, 12, 19]; however, closed loops may occasionally resolve with conservative therapy [11]. The bowel with irreversible ischemia (necrosis) needs to be resected, and bowel with reversible ischemia may be preserved by alternate adhesiolysis. As our results showed, CL-SBO without ischemia [22% (8 of 35) of the cases in this study] can be managed with conservative therapy with close monitoring or with waiting surgery. If CT can differentiate closed-loop bowel with irreversible ischemia from that with mild ischemia or without ischemia, the patients with CL-SBO will be triaged appropriately to an urgent laparotomy or conservative treatment, including waiting for surgery.
High-resolution imaging using MDCT has enabled easy detection of the closed-loop configuration, namely the dilated closed loop and proximal loop and the collapsed distal loop, by tracing the thin sectional imaging [7]. MPR facilitates tracing the tortuous small intestine and swirling or converging vessels in affected mesentery. Even using MDCT, the diagnosis of ischemia is often difficult, because ischemia cannot be predicted only by the configuration of the obstructed bowel. A meta-analysis by Mallo et al. of 15 separate studies found CT to be 83% sensitive (range, 63–100%) and 92% specific (range, 61–100%) for selecting patients with ischemic bowel in the setting of SBO [13, 18]. There have been various CT findings reported to be helpful for diagnosing bowel ischemia [2, 5, 8, 11, 12, 14, 27, 28]; however, some of the markers are debatable, and there has been no solely diagnosable finding. Furthermore, only a few reports refer to the difference in CT findings between bowel ischemia and necrosis [3, 5, 19].
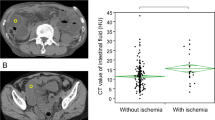
On the precontrast CT, high attenuation of the bowel wall, hemorrhagic changes in the mesentery, intestinal pneumatosis, small bowel feces sign [12, 21, 29], and portal venous gas are reported to indicate severe ischemia and infarction [5, 11]. In our study, however, logistic regression analysis showed that these signs in the CL-SBO were not a valuable predictor for bowel ischemia and necrosis. Although high attenuation of the bowel wall, which indicates hemorrhagic changes in the wall, showed high specificity for bowel necrosis (Figs. 1, 2), the subtle increase of the wall attenuation was difficult to detect subjectively (a κ value of 0.68).
A 58-year-old woman with CL-SBO caused by adhesive bands secondary to a prior laparotomy. A The precontrast CT scan shows a high-attenuated bowel wall of the closed loop (arrows) in contrast to a low-attenuated wall of the proximal loop (arrowheads). B The contrast-enhanced CT scan reveals decreased enhancement of the wall of the closed loop (arrow) and well-enhanced proximal loop (arrowhead). C, D On the axial contrast-enhanced image (80 s delay) (C) and oblique coronal MPR image (D), both the mesenteric arteries and veins in the closed loop are not depicted (arrows). The surgical laparotomy revealed CL-SBO with bowel necrosis.
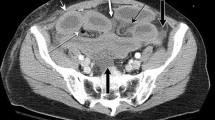
On contrast-enhanced CT, reduced bowel-wall enhancement (Figs. 1, 2) has been reported to be the only direct sign of vascular impairment of the small bowel and to be the most reliable sign of bowel ischemia in many studies [11, 12, 14, 18, 22, 24]. This finding was significant predictor for bowel necrosis (P < 0.05) in our multivariate analysis, but presented a sensitivity of 67% (18 of 27) for bowel ischemia or necrosis. It is sometimes difficult to recognize abnormal contrast enhancement of bowel wall by only single-phase images, especially in patients with poor circulation. We believe that two-phase contrast acquisitions, for example, 40 and 80 s delay in the study, are desirable to evaluate the decreased bowel-wall enhancement. However, further study is required to choose the adequate time to evaluate the bowel-wall enhancement. Additionally, we paid particular attention to the findings of the mesenteric arteries and veins in the closed loop (Figs. 1, 2, 3). To the best of our knowledge, no report in the literature has referred contrast enhancement of the mesenteric vessels in the affected mesentery of a closed loop. By precise tracing of the mesenteric vessels with MPR images of optional imaging planes, contrast enhancement of the affected vessels could be evaluated with sufficient interobserver correspondence (κ value 0.75–0.83). On early-phase contrast-enhanced images which are obtained 40 s after the injection of contrast material, mesenteric arterial enhancement was stronger than venous enhancement in normal mesenteries. On delayed-phase contrast-enhanced images obtained 80 s after the injection, the degree of arterial enhancement was equivalent to venous enhancement. Hence, the evaluation of both early- and delayed-phase images was desirable to differentiate mesenteric arteries from veins, especially because tracing the mesenteric vessels near the obstructive site was often difficult only using single-phase images. In both the necrosis group and ischemia or necrosis groups, reduced enhancement of the mesenteric veins showed higher sensitivities (88% and 70%, respectively) than the reduced enhancement of the mesenteric arteries (44% and 33%, respectively). This finding may represent that the venous return of the bowel loop is involved earlier than the cessation of the arterial influx.
An 80-year-old woman with CL-SBO caused by adhesive bands. A The precontrast CT scan shows a high-attenuated bowel wall of the closed loop (arrows). A small bowel feces sign (arrowheads) is demonstrated in the bowel loop. B, C The axial contrast-enhanced image (B) and oblique coronal MPR image (C) obtained 40 s after the injection of contrast material reveal decreased enhancement of the affected bowel wall (arrowheads) in contrast to normally enhanced other bowel loops (curved arrows). Although the mesenteric arteries (arrows) in the closed loop are enhanced, the veins are not depicted, in contrast to those in other mesenteries (open arrow). Transmural hemorrhagic necrosis was confirmed in the surgically resected bowel segment.
Some authors reported that engorgement of the mesenteric veins was found in bowel ischemia [11, 15]; however, this finding was demonstrated in the no-ischemia group, ischemia without necrosis group, and necrosis group in decreasing order of frequency in the present study. This result implies that this finding appears in early stages of CL-SBO with mild impairment of venous return and a viable bowel.
Pathologically, in CL-SBO, venous return from the involved bowel is initially impaired while arterial influx continues [11, 30]. Increased venous and capillary pressure in the bowel wall and mesentery leads to edema, engorgement of the veins, rupture of the small vessels, and intramural and mesenteric hemorrhage. Arterial insufficiency usually follows aggravating the anoxia and further contributing to the rapid development of ischemia [2, 11, 30]. CT findings would theoretically reflect these pathological changes. Multicollinearity was present among reduced bowel-wall enhancement, reduced enhancement of the mesenteric veins, and the lack of the engorgement of the mesenteric veins due to high correlations between each finding. In other words, evaluation of these three findings combined may be useful to increase the diagnostic accuracy of the findings.
A 60-year-old man with CL-SBO caused by adhesive bands. A The contrast-enhanced CT scan obtained 80 s after the injection of contrast material demonstrates a well-enhanced bowel wall and engorged mesenteric veins (arrowheads) near the obstruction site (arrow). B The mesenteric arteries (arrowheads) and veins (arrows) are well-enhanced and clearly recognized on contrast-enhanced images obtained 80 s after the injection. The surgical laparotomy revealed CL-SBO without bowel ischemia.
This study had several limitations. First, two patients without any finding of ischemia were diagnosed as no ischemia by laparotomy more than 2 days after CT. This inclusion makes precise correlation difficult, because CT findings might have changed during the intervals. Second, the patients who underwent only unenhanced CT scans were excluded from the study. When bowel necrosis is evident on an unenhanced CT, an immediate laparotomy is strongly suggested without CECT in a clinical setting. Some cases showing evident intestinal pneumatosis or high attenuation of the bowel wall may have been excluded from the study. Hence, the sensitivities of these findings may be underestimated.
In conclusion, reduced enhancements of the bowel wall and mesenteric vessels were reliable findings to detect bowel ischemia. On the contrary, engorgement of the mesenteric veins was a predictor of a viable bowel. We should pay attention to the findings of affected mesenteric arteries and veins in the closed loop.
References
Irvin TT (1989) Abdominal pain: a surgical audit of 1190 emergency admissions. Br J Surg 76:1121–1125
Balthazar EJ (1994) George W. Holmes Lecture. CT of small-bowel obstruction. AJR Am J Roentgenol 162:255–261
Fevang BT, Fevang J, Stangeland L, et al. (2000) Complications and death after surgical treatment of small bowel obstruction: a 35-year institutional experience. Ann Surg 231:529–537
Frager DH, Baer JW (1995) Role of CT in evaluating patients with small-bowel obstruction. Semin Ultrasound CT MR 16:127–140
Balthazar EJ, Birnbaum BA, Megibow AJ, et al. (1992) Closed-loop and strangulating intestinal obstruction: CT signs. Radiology 185:769–775
Frager D, Baer JW, Medwid SW, Rothpearl A, Bossart P (1996) Detection of intestinal ischemia in patients with acute small-bowel obstruction due to adhesions or hernia: efficacy of CT. AJR Am J Roentgenol 166:67–71
Kato K, Mizunuma K, Sugiyama M, et al. (2010) Interobserver agreement on the diagnosis of bowel ischemia: assessment using dynamic computed tomography of small bowel obstruction. Jpn J Radiol 28:727–732
Ha HK, Park CH, Kim SK, et al. (1993) CT analysis of intestinal obstruction due to adhesions: early detection of strangulation. J Comput Assist Tomogr 17:386–389
Balthazar EJ, Liebeskind ME, Macari M (1997) Intestinal ischemia in patients in whom small bowel obstruction is suspected: evaluation of accuracy, limitations, and clinical implications of CT in diagnosis. Radiology 205:519–522
Taourel PG, Fabre JM, Pradel JA, et al. (1995) Value of CT in the diagnosis and management of patients with suspected acute small-bowel obstruction. AJR Am J Roentgenol 165:1187–1192
Ha HK, Kim JS, Lee MS, et al. (1997) Differentiation of simple and strangulated small-bowel obstructions: usefulness of known CT criteria. Radiology 204:507–512
Sheedy SP, Earnest FT, Fletcher JG, Fidler JL, Hoskin TL (2006) CT of small-bowel ischemia associated with obstruction in emergency department patients: diagnostic performance evaluation. Radiology 241:729–736
Mallo RD, Salem L, Lalani T, Flum DR (2005) Computed tomography diagnosis of ischemia and complete obstruction in small bowel obstruction: a systematic review. J Gastrointest Surg 9:690–694
Zalcman M, Sy M, Donckier V, Closset J, Gansbeke DV (2000) Helical CT signs in the diagnosis of intestinal ischemia in small-bowel obstruction. AJR Am J Roentgenol 175:1601–1607
Nicolaou S, Kai B, Ho S, Su J, Ahamed K (2005) Imaging of acute small-bowel obstruction. AJR Am J Roentgenol 185:1036–1044
Burkill GJ, Bell JR, Healy JC (2001) The utility of computed tomography in acute small bowel obstruction. Clin Radiol 56:350–359
Furukawa A, Yamasaki M, Furuichi K, et al. (2001) Helical CT in the diagnosis of small bowel obstruction. Radiographics 21:341–355
Jancelewicz T, Vu LT, Shawo AE, et al. (2009) Predicting strangulated small bowel obstruction: an old problem revisited. J Gastrointest Surg 13:93–99
Makita O, Ikushima I, Matsumoto N, et al. (1999) CT differentiation between necrotic and nonnecrotic small bowel in closed loop and strangulating obstruction. Abdom Imaging 24:120–124
Idris M, Kashif N, Idris S, et al. (2011) Accuracy of 64-slice multidetector computed tomography scan in detection of the point of transition of small bowel obstruction. Jpn J Radiol 30:235–241
Zielinski MD, Eiken PW, Bannon MP, et al. (2010) Small bowel obstruction-who needs an operation? A multivariate prediction model. World J Surg 34:910–919
Boudiaf M, Soyer P, Terem C, et al. (2001) CT evaluation of small bowel obstruction. Radiographics 21:613–624
Silva AC, Pimenta M, Guimaraes LS (2009) Small bowel obstruction: what to look for. Radiographics 29:423–439
Assenza M, Ricci G, Macciucca Mde V, et al. (2007) Comparison among preoperative single-slice CT and multi-slice CT in simple, closed loop and strangulating bowel obstruction. Hepatogastroenterology 54:2017–2023
Maglinte DD, Howard TJ, Lillemoe KD, Sandrasegaran K, Rex DK (2008) Small-bowel obstruction: state-of-the-art imaging and its role in clinical management. Clin Gastroenterol Hepatol 6:130–139
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Megibow AJ, Balthazar EJ, Cho KC, et al. (1991) Bowel obstruction: evaluation with CT. Radiology 180:313–318
Federle MP, Chun G, Jeffrey RB, Rayor R (1984) Computed tomographic findings in bowel infarction. AJR Am J Roentgenol 142:91–95
Mayo-Smith WW, Wittenberg J, Bennett GL, et al. (1995) The CT small bowel faeces sign: description and clinical significance. Clin Radiol 50:765–767
Leffall LD Jr, Quander J, Syphax B (1965) Strangulation intestinal obstruction. Arch Surg 91:592–596
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakashima, K., Ishimaru, H., Fujimoto, T. et al. Diagnostic performance of CT findings for bowel ischemia and necrosis in closed-loop small-bowel obstruction. Abdom Imaging 40, 1097–1103 (2015). https://doi.org/10.1007/s00261-014-0335-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0335-2