Abstract
Background
The relationship between general obesity or abdominal obesity (abdominal circumference of ≥85 cm in men and ≥ 90 cm in women) and the heart-to-mediastinum ratio (HMR), a measure of cardiac sympathetic innervation, on cardiac iodine-123-metaiodobenzylguanidine scintigraphy (MIBG) in patients with heart failure with preserved ejection fraction (HFpEF) has not been clarified.
Methods
A total of 239 HFpEF patients with both MIBG and abdominal circumference data were examined. We divided these patients into those with abdominal obesity and those without it. In the cardiac MIBG study, early phase image was acquired 15–20 min after injection, and late phase image was acquired 3 h after the early phase. A HMR obtained from a low-energy type collimator was converted to that obtained by a medium-energy type collimator.
Results
Early and late HMRs were significantly lower in those with abdominal obesity, although washout rates were not significantly different. The incidence of patients with early and late HMRs <2.2 was significantly higher in those with abdominal obesity. Multivariate linear regression analysis revealed that abdominal obesity was independently associated with early HMR (standardized β = −0.253, P = 0.003) and late HMR (standardized β = −0.222, P = 0.010). Multivariate logistic regression analysis revealed that abdominal obesity was independently associated with early (odds ratio [OR] [95% confidence interval {CI}] = 4.25 [2.13, 8.47], P < 0.001) and late HMR < 2.2 (OR [95% CI] = 2.06 [1.11, 3.83], P = 0.022). Elevated BMI was not significantly associated with low early and late HMR. The presence of abdominal obesity was significantly associated with low early and late HMR even in patients without elevated BMI values.
Conclusion
Abdominal obesity, but not general obesity, in HFpEF patients was independently associated with low HMR, suggesting that visceral fat may contribute to decreased cardiac sympathetic activity in patients with HFpEF.
Trial registration
UMIN000021831.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heart failure with preserved ejection fraction (HFpEF) accounts for about half of all cases of heart failure [1, 2]. Although rates of hospitalization and all-cause mortality in patients with HFpEF are lower than those in patients with heart failure with reduced ejection fraction (HFrEF), absolute mortality is still high in patients with HFpEF [3]. The pathophysiology of HFpEF is not well understood and there is little evidence of ways to improve the prognosis.
General obesity is one of the common comorbidities in patients with HFpEF in Western countries [4]. The prevalence of general obesity is relatively low in Asian countries, especially in Japan [5, 6]. However, Asian people reportedly have a significantly greater amount of abdominal visceral fat relative to Caucasian populations [7], and abdominal obesity, defined with abdominal circumference is more prevalent than general obesity defined as high body mass index (BMI) [8] in Asian populations. Abdominal obesity, and not general obesity, is associated with a worse prognosis in patients with HFpEF [9].
Sympathetic nerve abnormality is an important pathophysiology associated with poor prognosis in patients with HFrEF [10, 11], whereas the significance in HFpEF remains unclear. Visceral fat reportedly affects muscle sympathetic nerve activity in healthy subjects [12]. Cardiac imaging with iodine-123-metaiodobenzylguanidine (MIBG), an analogue of norepinephrine, is a useful method to investigate cardiac sympathetic nerve activity in heart failure patients. Early and late heart to mediastinum ratio (HMR) and washout rate (WR) are generally acquired. A low late HMR is reportedly associated with poor prognosis in patients with HFrEF [13,14,15] and HFpEF [16]. Recently, it has been reported that HFrEF patients with BMI > 30 showed significantly lower early and late HMR, suggesting a causative link between high BMI and reduced cardiac sympathetic innervation [17]. However, there are no data regarding MIBG imaging in HFpEF patients with abdominal or general obesity. In this study, we examined the association between abdominal obesity vs. general obesity and cardiac sympathetic abnormalities in patients with HFpEF using cardiac MIBG scintigraphy.
Materials and methods
Study population
The PURSUIT-HFpEF registry is a prospective multicenter observational study which enrolled HFpEF patients admitted to participating hospitals in the Osaka region of Japan under the diagnosis of decompensated heart failure based on Framingham criteria (UMIN-CTR ID: UMIN000021831) [18]. The HFpEF was defined as left ventricular ejection fraction (LVEF) ≥ 50% and serum N-terminal pro brain natriuretic peptide (NT-proBNP) ≥ 400 pg/mL. Patients were registered when they were admitted in the hospital. The objectives of this registry are to collect uniform prospective data of patients with congestive heart failure that can be used to assess clinical variables, therapeutic procedures, and clinical events. Cardiac MIBG scintigraphy was performed in patients who were admitted to hospitals equipped to perform cardiac MIBG scintigraphy.
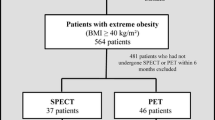
In this analysis, we examined 300 HFpEF patients that were enrolled in the PURSUIT-HFpEF registry between June 2016 and March 2020 and had cardiac MIBG scintigraphy data. We excluded patients without abdominal circumference data (n = 56), and those with amyloidosis (n = 4) and pulmonary arterial hypertension (n = 1). No patient met the exclusion criteria of treatment with serotonin-noradrenaline reuptake inhibitors or Parkinson disease. The resulting 239 patients were divided into those with and without abdominal obesity (abdominal circumference ≥ 85 cm in men and ≥ 90 cm in women) [19] (Fig. 1). General obesity was defined as BMI ≥ 25.
Data collection
Research cardiologists and specialized research nurses recorded the patients’ data during their hospital stays. In-hospital data were transmitted to the data collection center for processing and analysis. Collaborating hospitals were encouraged to enroll consecutive patients with HFpEF irrespective of their treatment. Blood samples and oral medications were obtained at admission and before discharge. We used these data on discharge as patient baseline characteristics.
In echocardiography, inferior vena cava diameter was measured using the standard method at expiration period. LVEF was measured using the Teichholz method. E/e’ was the mean of septal E/e’ and lateral E/e’. Tricuspid pressure gradient was measured using the simplified Bernoulli equation.
Cardiac MIBG scintigraphy
Sympathetic nerve activity was assessed by cardiac MIBG scintigraphy at the convalescent stage of decompensated heart failure during hospitalization. All patients received 111 MBq (3 mCi) of iodine-123-MIBG and underwent planar imaging of the anterior thorax on a nuclear camera equipped with low-energy, general-purpose collimators [20, 21]. Early phase image data were acquired 15–20 min after injection, and late phase data were acquired 3 h after the early phase. The HMR and washout rate were assessed by a standardized method for an automatic region of interest setting in the MIBG study [22]. The software of the automatic region of interest setting includes cross-calibration of HMR among hospitals that is caused by the differences in collimator types. The algorithm was based on the phantom studies in all participated hospitals, and a HMR obtained from a low-energy type collimator was converted to a value comparable to a medium-energy type collimator [23]. Washout rate was calculated from the initial and delayed images with background and decay correction. Readers blinded to clinical data in each participating hospital analyzed the images.
Statistical analysis
Continuous variables are expressed as median [interquartile range]. Categorical variables are presented as absolute values and percentages. Tests for significance were conducted using the unpaired t-test or nonparametric test for continuous variables, and the chi-squared test or Fisher’s exact test for categorical variables. Univariate and multivariate linear regression and logistic regression were performed to investigate the association between abdominal obesity and HMR. The multivariate model included presence or absence of abdominal obesity, age, sex, BMI ≥ 25, diabetes mellitus, estimated glomerular filtration rate < 60 mL/min/1.73m2, use of β-blockers, α-blockers, diuretics, and/or calcium channel-blockers, E/e’ and smoking status. These covariates were well-established confounding factors for abdominal obesity and sympathetic nerve activity reported in a previous study [24]. Statistical significance was defined as a p value <0.05. All analyses were performed using SPSS version 26 (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
Of the 239 patients, 89 patients (37%) had abdominal obesity and 150 (63%) patients were without (Fig. 1). Baseline characteristics are shown in Table 1. There was no significant difference in age between patients with and without abdominal obesity. Patients with abdominal obesity were more likely to be male, have a history of diabetes mellitus, and treatment with angiotensin II receptor-blockers or calcium channel antagonists compared with those without abdominal obesity. Patients with abdominal obesity had a higher BMI than those without. Notably, patients with abdominal obesity were more common than those with high BMI (> 25) in this study cohort (89 patients vs. 38 patients, respectively). Almost all patients with high BMI had abdominal obesity (36 of 38 patients), while the prevalence of high BMI was only 40% in patients with abdominal obesity (Table 1).
The association between abdominal obesity, general obesity, and cardiac MIBG scintigraphy data
Patients with abdominal obesity had lower early (2.3 [1.9, 2.6] vs. 2.6 [2.2, 2.8], P < 0.001) and late HMRs (2.0 [1.6, 2.3] vs. 2.2 [1.9, 2.6], P = 0.001) than those without abdominal obesity (Fig. 2). There was no significant difference in washout rate (35 [26, 44]% vs. 36 [24, 46]%, P = 0.688) between 2 groups (Fig. 2). We examined whether the presence of abdominal obesity was independently associated with low HMR values by linear regression. In this analysis, HMR was used as a continuous variable. Univariate and multivariate linear regression analysis adjusted for covariates revealed that the presence of abdominal obesity was independently associated with decrease in both early and late HMR (Table 2).
Comparison of heart-to-mediastinum ratio and washout rate between patients with or without abdominal obesity. Dark gray bars indicate early HMR, late HMR and washout rate in patients without abdominal obesity. Gray bars indicate early HMR, late HMR and washout rate in those with abdominal obesity (abdominal circumference of ≥85 cm in men and ≥ 90 cm in women). Error bar indicates the minimum value over the first quartile −1.5 × interquartile range and maximum value under the third quartile +1.5 × inter quartile range. Dark gray and gray dots are outliers. HMR: heart to mediastinum ratio
We examined the association between abdominal obesity and HMR < 2.2, since a previous report identified HMR = 2.2 as lower limit of normal range of HMR [22, 25]. The percentage of patients with early (45% vs. 24%, P = 0.001) and late HMR < 2.2 (64% vs. 46%, P = 0.007) was significantly higher in patients with abdominal obesity than those without abdominal obesity (Fig. 3). We examined whether abdominal obesity was associated with occurrence of early and late HMR < 2.2. Univariate and multivariate logistic regression analysis adjusted with covariates revealed that presence of abdominal obesity was independently associated with early and late HMR < 2.2. There were no significant associations between age, sex, presence of diabetes, estimated glomerular filtration rate, and HMR <2.2 (Table 3).
Frequency of HMR < 2.2 in patients with or without abdominal obesity. Dark gray bar indicates the frequency of HMR < 2.2 in patients without abdominal obesity (abdominal circumference of ≥85 cm in men and ≥ 90 cm in women). Gray bar indicates a frequency of HMR < 2.2 in those with abdominal obesity. HMR: heart to mediastinum ratio
Next, we examined the effects of general obesity on cardiac MIBG scintigraphy. Patients with general obesity (BMI ≥ 25, n = 38) showed a lower early HMR (2.2 [1.9, 2.5] vs. 2.5 [2.1, 2.8], P = 0.023) than those without general obesity (n = 200). There was no significant difference in late HMR (2.1 [1.7, 2.3] vs. 2.2 [1.8, 2.5], P = 0.068) and washout rate (33 [23, 40] vs. 36 [25, 46], P = 0.288) between the two groups (Fig. 4). General obesity was not significantly associated with early (standardized β = −0.014, P = 0.865) and late HMR (standardized β = −0.022, P = 0.784) in multivariate linear regression analysis (Table 4).
Comparison of HMR and washout rate between patients with or without abdominal obesity (abdominal circumference of ≥85 cm in men and ≥ 90 cm in women). Dark gray bars indicate early HMR, late HMR, and washout rate in patients without abdominal obesity. Gray bar indicates early HMR, late HMR and washout rate in those with abdominal obesity. Error bar indicates the minimum value over the first quartile −1.5 × interquartile range and maximum value under the third quartile +1.5 × inter quartile range. Dark gray and gray dots are outliers. HMR: heart to mediastinum ratio
Finally, to exclude the effect of general obesity on the relationship between abdominal obesity and the cardiac sympathetic system, we compared patients with abdominal obesity but without general obesity and those with neither abdominal obesity nor general obesity. Patients with abdominal obesity but without general obesity (n = 53) showed significantly lower early (2.3 [1.8, 2.6] vs. 2.6 [2.2, 2.8], P = 0.005) and late HMR (2.0 [1.5, 2.3] vs. 2.3 [1.9, 2.6], P = 0.012) than those with neither abdominal obesity nor general obesity (n = 147). Washout rate was not significantly different between these 2 groups (37 [26, 47] vs. 36 [24, 46], P = 0.209) (Fig. 5). Multivariate linear regression analysis revealed that abdominal obesity without general obesity was significantly associated with early and late HMR, but not with washout rate (Table 5).
Comparison of HMR and washout rate between patients with or without abdominal obesity in patients without general obesity. Dark gray bars indicate early HMR, late HMR and washout rate in patients without abdominal obesity. Gray bar indicates early HMR, late HMR and washout rate in those with. Error bar indicates the minimum value over the first quartile −1.5 × inter quartile range and maximum value under third quartile +1.5 × inter quartile range. Dark gray and gray dots are outliers. HMR: heart to mediastinum ratio
Discussion
In this study using cardiac MIBG scintigraphy data from a multicenter prospective observational study, we have shown that abdominal obesity diagnosed with clinical criteria for metabolic syndrome in Japan [19] was significantly associated with lower both early and late HMR and a higher frequency of both early and late HMR < 2.2 in patients with HFpEF. In addition, this association was independent of the presence of general obesity defined as BMI ≥ 25 as well as other factors which may affect sympathetic nerve condition, and also independent of LV filling pressures. This association was also observed in patients without high BMI. In contrast, BMI ≥ 25 was not associated with either early or late HMR values. This is the first report that demonstrates the association between abdominal obesity, but not general obesity, and sympathetic nervous system activity with cardiac MIBG scintigraphy in patients with HFpEF.
In this study, both early and late HMR were lower in patients with abdominal obesity than those without, whereas the washout rate was not significantly different between the two groups (Fig. 2). It has been widely accepted that the early HMR reflects integrity of presynaptic sympathetic nerve and sympathetic innervation, the late HMR reflects both sympathetic innervation and neuronal function of uptake and release of noradrenaline at nerve terminals, and washout rate is an index of the degree of sympathetic drive [26, 27]. Our results suggest that abdominal obesity is mainly associated with impairment of sympathetic nerve integrity and its innervation rather than with sympathetic drive.
A previous study of patients with dementia with Lewy body disease and Alzheimer disease reported that the normal range of HMR in the UK patients with larger body size was lower than that of Japanese patients with smaller body size [28], and two previous studies reported that obese patients had lower HMRs than non-obese patients [17, 29]. These findings raised a concern that lower HMR in patients with obesity may be due to increased attenuation or scatter rather than true differences in cardiac uptake. Considering that patients with abdominal obesity had higher BMIs than those without in our study (Table 1), the same concern may be raised. However, we demonstrated that the HMR was lower in patients with abdominal obesity than those without, even among patients without obesity (BMI < 25) (Fig. 4), which suggests that decreased HMR in patients with abdominal obesity may not be due to obesity. Komici et al. also proposed that greater body size or increased adipose tissue causes evenly-distributed attenuation of both heart and mediastinum, suggesting that HMR may not be significantly affected by attenuation [17]. Pellegrino et al. demonstrated that HMR was not significantly different between standard supine-position acquisition and prone-position acquisition, which can avoid the effect of attenuation from the liver or diaphragm [29], suggesting that the presence of abdominal visceral fat may not affect HMR. Taken together, the association between abdominal obesity and low HMR appears to be the result of differences in cardiac uptake related to abdominal obesity, and not attributable to attenuation or scatter mediated through body size or the presence of abdominal visceral fat.
In HFrEF patients, Komici et al. reported that patients with BMI > 30 showed lower early and late HMR but similar washout rates, but they did not examine the effect of abdominal obesity [17]. In our study, patients with high BMI did not show a significant association with lower HMR (Table 4), suggesting that the effect of high BMI on sympathetic nerve function may be different between patients with HFrEF and HFpEF. In contrast, the presence of abdominal obesity was significantly associated with both early and late HMR in HFpEF patients. Our cohort had a higher percentage of patients with abdominal obesity than those with BMI > 25 and 40% of patients with abdominal obesity also had a high BMI. Our multivariate linear regression analysis (Table 2) and the analysis in patients without high BMI (Fig. 4 and Table 4) clearly demonstrated that the presence of abdominal obesity was associated with low early and late HMR independent of BMI. Taken together, our data suggest that the presence of abdominal obesity may be an independent risk factor for sympathetic nerve abnormalities in patients with HFpEF. Considering that abdominal obesity is more common than high BMI in Asian populations [8], this association may be an important clue for identification of therapeutic targets in patients with HFpEF.
Our multivariable linear regression analysis demonstrated that the association between abdominal obesity and lower HMR was independent of LV filling pressure, suggesting that the enhancement of sympathetic nerve activity may be caused directly by abdominal obesity, but not by hemodynamic conditions due to diastolic dysfunction. This finding also suggests that abdominal obesity has a pathological role independent of diastolic dysfunction in HFpEF; therefore, improvement of abdominal obesity may have an additive therapeutic significance in the improvement of the hemodynamic condition in patients with HFpEF.
Limitation
This study has several limitations. First, although this study was a multicenter study, cardiac MIBG scintigraphy was performed in only a part of participating institutions that had nuclear imaging equipment. Therefore, selection bias may have occurred. Second, cardiac MIBG examinations were performed and analyzed at each institute. Although we set the identical interval between early and late scan, performed both background and decay corrections, and used the same software of the automatic region of interest setting in all hospitals, inter-institutional variation of data may not be avoided. Third, we diagnosed abdominal obesity with abdominal circumference not with computed tomography images, although abdominal circumference correlates well with visceral fat [30]. Finally, there is a possibility that unknown confounding factors may exist. Our data therefore need to be interpreted with caution, and further studies are required to evaluate our findings in patients with various characteristics.
Conclusion
Abdominal obesity but not general obesity was independently associated with low early and late HMR, suggesting that abdominal obesity is involved in sympathetic nerve abnormalities in HFpEF patients. Reducing visceral fat may be a possible treatment for patients with HFpEF.
Data availability
Not applicable.
References
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9.
Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75.
Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750–7.
Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2011;4:324–31.
Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, Yokota T, Goto D, Yokoshiki H, et al. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J. 2009;73:1893–900.
Nagai T, Yoshikawa T, Saito Y, Takeishi Y, Yamamoto K, Ogawa H, et al. Clinical characteristics, management, and outcomes of Japanese patients hospitalized for heart failure with preserved ejection fraction—a report from the Japanese Heart Failure Syndrome With Preserved Ejection Fraction (JASPER) Registry. Circ J. 2018;82:1534–45.
Tanaka S, Horimai C, Katsukawa F. Ethnic differences in abdominal visceral fat accumulation between Japanese, African-Americans, and Caucasians: a meta-analysis. Acta Diabetol. 2003;40:S302–4.
National Health and Nutrition Survey 2018, Health Japan 21. Available from: https://www.nibiohn.go.jp/eiken/kenkounippon21/en/eiyouchousa/kekka_shintai_chousa_nendo.html. Accessed 14 Aug 2020.
Tsujimoto T, Kajio H. Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol. 2017;70:2739–49.
Verberne HJ, Brewster LM, Somsen GA, van Eck-Smit BL. Prognostic value of myocardial 123I-metaiodobenzylguanidine (MIBG) parameters in patients with heart failure: a systematic review. Eur Heart J. 2008;29:1147–59.
Agostini D, Verberne HJ, Burchert W, Knuuti J, Povinec P, Sambuceti G, et al. I-123-mIBG myocardial imaging for assessment of risk for a major cardiac event in heart failure patients: insights from a retrospective European multicenter study. Eur J Nucl Med Mol Imaging. 2008;35:535–46.
Alvarez GE, Ballard TP, Beske SD, Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol. 2004;287:H414–8.
Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212–21.
Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, et al. A pooled analysis of multicenter cohort studies of (123)I-MIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging. 2013;6:772–84.
Parker MW, Sood N, Ahlberg AW, Jacobson AF, Heller GV, Lundbye JB. Relationship between quantitative cardiac neuronal imaging with 123I-meta-iodobenzylguanidine and hospitalization in patients with heart failure. Eur J Nucl Med Mol Imaging. 2014;41:1666–72.
Seo M, Yamada T, Tamaki S, Watanabe T, Morita T, Furukawa Y, et al. Prognostic significance of cardiac I-123-metaiodobenzylguanidine imaging in patients with reduced, mid-range, and preserved left ventricular ejection fraction admitted for acute decompensated heart failure: a prospective study in Osaka Prefectural Acute Heart Failure Registry (OPAR). Eur Heart J Cardiovasc Imaging. 2020:jeaa025.
Komici K, Bencivenga L, Paolillo S, Gargiulo P, Formisano R, Assante R, et al. Impact of body mass index on cardiac adrenergic derangement in heart failure patients: a 123 I-MIBG imaging study. Eur J Nucl Med Mol Imaging. 2020;47:1713–21.
Suna S, Hikoso S, Yamada T, Uematsu M, Yasumura Y, Nakagawa A, et al. Study protocol for the PURSUIT-HFpEF study: a prospective, multicenter, observational study of patients with heart failure with preserved ejection fraction. BMJ Open. 2020;10:e038294.
Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–62.
Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol. 2009;53:426–35.
Masuda M, Yamada T, Mizuno H, Minamiguchi H, Konishi S, Ohtani T, et al. Impact of atrial fibrillation ablation on cardiac sympathetic nervous system in patients with and without heart failure. Int J Cardiol. 2015;199:65–70.
Okuda K, Nakajima K, Hosoya T, Ishikawa T, Konishi T, Matsubara K, et al. Semi-automated algorithm for calculating heart-to-mediastinum ratio in cardiac Iodine-123 MIBG imaging. J Nucl Cardiol. 2011;18:82–9.
Nakajima K, Okuda K, Yoshimura M, Matsuo S, Wakabayashi H, Imanishi Y, et al. Multicenter cross-calibration of I-123 metaiodobenzylguanidine heart-to-mediastinum ratios to overcome camera-collimator variations. J Nucl Cardiol. 2014;21:970–8.
Bemelmans RH, van der Graaf Y, Nathoe HM, Wassink AM, Vernooij JW, Spiering W, et al. Increased visceral adipose tissue is associated with increased resting heart rate in patients with manifest vascular disease. Obesity. 2012;20:834–41.
Nakajima K, Matsumoto N, Kasai T, Matsuo S, Kiso K, Okuda K. Normal values and standardization of parameters in nuclear cardiology: Japanese Society of Nuclear Medicine working group database. Ann Nucl Med. 2016;30:188–99.
Kline RC, Swanson DP, Wieland DM, Thrall JH, Gross MD, Pitt B, et al. Myocardial imaging in man with I-123 meta-iodobenzylguanidine. J Nucl Med. 1981;22:129–32.
Marzullo P, Mariani G. From basic cardiac imaging to image fusion. Milano: Springer-Verlag Italia; 2013. p. 51–70.
Roberts G, Lloyd JJ, Kane JPM, Durcan R, Lawley S, Howe K, et al. Cardiac (123)I-MIBG normal uptake values are population-specific: results from a cohort of controls over 60 years of age. J Nucl Cardiol. 2019. https://doi.org/10.1007/s12350-019-01887-6.
Pellegrino T, Piscopo V, Boemio A, Russo B, De Matteis G, Pellegrino S, et al. Impact of obesity and acquisition protocol on (123)I-metaiodobenzylguanidine indexes of cardiac sympathetic innervation. Quant Imaging Med Surg. 2015;5:822–8.
Examination Committee of Criteria for “Obesity Disease” in Japan; Japan Society for the Study of Obesity. New criteria for “obesity disease” in Japan. Circ J. 2002;66:987–92.
Acknowledgements
The authors thank Nagisa Yoshioka, Kyoko Tatsumi, Satomi Kishimoto, Noriko Murakami, and Sugako Mitsuoka for their excellent assistance with data collection. We thank Libby Cone, MD, MA, from DMC Corp. (www.dmed.co.jp <http://www.dmed.co.jp/>) for editing a draft of this manuscript.
The OCVC-Heart Failure Investigators: Shunsuke Tamaki, Tetsuya Watanabe, and Takahisa Yamada, Osaka General Medical Center, Osaka, Japan; Takaharu Hayashi and Yoshiharu Higuchi, Osaka Police Hospital, Osaka, Japan; Masaharu Masuda, Mitsutoshi Asai, and Toshiaki Mano, Kansai Rosai Hospital, Amagasaki, Japan; Hisakazu Fuji, Kobe Ekisaikai Hospital, Kobe, Japan; Daisaku Masuda, Yoshihiro Takeda, Yoshiyuki Nagai, and Shizuya Yamashita, Rinku General Medical Center, Izumisano, Japan; Masami Sairyo, Yusuke Nakagawa and Shuichi Nozaki, Kawanishi City Hospital, Kawanishi, Japan; Haruhiko Abe, Yasunori Ueda, Masaaki Uematsu, and Yukihiro Koretsune, National Hospital Organization Osaka National Hospital, Osaka, Japan; Kunihiko Nagai, Ikeda Municipal Hospital, Ikeda, Japan; Masamichi Yano, Masami Nishino, and Jun Tanouchi, Osaka Rosai Hospital, Sakai, Japan; Yoh Arita and Shinji Hasegawa, Japan Community Health Care Organization Osaka Hospital, Osaka, Japan; Takamaru Ishizu, Minoru Ichikawa and Yuzuru Takano, Higashiosaka City Medical Center, Higashiosaka, Japan; Eisai Rin, Kawachi General Hospital, Higashiosaka, Japan; Yukinori Shinoda and Shiro Hoshida, Yao Municipal Hospital, Yao, Japan; Masahiro Izumi, Kinki Central Hospital, Itami, Japan; Hiroyoshi Yamamoto and Hiroyasu Kato, Japan Community Health Care Organization, Osaka Minato Central Hospital, Osaka, Japan; Kazuhiro Nakatani and Yuji Yasuga, Sumitomo Hospital, Osaka, Japan; Mayu Nishio and Keiji Hirooka, Saiseikai Senri Hospital, Suita, Japan; Takahiro Yoshimura and Yoshinori Yasuoka, National Hospital Organization Osaka Minami Medical Center, Kawachinagano, Japan; Akihiro Tani, Kano General Hospital, Osaka, Japan; Yasushi Okumoto and Hideharu Akagi, Kinan Hospital, Tanabe, Japan; Yasunaka Makino, Hyogo Prefectural Nishinomiya Hospital, Nishinomiya, Japan; Toshinari Onishi and Katsuomi Iwakura, Sakurabashi Watanabe Hospital, Osaka, Japan; Nagahiro Nishikawa and Yoshiyuki Kijima, Japan Community Health Care Organization, Hoshigaoka Medical Center, Hirakata, Japan; Takashi Kitao and Hideyuki Kanai, Minoh City Hospital, Minoh, Japan; Wataru Shioyama and Masashi Fujita, Osaka International Cancer Institute, Osaka, Japan; Koichiro Harada, Suita Municipal Hospital, Suita, Japan; Masahiro Kumada and Osamu Nakagawa, Toyonaka Municipal Hospital, Toyonaka, Japan; Ryo Araki and Takayuki Yamada, Otemae Hospital, Osaka, Japan; Akito Nakagawa and Yoshio Yasumura, Amagasaki Chuo Hospital, Amagasaki, Japan; and Taiki Sato, Akihiro Sunaga, Bolrathanak Oeun, Hirota Kida, Takayuki Kojima, Yohei Sotomi, Tomoharu Dohi, Kei Nakamoto, Katsuki Okada, Fusako Sera, Shinichiro Suna, Hidetaka Kioka, Tomohito Ohtani, Toshihiro Takeda, Daisaku Nakatani, Hiroya Mizuno, Shungo Hikoso, Yasushi Matsumura and Yasushi Sakata, Osaka University Graduate School of Medicine, Suita, Japan.
Code availability
Not applicable.
Funding
This study was funded by Roche diagnostic and FUJIFILM Toyama Chemical.
Author information
Authors and Affiliations
Consortia
Contributions
Akihiro Sunaga: conceptualization, formal analysis, investigation, methodology, project administration, visualization, writing—original draft. Shungo Hikoso: project administration, supervision, writing—review & editing. Takahisa Yamada: resources, supervision, writing—review & editing. Yoshio Yasumura, resources, writing—review & editing. Masaaki Uematsu; Resources, writing—review & editing. Haruhiko Abe; Resources, writing—review & editing. Yusuke Nakagawa, Resources, writing—review & editing. Yoshiharu Higuchi: resources, writing—review & editing. Hisakazu Fuji; Resources, writing—review & editing. Toshiaki Mano; Resources, writing—review & editing. Hiroyuki Kurakami: data curation, formal analysis. Tomomi Yamada: data curation, formal analysis. Tetsuhisa Kitamura: writing—review & editing. Taiki Sato: writing—review & editing. Bolrathanak Oeun: writing—review & editing. Hirota Kida: writing—review & editing. Takayuki Kojima; writing—review & editing. Yohei Sotomi: data curation, writing—review & editing. Tomoharu Dohi: writing—review & editing. Katsuki Okada: writing—review & editing. Shinichiro Suna: writing—review & editing. Hiroya Mizuno: writing—review & editing. Daisaku Nakatani: writing—review & editing. Yasushi Sakata: funding acquisition, project administration, supervision, writing—review & editing.
Corresponding author
Ethics declarations
Ethics approval
The protocol was approved by the Institutional Review Board (IRB) of Osaka University Hospital on 24 February 2016 (ID: 15471), and by the IRBs of the all participating facilities.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Consent for publication was obtained from all individual participants included in the study.
Conflict of interest
Shungo Hikoso has received remuneration from Daiichi Sankyo Company, and received research funding from Roche Diagnostics, FUJIFILM Toyama Chemical, and Actelion Pharmaceuticals. Yoshiharu Higuchi has received remuneration from Daiichi Sankyo Company. Hiroya Mizuno has received endowed department from Terumo. Yohei Sotomi has received remuneration from Abbott Vascular Japan, Boston Scientific Japan, and received research funding from Abbott Vascular Japan, and endowed department form Terumo. Yasushi Sakata received remuneration from Otsuka Pharmaceutical, Ono Pharmaceutical, Daiichi Sankyo Company and AstraZeneca K.K., and received research funding form Otsuka Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, Astellas Pharma, Kowa Company, Boehringer Ingelheim Japan, and Biotronik. The other authors have nothing to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology
Rights and permissions
About this article
Cite this article
Sunaga, A., Hikoso, S., Yamada, T. et al. Abdominal obesity, and not general obesity, is associated with a lower 123I MIBG heart-to-mediastinum ratio in heart failure patients with preserved ejection fraction. Eur J Nucl Med Mol Imaging 49, 609–618 (2022). https://doi.org/10.1007/s00259-021-05280-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05280-9