Abstract
Purpose
Germline mutations in genes encoding succinate dehydrogenase (SDH) are frequent in patients with pheochromocytoma and paraganglioma (PPGL). They lead to SDH inactivation, mediating a massive accumulation of succinate, which constitutes a highly specific biomarker of SDHx-mutated tumors when measured in vitro. In a recent pilot study, we showed that magnetic resonance spectroscopy (1H-MRS) optimized for succinate detection (SUCCES) could detect succinate in vivo in both allografted mouse models and PPGL patients. The objective of this study was to prospectively assess the diagnostic performances of 1H-MRS SUCCES sequence for the identification of SDH deficiency in PPGL patients.
Methods
Forty-nine patients presenting with 50 PPGLs were prospectively enrolled in our referral center for 1H-MRS SUCCES. Two observers blinded to the clinical characteristics and genetic status analyzed the presence of a succinate peak and confronted the results to a composite gold standard combining PPGL genetic testing and/or in vitro protein analyses in the tumor.
Results
A succinate peak was observed in 20 tumors, all of which had proven SDH deficiency using the gold standard (17 patients with germline SDHx mutations, 2 with a somatic SDHD mutation, and 1 with negative SDHB IHC and SDH loss of function). A false negative result was observed in 3 tumors. Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 1H-MRS SUCCES were respectively 87%, 100%, 100%, 90%, and 94%.
Conclusions
Detection of succinate using 1H-MRS is a highly specific and sensitive hallmark of SDH-deficiency in PPGLs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
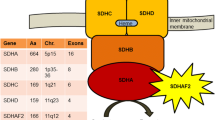
Pheochromocytomas and paragangliomas (PPGL) are characterized by a very strong genetic determinism with almost 20 susceptibility genes identified so far, which mutations can impact the prognosis, clinical outcome [1], and response to treatment [2]. More than half of inherited PPGL involve mutations in genes encoding mitochondrial enzymes or transporters. Among them, SDHx genes (SDHA, SDHB, SDHC, SDHD, and SDHAF2) encode the four subunits and the assembly factor of succinate dehydrogenase (SDH), which oxidizes succinate to fumarate in the tricarboxylic acid (TCA) cycle and constitutes the complex II of the electron transport chain. SDHx mutations predispose to multiple PPGL and SDHB mutated tumors are of strongly increased risk of metastatic progression [3, 4]. While SDHB mutations are found in around 10% of PPGL patients, it is estimated that more than one third of patients with metastatic PPGL carry an SDHB germline mutation. Similarly, it is estimated that approximately 15% of PPGL patients will ultimately develop a metastatic disease, versus 50% of SDHB-mutation carriers. Patients with hereditary SDHx-related PPGL carry a germline heterozygous mutation, which is associated with the somatic inactivation of the normal allele by somatic mutation or loss of heterozygosity according to the Knudson’s 2-hit model for tumor suppressor genes. This bi-allelic inactivation induces a complete loss of SDH activity in the tumor, leading to the accumulation of succinate, which acts as an oncometabolite and is suspected to mediate most tumorigenic effects related to SDHx mutations [5,6,7,8].
In SDHx-mutated PPGL, succinate concentrations increase dramatically, up to 100-fold with respect to tumors without SDHx mutations [9,10,11]. We recently adapted a magnetic resonance spectroscopy (MRS) method to detect succinate in tumors (SUCCinate Estimation by Spectroscopy (SUCCES)) relying on a monovoxel PRESS asymmetric « PROBE » sequence. This pilot study [12] performed in 9 patients with PPGL (5 with SDHx mutations and 4 sporadic cases) demonstrated that succinate accumulation was indeed detectable in the tumors of all SDHx-mutated patients but not in non-SDHx tumors. An unexplained choline peak was also observed in SDHx-mutated cases. These results supported the proof-of-concept that noninvasive detection of succinate using in vivo 1H-MRS may constitute a specific biomarker of SDHx mutations. Following this first-in-man study, we here report the results of a prospective study aiming at evaluating the clinical relevance of 1H-MRS SUCCES in the routine care of PPGL patients.
Materials and methods
Patients
Patients explored for PPGL in the hypertension unit of Hôpital Européen Georges Pompidou, Paris (France), were included prospectively if they met the following criteria: (1) age ≥ 18 years old; (2) PPGL diagnosis in accordance with international clinical practice guidelines in a reference center, relying on biochemistry, cross-sectional, and functional imaging [13]; (3) signed informed consent for PPGL genetic testing; and (4) no contraindications to MRI (i.e., metallic implants, claustrophobia,...).
Ethical approval for the study was obtained from the institutional review board (Comité de Protection des Personnes (CPP) Ile de France II). All patients provided written informed consent for participation to the study, collection of samples, and subsequent analyses.
When a surgical procedure was performed, fresh tumor samples were frozen immediately after surgical resection and subsequently stored at − 80 °C until processing following the COMETE collection procedures [14]. Histologic diagnosis was performed on paraffin-embedded formalin-fixed samples.
Succinate detection using 1H-MRS at 3 Tesla
Combined MR images and MR spectroscopic scans of patients were acquired in a 3 Tesla MRI clinical scanner (Discovery MR750w GEMSOW, GE Medical Systems, Milwaukee, WI), as previously described [12]. 1H-MRS spectra were acquired using the optimized SUCCES sequence: asymmetric Point REsolved SpectroScopy (PRESS) monovoxel acquisition based on the PROBE monovoxel sequence [15], with TR: 2500 ms; TE: 144 ms; Nex: 512 (22 min acquisition) or 1024 (44 min acquisition).
Detection of tumors and VOI (Volume of Interest) positioning were performed on thin-section high-resolution T2-weighted fast spin-echo imaging in at least two orthogonal planes with the following parameters: TR: 2500 ms; TE: 85 ms; echo train length: 19; slice thickness: 2 mm; spacing: 0.3; field of view: 14 × 14 cm for neck or 42 × 42 cm for whole body coil; matrix: 320 × 320 or on thin-section high-resolution balanced steady-state gradient echo sequence (FIESTA) in at least two orthogonal planes with the following parameters: TR: 4.9 ms; TE: 1.4 ms; NEX:1; Flip angle: 50; slice thickness: 3 mm; spacing: 1; field of view: 14 × 14cm for neck or 42 × 42 cm for whole body coil; and matrix: 256 × 256.
Immunochemistry
SDHA and SDHB protein expression were assessed on formalin-fixed paraffin embedded (FFPE) tumor samples by immunohistochemistry (IHC) as previously described [16,17,18] using the following antibodies and conditions: anti-SDHA (ab14715, Abcam; 1:1000) and anti-SDHB (HPA002868, Sigma-Aldrich Corp; 1:500).
SDH activity
SDH activity was investigated, when necessary, on frozen tumor samples, homogenized using a 1-ml glass Potter-Elvehjem, using a spectrophotometric assay, as previously described [19].
Genetic analysis
The DNA extracted from leukocytes was genotyped in all included patients, as well as the DNA extracted from frozen or FFPE-tumors when available. Genetic analyses were performed through next generation sequencing (NGS) using the “MASTR plus SDHv2” targeted panel (Multiplicom, Agilent technologies) as previously reported and in accordance with international guidelines [20, 21].
Data analysis
Two independent investigators (CLL and ABE), blinded from clinical observations and genetic test results at the time of analyses, examined all spectra in a qualitative manner and scored for the presence or absence of a succinate peak at 2.44 ppm, lactate/lipid signal (0.9 to 1.3 ppm), and choline peak (3.2 ppm). When spectra interpretation was different between the two investigators, discordance(s) have been resolved by consensus by a panel of experts (CLL, ABE, JF, and BT). Tumor and VOI size as well as tumor heterogeneity were evaluated on the FIESTA or the T2-weighted fast spin-echo sequence.
1H-MRS data were initially compared with the genetic gold standard of SDH deficiency assessed using germline genetic testing. In cases of discrepancies between germline genetic test and 1H-MRS results, a composite gold standard was applied by considering the results of tumor tissue analyses (somatic NGS, SDHB IHC, or SDH activity measurement in the tumor). Tumors were thus considered as SDH deficient when (i) an SDHx mutation was detected on germline and/or (ii) tumor DNAs, or (iii) when SDH inactivation was supported by both protein analyses of tissue samples (SDHB/A negative immunochemistry and loss of SDH activity).
Sensitivity (Se), specificity (Sp), positive/ negative predictive values (PPV/NPV), and accuracy of the method were calculated using the SISA (Simple Interactive Statistical Analysis) procedure with continuity corrected Wilson 95% confidence intervals presentation.
Results
Patients
Fifty-six patients, including the 9 from the original pilot study [12], were prospectively enrolled between January 2015 and March 2019, leading to the exploration of 57 tumors with the 1H-MRS SUCCES sequence (one abdominal PGL and one head and neck PGL were explored in patient 1 (previously reported in the pilot study, Table S1) carrying an SDHB mutation [12]). Seven patients were excluded. One presented a surgical clip, one a non-evaluable highly necrotic tumor, and one diagnosed with a poorly differentiated neuroendocrine tumor instead of a PPGL following pathological analysis of the tumor and the “gold standard” data could not be obtained for four of them. Overall, 49 patients with 50 tumors were analyzed (Fig. 1) and their main characteristics are presented in Table 1. Median age at the time of 1H-MRS examination was 44.7 years old [20–75]. The 50 tumors were located as follows: 24 head and neck PGL, 9 abdominal PGL, and 17 pheochromocytomas (PCC).
1H-MRS SUCCES performance and added value for management of patients
A succinate peak at 2.44 ppm was observed in 20 tumors (Fig. 2), while a choline peak was detected in 27 tumors, including 17 with an associated succinate peak. Genetic status was known at the time of 1H-MRS for 9 patients (18%). For the remaining 40 patients, genetic status was available for clinician after genetic counseling and NGS analyses of germline and somatic DNA in average 12.2 months (± 8.6 months) after 1H-MRS results. Among the 19 patients (20 tumors) showing a tumor succinate accumulation, 16 (17 tumors) were confirmed to carry a germline SDHx mutation: 1 SDHA, 4 SDHB, 2 SDHC, and 9 SDHD.
In 3 patients, 1H-MRS SUCCES suggested SDH deficiency despite a negative genetic test. In 2 of them, NGS analysis of tumor DNA identified an SDHD somatic mutation (patients 20 and 21, Table S1). The third patient (patient 22, Table S1) was a 42-two year-old patient with a familial history PPGL (his father developed bilateral carotid body PGL diagnosed at 40 years old). The patient suffered from a right vagal PGL operated 10 years earlier and a left vagal PGL treated at the same period using external beam radiotherapy. 1H-MRS SUCCES was performed in a metastatic relapse of the left vagal PGL and showed a succinate peak. PPGL genetic testing was negative both in germline and in somatic DNA, but SDH deficiency was demonstrated by negative SDHB IHC and complete inhibition of SDH enzymatic activity in the tumor (Fig. 3). Hence, there was no false positive finding.
Patient 22 with SDH deficiency demonstrated by functional studies. a Axial T2-weighted MRI of the left vagal PGL. b Maximal intensity projection of 18F-FDopa-PET/CT. c Axial fused maximal 18F-FDopa-PET/CT. d1H-MRS spectrum showing a succinate (Su) peak at 2.44 ppm associated with a choline (Ch) and a lipids/lactate (L) peak. e SDHB immunohistochemsitry in a non-SDH PGL shows a typical mitochondrial granular staining while f the patient’s tumor displays a weak diffuse SDHB staining frequently observed in SDHD-mutated PGL (ref) and g a strong SDHA positive labeling. h Analysis of succinate cytochrome C reductase (SCCR) activity in the patient’s tumor compared with a non-SDH and an SDH-mutated PGL confirms loss of SDH activity
1H-MRS SUCCES failures in SDH-deficient tumors. a Left panel shows both the cervical PGL including the carotid artery and the jugular vein and a small metastatic lymph node (arrows) developed by a 35-year-old patient carrying a germline SDHB gene mutation (patient 7). Positioning a small voxel of 0.7 cm3 (middle pannel) did not allow revealing a succinate peak (right). b T2 (left) and T1-weighted MRI (middle) show a right cervical PGL with hemorrhagic and necrotic spots in a 68-year-old patient with a germline SDHD gene mutation (patient 9) and the absence of succinate peak. c Small left-sided PCC (voxel size 1.5 cm3) in a 20 years old patient followed in a familial context of germline SDHD gene mutation (patient 6) without succinate peak
Thirty tumors (30 patients) showed no succinate accumulation detectable by 1H-MRS SUCCES. Gold standard confirmed the absence of SDH deficiency in 27 cases (Table S2). Two patients carried a germline mutation in the VHL gene, while germline genotyping was negative in 25 patients. In the latter cases, NGS identified 10 with somatic mutations (2 in EPAS1, 4 in HRAS, 3 in VHL, and 1 in NF1 gene).
A false negative result (i.e., no succinate peak in a patient with an SDHx mutation) was observed in 3 tumors (Fig. 4). The first patient (SDHB mutated, patient 7) presented with a cervical PGL that included the carotid artery and the jugular vein, preventing the use of the 1H-MRS SUCCES which had to be performed on a very small metastatic lymph node (voxel size 0.7 cm), which may have been a limiting factor. The second patient (patient 9) carried a germline SDHD gene mutation and suffered from a right cervical PGL with numerous hemorrhagic and necrotic spots (voxel size 2.5 cm3). The third one was a 20-year-old patient (patient 6) followed in a familial context of germline SDHD gene mutation with a small left-sided PCC (voxel size 1.5 cm3), for which respiratory motion may have compromised the result.
Discordance between the 2 investigators (CLL and ABE) were observed for 2 cases (patients 13 and 36, Tables S1 and S2) and resolved by consensus by the panel of experts.
Altogether, at the lesion level, sensitivity, specificity, PPV, NPV, and accuracy of 1H-MRS SUCCES were respectively of 87% (Wilson CI 59.2–97.4), 100% (Wilson CI 79–99.8), 100% (Wilson CI 72.6–99.8), 90% (Wilson CI 66.8–98), and 94% (Wilson CI 78.5–98.8).
Discussion
To our knowledge, this is the first prospective validation study demonstrating high sensitivity and specificity of non-invasive detection of succinate using in vivo 1H-MRS. Our results qualify the presence of a succinate peak at 2.44 ppm as a specific bona fide biomarker of SDH deficiency in patients suffering from PPGL, whatever the SDHx gene mutated (SDHA, B, C, and D).
We clearly show that 1H-MRS SUCCES allows early suspicion of SDH deficiency in routine clinical practice. Indeed, for almost 80% of patients, results of 1H-MRS SUCCES sequence were provided to clinicians before the results of genetic test. A recent multicentric retrospective study demonstrated that early knowledge of genetic status has a positive impact on the management and clinical outcome of patients with a germline SDHx mutation [1]. Identification of a succinate peak with 1H-MRS SUCCES sequence should therefore accelerate genetic testing in these patients, focusing and facilitating the interpretation of genetic variations in SDHx genes provided by NGS. In a patient with initial PPGL diagnosis, early assessment of SDHx mutational status may impact patient’s management, by taking into account the higher risk of metastasis of SDHB-related tumors, or the high rate of multiple cervical PGL for SDHD-mutated patients. Finally, early identification of patients with genetic predisposition will also accelerate the familial pre-symptomatic genetic screening and therefore a benefit for the relatives of index patients.
Importantly, we demonstrate that, in case of negative germline genotyping, the finding of a positive succinate peak using 1H-MRS SUCCES should definitively prompt to search for SDHx somatic mutations in tumor DNA. In our series, this approach led to the identification of 2 tumors with somatic SDHD variants, which have only been very rarely reported in the past [20, 22, 23]. It could also prompt to go deeper in the exploration of some patients like in patient 22, familial case presenting with an SDH deficiency despite the absence of germline and somatic SDHx variants. Several hypotheses such as an intronic mutation on germline DNA not detected by NGS analyses, epigenetic mutations in SDHx promoters, or mutation in a regulator gene of SDHx genes expression, are under evaluation to go further in this case by performing a whole genome sequencing.
Moreover, the identification of a succinate peak in a tumor is a major argument to support the pathogenicity of a variant of unknown significance (VUS) in an SDHx gene, which is essential to assess the risk of recurrence, to adapt the follow-up and to manage the genetic counseling to relatives [2, 20]. In our cohort, a VUS was detected in 3 patients and classified as deleterious or not (a likely benign VUS in SDHA was ruled out, and 2 VUS, 1 on SDHB (patient 1) and 1 on SDHD (patient 14) genes, were eventually classified as pathogenic), based on in silico predictions and SDHB IHC. In these 3 patients, 1H-MRS results were concordant with IHC results, suggesting that 1H-MRS SUCCES is a useful contribution for patient management, especially whenever the absence of tumor tissue precludes immunohistochemical analyses. Assessing this tumor hallmark in vivo in patients with SDHx-related tumors will present benefits in other aspects. In a patient carrying a germline SDHx variant it could help: (i) to classify a suspicious lesion detected during follow-up and (ii) to confirm the metastatic development of the disease after the identification of one (or several) dubious lesion(s) in extra-paraganglionic sites on imaging follow-up. SDHx mutations also predispose to other types of tumors such as GIST (gastrointestinal stromal tumors), rare cases of renal cell carcinomas, and of pituitary adenomas, which are still debated [24,25,26,27]. Interestingly, in a recent study, the application of 1H-MRS was successfully extended to metastasis of PPGL and GIST and permitted to exclude SDH deficiency in pituitary adenomas developed in an SDHB mutation carrier [28]. However, it is important to note that in our study, only 2 patients with an SDHx mutation were explored for an abdominal PGL. Additional studies should be performed in the future to evaluate 1H-MRS SUCCES accuracy for this particular location.
Regarding clinical relevance, we suggest to perform 1H-MRS SUCCES in all patients with a newly discovered PPGL, in patients with negative germline genetic testing in case of multiple tumors and/or a metastatic development of the disease to tailor therapeutic choices, and in patients already identified to carry a germline SDHx mutation in case of dubious finding on imaging follow-up. By contrast, 1H-MRS SUCCES would not present any relevance in patients with a known mutation on a non-SDHx susceptibility gene.
Imaging of patients with PPGL is crucial in every step of their management and functional imaging using PET tracers providing important information on tumor biology, highly connected with genetic status [29]. Recent development of PET/MR devices would allow to combine functional imaging and 1H-MRS, providing simultaneously and non-invasively coregistered anatomical, metabolic (i.e., FDG uptake), and metabolomic (succinate accumulation) information on the tumor.
1H-MRS SUCCES allowed assessing for the presence (or absence) of a succinate peak in a qualitative manner. However, quantification of succinate in vivo with 1H-MRS is feasible and development of a specific post-processing method allowing assessment of succinate as a quantitative biomarker over the time course of the disease would be of great interest especially for the evaluation of response to treatment.
It should also be stressed that SUCCES has the same limitations as any 1H-MRS approach, in particular sensitivity in the low millimolar range. Accordingly, its capacity to detect succinate in PPGL lesions is dependent on: (i) the size of the tumor, which is directly related to detection sensitivity: a voxel size smaller than 1 cm3 is associated with a high risk of failure (Table 2) and (ii) the presence of hemorrhagic or necrotic spots, which are sources of technical flaws: a large necrotic area or inclusion of blood vessel within a tumor may discourage exploration by 1H-MRS SUCCES (Fig. 4). Nevertheless, there is still room for progress and in this respect, several adjustments may improve sensitivity and decrease the sequence duration. These include increasing MRI field using the new research-dedicated 7-tesla devices, post-processing of the spectra, or respiratory gating for abdominal tumours. Indeed, respiratory gating would be a possible adjustment for abdominal tumors and in particular for PCC but with an increase in total duration of the sequence, a risk of patient discomfort, and then motion. Nevertheless, one solution could be to analyze respiratory-gated acquisition combined with an increased MRI field (7 tesla) that could allow reducing average number and thus sequence duration.
In conclusion, using 1H-MRS to detect specific tumor phenotypes induced by genetic mutations is innocuous (no tissue sampling, no injection of radiopharmaceutical or contrast agent are needed) and precise. Following the initial pilot studies [12, 28, 30], this study crosses the second translational gap and demonstrates that 1H-MRS SUCCES can be performed in a radiology department during routine clinical practice. 1H-MRS improves genetic diagnosis, providing VUS characterization, and guiding geneticists to extend investigations to somatic NGS. 1H-MRS SUCCES is ideal for assessing the presence of succinate repeatedly over the time course of the disease, for clinical surveillance, postoperative follow-up, and evaluation of treatment efficacy.
References
Buffet A, Ben Aim L, Leboulleux S, Drui D, Vezzosi D, Libe R, et al. Positive impact of genetic test on the management and outcome of patients with paraganglioma and/or pheochromocytoma. J Clin Endocrinol Metab. 2019;104(4):1109–18. https://doi.org/10.1210/jc.2018-02411.
Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101–11. https://doi.org/10.1038/nrendo.2014.188.
Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92(10):3822–8. https://doi.org/10.1210/jc.2007-0709.
Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, et al. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63(17):5615–21.
Favier J, Briere JJ, Burnichon N, Riviere J, Vescovo L, Benit P, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4(9):e7094. https://doi.org/10.1371/journal.pone.0007094.
Letouze E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23(6):739–52. https://doi.org/10.1016/j.ccr.2013.04.018.
Morin A, Letouze E, Gimenez-Roqueplo AP, Favier J. Oncometabolites-driven tumorigenesis: from genetics to targeted therapy. Int J Cancer. 2014;135(10):2237–48. https://doi.org/10.1002/ijc.29080.
Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77–85. https://doi.org/10.1016/j.ccr.2004.11.022.
Pollard PJ, Briere JJ, Alam NA, Barwell J, Barclay E, Wortham NC, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14(15):2231–9. https://doi.org/10.1093/hmg/ddi227.
Richter S, Peitzsch M, Rapizzi E, Lenders JW, Qin N, de Cubas AA, et al. Krebs cycle metabolite profiling for identification and stratification of pheochromocytomas/paragangliomas due to succinate dehydrogenase deficiency. J Clin Endocrinol Metab. 2014;99(10):3903–11. https://doi.org/10.1210/jc.2014-2151.
Rao JU, Engelke UF, Rodenburg RJ, Wevers RA, Pacak K, Eisenhofer G, et al. Genotype-specific abnormalities in mitochondrial function associate with distinct profiles of energy metabolism and catecholamine content in pheochromocytoma and paraganglioma. Clin Cancer Res. 2013;19(14):3787–95. https://doi.org/10.1158/1078-0432.CCR-12-3922.
Lussey-Lepoutre C, Bellucci A, Morin A, Buffet A, Amar L, Janin M, et al. In vivo detection of succinate by magnetic resonance spectroscopy as a Hallmark of SDHx mutations in paraganglioma. Clin Cancer Res. 2016;22(5):1120–9. https://doi.org/10.1158/1078-0432.CCR-15-1576.
Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–42. https://doi.org/10.1210/jc.2014-1498.
Plouin PF, Gimenez-Roqueplo AP, Bertagna X. COMETE, a network for the study and management of hypersecreting adrenal tumors. Bull Acad Natl Med. 2008;192(1):73–82 discussion 3-5.
Webb PG, Sailasuta N, Kohler SJ, Raidy T, Moats RA, Hurd RE. Automated single-voxel proton MRS: technical development and multisite verification. Magn Reson Med. 1994;31(4):365–73.
Korpershoek E, Favier J, Gaal J, Burnichon N, van Gessel B, Oudijk L, et al. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab. 2011;96(9):E1472–6. https://doi.org/10.1210/jc.2011-1043.
Menara M, Oudijk L, Badoual C, Bertherat J, Lepoutre-Lussey C, Amar L, et al. SDHD immunohistochemistry: a new tool to validate SDHx mutations in pheochromocytoma/paraganglioma. J Clin Endocrinol Metab. 2015;100(2):E287–91. https://doi.org/10.1210/jc.2014-1870.
van Nederveen FH, Gaal J, Favier J, Korpershoek E, Oldenburg RA, de Bruyn EM, et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol. 2009;10(8):764–71. https://doi.org/10.1016/S1470-2045(09)70164-0.
Benit P, Goncalves S, Philippe Dassa E, Briere JJ, Martin G, Rustin P. Three spectrophotometric assays for the measurement of the five respiratory chain complexes in minuscule biological samples. Clin Chim Acta. 2006;374(1–2):81–6. https://doi.org/10.1016/j.cca.2006.05.034.
Ben Aim L, Pigny P, Castro-Vega LJ, Buffet A, Amar L, Bertherat J, et al. Targeted next-generation sequencing detects rare genetic events in pheochromocytoma and paraganglioma. J Med Genet. 2019. https://doi.org/10.1136/jmedgenet-2018-105714.
Group NGSiPS, Toledo RA, Burnichon N, Cascon A, Benn DE, Bayley JP, et al. Consensus statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nat Rev Endocrinol. 2017;13(4):233–47. https://doi.org/10.1038/nrendo.2016.185.
Curras-Freixes M, Inglada-Perez L, Mancikova V, Montero-Conde C, Leton R, Comino-Mendez I, et al. Recommendations for somatic and germline genetic testing of single pheochromocytoma and paraganglioma based on findings from a series of 329 patients. J Med Genet. 2015;52(10):647–56. https://doi.org/10.1136/jmedgenet-2015-103218.
van Nederveen FH, Korpershoek E, Lenders JW, de Krijger RR, Dinjens WN. Somatic SDHB mutation in an extraadrenal pheochromocytoma. N Engl J Med. 2007;357(3):306–8. https://doi.org/10.1056/NEJMc070010.
Boikos SA, Pappo AS, Killian JK, LaQuaglia MP, Weldon CB, George S, et al. Molecular subtypes of KIT/PDGFRA wild-type gastrointestinal stromal tumors: a report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol. 2016;2(7):922–8. https://doi.org/10.1001/jamaoncol.2016.0256.
Evenepoel L, Papathomas TG, Krol N, Korpershoek E, de Krijger RR, Persu A, et al. Toward an improved definition of the genetic and tumor spectrum associated with SDH germ-line mutations. Genet Med. 2015;17(8):610–20. https://doi.org/10.1038/gim.2014.162.
Mason EF, Hornick JL. Conventional risk stratification fails to predict progression of succinate dehydrogenase-deficient gastrointestinal stromal tumors: a clinicopathologic study of 76 cases. Am J Surg Pathol. 2016;40(12):1616–21. https://doi.org/10.1097/PAS.0000000000000685.
Ricketts C, Woodward ER, Killick P, Morris MR, Astuti D, Latif F, et al. Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst. 2008;100(17):1260–2. https://doi.org/10.1093/jnci/djn254.
Casey RT, McLean MA, Madhu B, Challis BG, ten Hoopen R, Roberts T, et al. Translating in vivo metabolomic analysis of succinate dehydrogenase deficient tumors into clinical utility. JCO Precis Oncol. 2019;2:1–12. https://doi.org/10.1200/PO.17.00191.
Taieb D, Jha A, Treglia G, Pacak K. Molecular imaging and radionuclide therapy of paraganglioma and pheochromocytoma. Endocr Relat Cancer. 2019. https://doi.org/10.1530/ERC-19-0165.
Varoquaux A, le Fur Y, Imperiale A, Reyre A, Montava M, Fakhry N, et al. Magnetic resonance spectroscopy of paragangliomas: new insights into in vivo metabolomics. Endocr Relat Cancer. 2015;22(4):M1–8. https://doi.org/10.1530/ERC-15-0246.
Acknowledgment
We thank Catherine Tritscher for the administrative assistance and Estelle Robidel, Anne-Laure Gauthier, and Benjamin Banting for the technical assistance.
Funding
This work has received funding from the Paradifference Foundation, the European Union’s Horizon 2020 research and innovation program under grant agreement No 633983, the Institut National du Cancer and the Direction Générale de l’Offre de Soins (PRT-K 2014, COMETE-TACTIC, INCa-DGOS_8663), and the Cancer Research for Personalized Medicine-CARPEM project (Site de Recherche Intégré sur le Cancer-SIRIC). Our team is supported by the Ligue Nationale contre le Cancer (Equipe Labellisée). The genetical and phenotyping parts of this study were granted by the Institut National du Cancer and the Direction Générale de l’Offre de Soins (PRT-K 2014, COMETE-TACTIC, INCa-DGOS_8663).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval for the study was obtained from the institutional review board (Comité de Protection des Personnes (CPP) Ile de France II).
Informed consent
All patients provided written informed consent for participation to the study, collection of samples, and subsequent analyses.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Endocrinology.
Rights and permissions
About this article
Cite this article
Lussey-Lepoutre, C., Bellucci, A., Burnichon, N. et al. Succinate detection using in vivo 1H-MR spectroscopy identifies germline and somatic SDHx mutations in paragangliomas. Eur J Nucl Med Mol Imaging 47, 1510–1517 (2020). https://doi.org/10.1007/s00259-019-04633-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04633-9