Abstract
Purpose
To conduct a systematic review of articles on PET imaging of carotid atherosclerosis with emphasis on clinical usefulness and comparison with other imaging modalities.
Methods
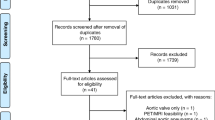
Research articles reporting carotid artery PET imaging with different radiotracers until 30 November 2018 were systematically searched for in Medline/PubMed, Scopus, Embase, Google Scholar, and Cochrane Library. Duplicates were removed, and editorials, case studies, and investigations on feasibility or reproducibility of PET imaging and of patients with end-stage diseases or immunosuppressive medications were omitted. After quality assessment of included articles using Joanna Briggs Institute checklists, all eligible articles were reviewed.
Results
Of 1718 primary hits, 53 studies comprising 4472 patients, aged 47–91 years (78.8% males), were included and grouped under the following headlines: diagnostic performance, risk factors, laboratory findings, imaging modalities, and treatment. 18F-fluorodeoxyglucose (FDG) (49/53) and 18F-sodium fluoride (NaF) (5/53) were the most utilized tracers to visualize carotid wall inflammation and microcalcification, respectively. Higher carotid FDG uptake was demonstrated in patients with than without symptomatic carotid atherosclerosis. Normal carotid arteries presented with the lowest FDG uptake. In symptomatic atherosclerosis, carotid arteries ipsilateral to a cerebrovascular event had higher FDG uptake than the contralateral carotid artery. FDG uptake was significantly associated with age, male gender, and body mass index in healthy individuals, and in addition with arterial hypertension, hypercholesterolemia, and diabetes mellitus in patients. Histological assessment indicated a strong correlation between microcalcification and NaF uptake in symptomatic patients. Histological evidence of calcification correlated inversely with FDG uptake, which was associated with increased macrophage and CD68 count, both accounting for increased local inflammatory response.
Conclusion
FDG-PET visualizes the inflammatory part of carotid atherosclerosis enabling risk stratification to a certain degree, whereas NaF-PET seems to indicate long-term consequences of ongoing inflammation by demonstrating microcalcification allowing discrimination of atherosclerotic from normal arteries and suggesting clinically significant carotid atherosclerosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiovascular and cerebrovascular diseases are the number one causes of mortality worldwide [1], and thromboembolic events caused by carotid artery diseases are the main reason for cerebrovascular diseases [2]. We aimed to elucidate what is known from the literature about positron emission tomography (PET) imaging of carotid artery disease and to what degree this provides insight into disease mechanisms and handles for improved management.
Ongoing arterial wall inflammation causes lipid accumulation and calcification. Formed atherosclerotic lesions with thrombus formation may result in carotid artery occlusion and/or emboli giving rise to downstream cerebral artery occlusion [3]. Since carotid atherosclerosis is usually symptomless, most diagnostic approaches are in symptomatic patients to detect obstruction, which requires that the carotid lesion has grown to a size above the detection limit. Therefore, most details of the processes leading to progressive carotid atherosclerosis remain unknown [4]. PET has brought a new dimension by targeting molecular elements of the atherosclerotic process including focally increased glucose metabolism and arterial wall microcalcification by means of 18F-fluorodeoxyglucose (FDG) and 18F-sodium fluoride (NaF), respectively [5, 6].
Recent studies using PET have reported promising results regarding the detection of subtle and primary atherosclerotic changes in arteries based on inflammation and/or injury rather than demonstrating anatomical features of the atherosclerotic process [7]. This would promise early detection of atherosclerosis, risk stratification of developed atherosclerotic lesions, and potentially timely treatment options. We aimed to systemically review the literature with regard to studies utilizing PET to investigate early carotid atherosclerosis and the relation of PET findings to clinical, paraclinical, and therapeutic factors.
Methods
Search protocol
This systematic review was conducted using the PRISMA guidelines [8] (for details see Online Resource 1). Search strings were “Carotid,” “Artery,” “Atherosclerosis,” “Plaque,” “PET,” “Fluorodeoxyglucose,” “Sodium Fluoride,” and their equivalent terms as the keywords. The search strategy and PICOS questions (Problem, Indicator, Comparison, Outcome and Study types) to help guide a standardized and disciplined way of formulating the clinical research question are outlined in Online Resource 2 and 3, respectively.
The databases Medline/PubMed, Scopus, Embase, Google Scholar, and Cochrane Library were searched. All human studies utilizing PET imaging with clinically available radiotracers to visualize carotid arteries or carotid plaque published after 2000 were included. Editorials, letters, commentaries, perspectives, reviews, case studies, conference abstracts, and articles on feasibility, methodology, and reproducibility were omitted together with studies including patient populations with end-stage diseases. Only original articles in English were considered.
Study selection
The primary results of the search were compiled using EndNote (Package for Windows version 8). After deletion of duplicates, titles and abstracts were reviewed and screened. Then, full-text articles were extracted and evaluated for eligibility using the Joanna Briggs Institute assessment tools [9] and rated with regard to evidence class according to Oxford Centre for Evidence-based Medicine [10]. All steps were conducted by one author (RP).
Data extraction and study analysis
Extracted data included first author, publication year, study design, demographics (number of patients, age, and gender), clinical conditions (inclusion criteria) (Online Resource 4), radiotracer type, acquisition time, radiotracer uptake scale (Online Resource 5), hypothesis, results, and limitations (Online Resource 6). The included studies were (a) categorized based on PET findings in different clinical conditions, (b) correlated with paraclinical findings, and (c) assessed with respect to PET’s potential to influence atherosclerosis treatment. The categories included diagnostic and prognostic performance, risk factors, laboratory findings, imaging modalities, and treatment.
Results
General characteristics
In the initial literature search, 1718 studies were identified. After assessment, 53 were included for analysis (Fig. 1, Online Resource 4). In these, a total of 4472 subjects with mean age of 58 years (range 47–91) were studied comprising 3526 (78.8%) males. Study designs included cross-sectional (45/53), case-control (4/53), and interventional trials (4/53). FDG (49/53) and NaF (5/53) were the most used tracers. The numbers of studies within the chosen categories were as follows: diagnostic and prognostic performance (n = 21), risk factors (n = 17), laboratory findings (n = 13), immunohistochemistry (n = 21), other imaging modalities (n = 25), and treatment (n = 9); some studies included multiple categories. The mean level of evidence of the included studies was 3.76 (from the highest level of evidence 1 to the lowest 5). Descriptive statistics of included studies categorized based on their study design and category is shown in Table 1.
Diagnostic and prognostic performance
Carotid FDG uptake in patients with atherosclerosis was mostly compared with that in subjects without atherosclerosis. Uptake of FDG [11,12,13,14,15,16,17] was more pronounced in atherosclerotic patients with than without symptoms except that in one study this difference was not present [18]. FDG uptake was higher in carotid artery specimens of patients with more severe disease (stroke vs. transient ischemic attack; maximum standardized uptake value (SUVmax), i.e., FDG uptake corrected by the background mean uptake by micro-PET, 5.3 vs. 3.7, p < 0.05) [13] or with an “acute” cerebrovascular event (< 90 days vs. > 90 days: SUVmax 3.1 vs. 2.5, p = 0.01) [12]. Kim et al. showed in a recent study that patients with high carotid FDG uptake are more prone to early recurrence of ischemic stroke [19], although a prior study by Arauz et al. was unable to demonstrate this prognostic trend [20]. In patients with symptomatic atherosclerosis, ipsilateral carotid FDG uptake correlated with the contralateral carotid FDG uptake [21, 22], and such that the ipsilateral was higher than contralateral FDG uptake [12, 22,23,24,25]. In contrast, studies performed early after a cerebrovascular event (less than 10 days) showed no difference between ipsilateral and contralateral FDG uptake [26, 27]. However, in a study by Kwee et al., the difference in FDG uptake between ipsilateral and contralateral carotid arteries was significant until 38 days after initiation of symptoms, but not longer than that [22].
NaF uptake was shown to be lower in normal than in atherosclerotic carotid arteries (percentage uptake of total incubation dose per gram 0.44 vs. 2.3, p < 0.001) [28], whereas this difference was not significantly present when comparing atherosclerotic carotid arteries in symptomatic and asymptomatic patients [28, 29]. Furthermore, there was no difference between contralateral and ipsilateral carotid arteries of patients with atherosclerosis [27, 30].
Risk factors and laboratory findings
The correlation between different physiological parameters, medical history, and drug history varied widely. In the general population (asymptomatic subjects), higher FDG uptake was associated with older age [7, 31,32,33,34], hypertension [31, 33, 35, 36], diabetes [35,36,37], central obesity [17, 31, 36], hypercholesterolemia [7, 31, 38, 39], male gender [34, 36], history of cardiovascular disease [34, 35], increased abdominal fat [31, 32], cardiovascular risk [7, 31, 36], increased triglyceride [36, 39], increased hs-CRP (C-reactive protein) [35, 40], leukocytosis [41], peripheral arterial disease [37], metabolic syndrome [36], periodontal disease [42], and non-alcoholic fatty liver disease [32]. Other studies did not show any correlation between FDG uptake and hs-CRP [31, 43,44,45], CRP [19, 21, 46], lipid profile [35, 45], leukocytosis [19], smoking [37], gender [37], and body weight [37]. Finally, in a few studies, HDL level [39, 40], diabetes [34], and previous statin use [35] were inversely correlated with FDG uptake.
In patients with atherosclerosis, smoking [12, 21, 39, 43], previous anti-coagulant or statin therapy [12, 21, 39, 43], impaired lipid profile [12, 21, 43], male gender [21, 43], hypertension [12, 21, 43], old age [21, 43], diabetes [12, 43], history of cardiovascular diseases [21], high body mass index [21], and metabolic syndrome [39] appeared not to be associated with a higher FDG uptake. In contrast, some studies showed significant correlation between FDG uptake and male gender [39, 41], age [41], lipid profile [39], and history of cerebrovascular disease [41] in atherosclerotic patients. Regarding NaF uptake in the general population, it was shown that increased NaF uptake was associated with aging, male gender, hypertension, and hypercholesterolemia, but not with smoking, diabetes, obesity, and history of cardiovascular disease [47].
Immunohistochemistry
In histological studies performed on samples acquired during endarterectomy, it was shown that FDG uptake was associated with an increase in parameters reflecting inflammatory cell infiltration—e.g., percentage of inflammatory cells and absolute macrophage area [11, 13, 16, 21, 43, 48,49,50], neovascularization, and loose extracellular matrix [49]. On the other hand, FDG uptake correlated inversely with calcium deposition in histological studies [13, 16, 24, 48, 49] and fibrous tissue [49] in histological assessment. Regarding lipid deposition, Saito et al. concluded that FDG uptake correlates positively with lipid deposition [51], whereas Menezes et al. did not [24]. NaF uptake was correlated with calcium deposition in histological assessment [28, 30].
Based on the immunohistochemical studies, FDG uptake correlated with CD68 [12, 23, 24, 43, 48, 51,52,53,54,55], cathepsin K [52, 53, 55], CD45 [12], MMP-9 [51], IL-18 [52], GLUT-1 [55], and VEGF [24] expression. However, some studies reported that FDG does not correlate with CD68 [26], MMP-9 [53], and VEGF [53]. FDG uptake was found to be inversely correlated with hexokinase 2 [55], and CD34 [53] expression. There was no significant relationship between NaF uptake and CD68 expression, but arterial wall NaF uptake correlated inversely with α-smooth muscle antigen [30].
Imaging modalities
In ultrasonographic studies of carotid arteries, it was shown that carotid plaque echogenicity correlates inversely with FDG uptake [11, 23, 40]. Also, microembolous signals in transcranial Doppler sonography were associated with higher FDG uptake [15, 56, 57]. Intima-media thickness was found to be correlated with FDG uptake in the general population [17, 31]. In patients with atherosclerosis, the degree of stenosis in carotid plaque did not correlate with FDG uptake [18, 19, 22, 39], whereas Shaikh et al. reported the opposite [26]. Carotid plaque surface irregularity did not correlate with FDG uptake [19].
In CT studies, FDG uptake correlated with remodeling, low attenuation [48], vessel wall volume, lipid-rich necrotic core volume, fibrous tissue volume, and degree of stenosis [20, 22]. In contrast, FDG uptake correlated inversely with calcium score [20]. Calcium score was found to correlate with NaF uptake in asymptomatic patients, but not in symptomatic patients [28, 30, 47].
When compared with MRI, FDG uptake correlated with vessel wall volume, fibrous tissue volume [22], the presence of lipid-rich necrotic cores, intra-plaque hemorrhage, the presence of rupture in plaque, and high-risk characteristics of plaque [58]. Other studies found that FDG uptake does not correlate with plaque thickness [43] or any other anatomical features [59]. Findings of T1-phase MRI [51], diffusion-weighted MRI [56], and contrast-enhanced MRI [60] did not correlate with FDG-PET observations. In a study by Calcagno et al., it was concluded that Ktrans, Kep, and vp (which all are parameters of perfusion in tissue) correlated inversely with FDG uptake [59].
Treatment
The main therapies applied against carotid artery atherosclerosis were statins. Among patients undergoing endarterectomy, those who also had used statins had a more significant decrease in FDG uptake 3 months after surgery [21]. The effect of statins on carotid FDG uptake in atherosclerotic patients was examined in a retrospective [39] and a prospective [44] study and was not significant in any of them, although Watanabe et al. showed that pitavastatin administration (2 mg/day) resulted in a significant reduction in FDG uptake after 6 months of follow-up [38]. Furthermore, patients undergoing treatments with antiplatelets, e.g., clopidogrel (75 mg/day for 6 months) and ticagrelor (90 mg/day for 6 months) [45], or antidiabetics, e.g., pioglitazone (15–30 mg/day for 4 months) [46], or even bariatric surgery [61] have been shown to have lower FDG uptake compared with baseline.
Discussion
Literature search
Our systematic literature search yielded 1718 reports of which 53 were included in the analysis. Since the first article from Rudd et al. [25], about 4 articles have been published each year. Of these, the vast majority were cross-sectional studies targeting elements of inflammation using FDG in 92% of all included studies. The only other process studied was arterial wall microcalcification targeted by means of NaF as reported in 5 of the 53 studies. Several studies belonged to more than one category. Thus, 21 studies dealt with diagnostic and prognostic performance, 17 with risk factors, 13 with laboratory findings, 21 with immunohistochemistry, 25 with other imaging modalities, and 7 with treatments, while 23 studies belonged to only one category.
Diagnostic and prognostic performance
Different scenarios may apply to developing carotid atherosclerosis (Fig. 2a–c). It was shown that FDG uptake increased as carotid atherosclerosis advanced toward complication (normal → asymptomatic atherosclerosis → symptomatic atherosclerosis). Carotid atherosclerotic lesions may lead to acute vascular complications or undergo inflammatory regression until long-term traces of atherosclerosis prevail in the shape of developing or consolidated lesions [62]. The underlying mechanism splitting the atherosclerosis evolution process into two different downstream courses, i.e., complications due to thrombosis/occlusion or organized and stable atherosclerotic plaque, is not known. Furthermore, it is not known whether this mechanism changes inflammation status—and subsequently FDG uptake—in the atherosclerotic plaque or the arteries of the general population (Fig. 2a, ii). The reason for this ignorance is that no researcher has performed repeat PET imaging in asymptomatic atherosclerotic patients to follow them until a complication occurs. Higher carotid FDG uptake appears in neurologically asymptomatic patients with neoplastic diseases to be associated with a higher risk of cerebrovascular events [63]. So, a difference in inflammation intensity may exist between asymptomatic and symptomatic atherosclerotic plaques before complications occur (Fig. 2a, ii). Plaque calcification is thought to be induced by the osteogenic transformation of the surrounding vascular smooth muscle cell secondary to release of inflammatory cytokines, which induce a positive feedback loop by increasing inflammation secondary to microcalcification [64]. NaF-PET is routinely used to trace skeletal mineralization due to metastatic cancers, but is highly sensitive in visualizing vascular microcalcification [65, 66]. In a recent study, NaF uptake in atherosclerotic carotid arteries was found to be clearly higher than that in healthy control subjects (Fig. 2b) [28].
Conceptual diagrams demonstrating trends in FDG (a) and NaF (b) uptake in healthy subjects and patients with and without atherosclerosis. For the FDG uptake, the changes are divided into three time periods: (i) before initiation of atherosclerosis, (ii) after i and before iii, and (iii) after atherosclerosis complication. Note that it is not known yet whether complication of atherosclerotic plaque is a process started as soon as formation of atherosclerosis or a sudden incident giving rise to FDG uptake (between two dotted vertical lines). c Conceptual diagram depicting the risk of complication caused by carotid atherosclerotic plaque (black line), whether due to risk of rupture (red line) or due to stenosis (blue line)
The mentioned limitations (ethical and technical) are abolished after a cerebrovascular event making imaging more justifiable among symptomatic than asymptomatic patients. In the complicated atherosclerotic plaque, local inflammation in the plaque may cause a flare-up due to local pathophysiological processes such as initiation of the coagulation cascade and release of pro-inflammatory cytokines [67, 68]. This is reflected by a significantly higher FDG uptake in a symptomatic compared with an asymptomatic carotid plaque; meanwhile, FDG uptake in the carotid artery contralateral to the culprit plaque appears to be higher in symptomatic than in asymptomatic cases potentially due to initiation of a systemic inflammation in the entire body after the cerebrovascular event [69]. The inflammation in the contralateral carotid artery has a more protracted initiation sequence than in the complicated carotid plaque, meaning that the side difference in inflammation intensity is significant initially, but wanes as the local inflammation in the complicated carotid plaque decreases and the inflammation in the contralateral carotid artery evolves (Fig. 2a, iii). The outlined course of development was derived from PET findings at different time points after a cerebrovascular event [11,12,13,14,15,16,17,18,19, 21,22,23,24,25,26,27,28,29,30, 37]. In addition, it was shown that increased FDG uptake in patients with symptomatic atherosclerotic plaques is associated with a higher risk of recurrence [19].
Risk factors and laboratory findings
The most common risk factors correlated with higher FDG uptake were aging, gender, hypertension, and hyperlipidemia in the general population. These findings are justifiable based on histological studies. Aging has been shown to be associated with some phenotypic changes in inflammatory vascular smooth muscle and endothelial cells including increased pro-inflammatory cytokine secretion and altered adhesion molecules, all leading to increased migration and activation of inflammatory cells, especially macrophages [70]. In detail, FDG uptake increases in carotid arteries as age does, but this process is more pronounced in patients bearing risk factors such as hypertension, diabetes, and high cholesterol and triglyceride than in healthy controls. This is understandable, as the stress-induced inflammation is accumulated with age and accelerated by the aforementioned risk factors [71]. Although there are no reports on the measurement of arterial FDG uptake in childhood or adolescence to compare with older ages (Fig. 2a, i), there is evidence from postmortem studies of the existence of fatty streaks as a surrogate for initiation of underlying inflammation in early ages [72]. The effect of gender on inflammatory status of vascular structures is mostly debated through the role of sex steroids [73], but the role of these or other gender-related factors is not known. The influence of sex hormones on inflammation in the cardiovascular system depends on hormone level, whether physiologic or therapeutic, resulting in anti- or pro-inflammatory effects [74]. Endothelium has an important role in maintaining the anti- and pro-inflammatory balance in arteries especially by nitric oxide production [75]. Hypertension and hypercholesterolemia induce endothelial dysfunction and impaired nitric oxide production, which leads to the dominance of pro-inflammatory factors [76, 77]. Interestingly, most of the mentioned risk factors did not correlate with NaF-PET findings in patients with carotid atherosclerotic disease, which is in contrast to what has been reported in other parts of the arterial system [78].
Immunohistochemistry
Histological and immunohistochemical findings were the most coherent with PET findings because of the shared focus on molecular aspects. It was shown that pro-inflammatory changes especially those in relation to count and activity of macrophages in carotid arteries correlated with PET findings. FDG was used to trace the inflammation in carotid plaques as indicated by a higher radiotracer uptake. Inflammation in carotid arteries is reflected by an increased count of inflammatory cells especially macrophages in histological studies [79]. In immunohistochemical studies, atherosclerotic carotid arteries have an increased expression of CD68 and cathepsin K, which is also characteristic for monocyte lineage [80]. Microcalcification as another aspect inspected in histological studies was co-located with NaF uptake (Fig. 2b), whereas FDG uptake was inversely correlated with microcalcification. This is in agreement with the fact that plaques bearing more calcification have a lower risk of complication and lower FDG uptake [49]. To sum up, lipid and necrotic debris in plaques, known to increase plaque vulnerability, are replaced by microcalcification and eventually consolidated calcifications during atherosclerosis development, and meanwhile, plaque size increases and may lead to stenosis (Fig. 2c).
Imaging modalities
Findings of molecular imaging by PET were also assessed in comparison with conventional imaging modalities. Echolucency was found to correlate with FDG uptake, although agreement on correlation between FDG uptake and degree of stenosis or intima-media thickness was poor [18]. It has previously been shown that echolucent carotid plaques (lipid-rich) have higher macrophage infiltration [81] justifying increased FDG uptake. However, considering intima-media thickness and stenosis as relatively macroscopic features and the fact that a stenotic carotid artery could both bear both a stable large calcified and an inflamed and complicated atherosclerotic plaque [82, 83], correlating FDG uptake with these would be unreliable. Also, it was observed that findings by CT and MRI such as presence and volume of lipid core and accompanied soft tissue, intra-plaque hemorrhage, and presence of rupture are correlated with FDG uptake [58, 84]. Previously, it was shown that low attenuation by CT [85] and intra-plaque hemorrhage and increased lipid content on MRI [86] are findings associated with increased inflammation and more frequent among symptomatic atherosclerotic patients. Ktrans, Kep, and vp (parameters of perfusion in tissue) in MRI have been shown to correlate with histological evidence of inflammation [87], but in a recent study, there was no consensus on the correlation between FDG uptake and these parameters [35, 36, 50]. Regarding the detection of calcification, calcium score on CT was found to be correlated with NaF uptake in asymptomatic patients, but not in symptomatic patients. This can be interpreted in view of the fact that as carotid plaques grow in size so does calcium percentage in histological studies [85], and a higher calcium score in CT is associated with a decrease in complication risk [88]. Therefore, continued calcification is a factor resulting in plaque stability, although the calcification process is initiated as early as inflammation itself [28] (Fig. 2b). In other words, the risk of plaque rupture is a result of a process in which inflammation increases and calcification decreases the end result.
Treatment
Most interventions directed to reduce carotid atherosclerosis burden focus on controlling modifiable risk factors, especially impaired lipid profile and diabetes. Statins, as the most common lipid-lowering drugs, have been shown to have anti-inflammatory effects on carotid atherosclerotic plaques [89], but the information retrieved from the current review on this theme was inconsistent among others due to different potency and dosages of the statins proscribed.
Limitations
A main limitation in the current review was the different measures and scales used to express tracer uptake restraining us to perform a meta-analysis. Another limitation was the variation in PET imaging acquisition technique, timing of PET imaging following a complication due to a carotid plaque, and the fact that PET was used for multiple, not always precisely defined, purposes like identification of vulnerable plaques or just to demonstrate the presence of inflammation in the shape of increased FDG uptake. In addition, several other factors should be taken into account to underline the complexity of PET imaging of carotid atherosclerosis. The small size of the vessel and the limited spatial resolution of PET in assessing plague structures no more than 100–200 μm in size are challenges in particular when analyzing tracer uptake in the right carotid artery. The timing of imaging is critical since imaging 1 h following tracer administration will substantially degrade the contrast between plaque and background activity because of which delayed imaging is preferable in detecting atherosclerosis in the carotid and other arteries. Most literature dealt with FDG, but the role of FDG for detection of inflammatory plaques is questionable because of its non-specificity; not only is FDG taken up by inflammatory cells in the plaques, it is also incorporated into smooth muscles and other arterial wall structures which adversely affects the specificity in identifying the intended structures. Therefore, it is unclear whether FDG will remain a major player in assessing atherosclerosis. Among other tracers tested over the years, no other tracer than NaF, in use for detection of arterial wall microcalcification, appears to have a role to play in detecting and quantifying atherosclerosis in carotid arteries. Finally, many of the “healthy” individuals used for comparison were patients with suspected or confirmed malignancies, which could also cast doubt on the results reported and the deductions made from them.
Conclusion
Molecular PET imaging appears to be an interesting modality for investigation of atherosclerotic carotid artery disease. FDG-PET visualizes different inflammatory phases of carotid atherosclerosis development and its complications, while NaF-PET seems to mirror and monitor more long-term consequences by demonstrating arterial wall microcalcification allowing discrimination between atherosclerotic and normal carotid arteries.
References
Organization WH. World health statistics 2016: monitoring health for the SDGs sustainable development goals. World Health Organization; 2016.
Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke. 2006;37(1):87–92.
Polak JF, Pencina MJ, O’leary DH, D’agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in multi-ethnic study of atherosclerosis. Stroke. 2011;42(11):3017–21.
Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–38.
Raynor W, Houshmand S, Gholami S, Emamzadehfard S, Rajapakse CS, Blomberg BA, et al. Evolving role of molecular imaging with 18 F-sodium fluoride PET as a biomarker for calcium metabolism. Curr Osteoporos Rep. 2016;14(4):115–25.
McKenney-Drake ML, Moghbel MC, Paydary K, Alloosh M, Houshmand S, Moe S, et al. 18 F-NaF and 18 F-FDG as molecular probes in the evaluation of atherosclerosis. Eur J Nucl Med Mol Imaging. 2018:1–11.
Blomberg BA, Thomassen A, Takx RA, Hildebrandt MG, Simonsen JA, Buch-Olsen KM, et al. Delayed 18 F-fluorodeoxyglucose PET/CT imaging improves quantitation of atherosclerotic plaque inflammation: results from the CAMONA study. J Nucl Cardiol. 2014;21(3):588–97.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Joanna Briggs Institute. Joanna Briggs Institute reviewers’ manual 2014. Adelaide: The Joanna Briggs Institute; 2014.
Howick J, Phillips B, Ball C, Sackett D, Badenoch D, Straus S et al. Oxford Centre for Evidence-based Medicine—levels of evidence (March 2009). Centre for Evidence Based Medicine. 2009.
Skagen K, Johnsrud K, Evensen K, Scott H, Krohg-Sørensen K, Reier-Nilsen F, et al. Carotid plaque inflammation assessed with 18F-FDG PET/CT is higher in symptomatic compared with asymptomatic patients. Int J Stroke. 2015;10(5):730–6.
Cocker MS, Spence JD, Hammond R, Lum C, Wells G, Bernick J, et al. [18F]-Fluorodeoxyglucose PET/CT imaging as a marker of carotid plaque inflammation: comparison to immunohistology and relationship to acuity of events. Int J Cardiol. 2018;271:378–86.
Masteling MG, Zeebregts CJ, Tio RA, Breek J-C, Tietge UJ, de Boer JF, et al. High-resolution imaging of human atherosclerotic carotid plaques with micro 18 F-FDG PET scanning exploring plaque vulnerability. J Nucl Cardiol. 2011;18(6):1066–75.
Kashiwazaki D, Yamamoto S, Akioka N, Kuwayama N, Noguchi K, Kuroda S. Inflammation coupling between unstable carotid plaque and spleen—a 18F-fluorodeoxyglucos positron emission tomography study. J Stroke Cerebrovasc Dis. 2018;27(11):3212–7.
Müller HFG, Viaccoz A, Fisch L, Bonvin C, Lovblad K-O, Ratib O, et al. 18FDG-PET-CT: an imaging biomarker of high-risk carotid plaques. Correlation to symptoms and microembolic signals. Stroke. 2014;45(12):3561–6.
Demeure F, Bouzin C, Roelants V, Bol A, Verhelst R, Astarci P, et al. Head-to-head comparison of inflammation and neovascularization in human carotid plaques: implications for the imaging of vulnerable plaques. Circ Cardiovasc Imaging. 2017;10(5):e005846.
Tahara N, Kai H, Nakaura H, Mizoguchi M, Ishibashi M, Kaida H, et al. The prevalence of inflammation in carotid atherosclerosis: analysis with fluorodeoxyglucose–positron emission tomography. Eur Heart J. 2007;28(18):2243–8. https://doi.org/10.1093/eurheartj/ehm245.
Noh S-M, Choi WJ, Kang B-T, Jeong S-W, Lee DK, Schellingerhout D, et al. Complementarity between 18F-FDG PET/CT and ultrasonography or angiography in carotid plaque characterization. J Clin Neurol. 2013;9(3):176–85.
Kim H-J, Oh M, Moon DH, Yu K-H, Kwon SU, Kim JS, et al. Carotid inflammation on 18F-fluorodeoxyglucose positron emission tomography associates with recurrent ischemic lesions. J Neurol Sci. 2014;347(1–2):242–5.
Arauz A, Hoyos L, Zenteno M, Mendoza R, Alexanderson E. Carotid plaque inflammation detected by 18F-fluorodeoxyglucose-positron emission tomography: pilot study. Clin Neurol Neurosurg. 2007;109(5):409–12. https://doi.org/10.1016/j.clineuro.2007.02.012.
Font MA, Fernandez A, Carvajal A, Gamez C, Badimon L, Slevin M, et al. Imaging of early inflammation in low-to-moderate carotid stenosis by 18-FDG-PET. Front Biosci. 2009;14:3352–60.
Kwee R, Truijman M, Mess W, Teule G, ter Berg J, Franke C, et al. Potential of integrated [18F] fluorodeoxyglucose positron-emission tomography/CT in identifying vulnerable carotid plaques. Am J Neuroradiol. 2011;32(5):950–4.
Græbe M, Pedersen SF, Højgaard L, Kjær A, Sillesen H. 18FDG PET and ultrasound echolucency in carotid artery plaques. JACC Cardiovasc Imaging. 2010;3(3):289–95.
Menezes LJ, Kotze CW, Agu O, Richards T, Brookes J, Goh VJ, et al. Investigating vulnerable atheroma using combined 18F-FDG PET/CT angiography of carotid plaque with immunohistochemical validation. J Nucl Med. 2011;52(11):1698–703.
Rudd JH, Warburton E, Fryer TD, Jones H, Clark J, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–11.
Shaikh S, Welch A, Ramalingam S, Murray A, Wilson H, McKiddie F, et al. Comparison of fluorodeoxyglucose uptake in symptomatic carotid artery and stable femoral artery plaques. Br J Surg. 2014;101(4):363–70.
Quirce R, Martínez-Rodríguez I, Banzo I, Jiménez-Bonilla J, Martínez-Amador N, Ibáñez-Bravo S, et al. New insight of functional molecular imaging into the atheroma biology: 18F-NaF and 18F-FDG in symptomatic and asymptomatic carotid plaques after recent CVA. Preliminary results. Clin Physiol Funct Imaging. 2016;36(6):499–503. https://doi.org/10.1111/cpf.12254.
Hop H, de Boer SA, Reijrink M, Kamphuisen PW, de Borst MH, Pol RA, et al. 18F-sodium fluoride positron emission tomography assessed microcalcifications in culprit and non-culprit human carotid plaques. J Nucl Cardiol. 2018. https://doi.org/10.1007/s12350-018-1325-5.
Quirce R, Martínez-Rodríguez I, De Arcocha TM, Jiménez-Bonilla JF, Banzo I, Rebollo M, et al. Contribution of 18F-sodium fluoride PET/CT to the study of the carotid atheroma calcification. Rev Esp Med Nucl Imagen Mol. 2013;32(1):22–5. https://doi.org/10.1016/j.remnie.2012.11.009.
Zhang Y, Li H, Jia Y, Yang P, Zhao F, Wang W, et al. Noninvasive assessment of carotid plaques calcification by 18F-sodium fluoride accumulation: correlation with pathology. J Stroke Cerebrovasc Dis. 2018;27(7):1796–801. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.02.011.
Noh TS, Moon S-H, Cho YS, Hong SP, Lee EJ, Choi JY, et al. Relation of carotid artery 18F-FDG uptake to C-reactive protein and Framingham risk score in a large cohort of asymptomatic adults. J Nucl Med. 2013;54(12):2070–6.
Moon SH, Noh TS, Cho YS, Hong SP, Hyun SH, Choi JY, et al. Association between nonalcoholic fatty liver disease and carotid artery inflammation evaluated by 18F-fluorodeoxyglucose positron emission tomography. Angiology. 2015;66(5):472–80. https://doi.org/10.1177/0003319714537872.
Bucerius J, Duivenvoorden R, Mani V, Moncrieff C, Rudd JHF, Calcagno C, et al. Prevalence and risk factors of carotid vessel wall inflammation in coronary artery disease patients. FDG-PET and CT Imaging Study. 2011;4(11):1195–205. https://doi.org/10.1016/j.jcmg.2011.07.008.
Bucerius J, Mani V, Wong S, Moncrieff C, Izquierdo-Garcia D, Machac J, et al. Arterial and fat tissue inflammation are highly correlated : a prospective 18F-FDG PET/CT study. Eur J Nucl Med Mol Imaging. 2014;41(5):934–45. https://doi.org/10.1007/s00259-013-2653-y.
Kim S, Lee S, Kim JB, Na JO, Choi CU, Lim H-E, et al. Concurrent carotid inflammation in acute coronary syndrome as assessed by 18F-FDG PET/CT: a possible mechanistic link for ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24(11):2547–54. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.07.004.
Lee DH, Lee SJ, Lee D-J, Kwon SH, Jo K-S, An Y-S, et al. Carotid artery FDG uptake may serve as a biomarker for cardiovascular risk stratification in asymptomatic adults. Nucl Med Mol Imaging. 2014;48(3):196–202. https://doi.org/10.1007/s13139-014-0277-1.
Bernelot Moens SJ, Stoekenbroek RM, van der Valk FM, Verweij SL, Koelemay MJW, Verberne HJ, et al. Carotid arterial wall inflammation in peripheral artery disease is augmented by type 2 diabetes: a cross-sectional study. BMC Cardiovasc Disord. 2016;16(1):237. https://doi.org/10.1186/s12872-016-0397-x.
Watanabe T, Kawasaki M, Tanaka R, Ono K, Kako N, Saeki M, et al. Anti-inflammatory and morphologic effects of pitavastatin on carotid arteries and thoracic aorta evaluated by integrated backscatter trans-esophageal ultrasound and PET/CT: a prospective randomized comparative study with pravastatin (EPICENTRE study). Cardiovasc Ultrasound. 2015;13(1):17.
Chróinín DN, Marnane M, Akijian L, Merwick Á, Fallon E, Horgan G, et al. Serum lipids associated with inflammation-related PET-FDG uptake in symptomatic carotid plaque. Neurology. 2014;82(19):1693–9.
Choi Y-S, Youn H-J, Chung W-B, Hwang H-J, Lee D-H, Park C-S, et al. Uptake of F-18 FDG and ultrasound analysis of carotid plaque. J Nucl Cardiol. 2011;18(2):267–72.
Kim J, Choi K-H, Song H-C, Kim J-T, Park M-S, Cho K-H. 18 F-FDG PET/CT imaging factors that predict ischaemic stroke in cancer patients. Eur J Nucl Med Mol Imaging. 2016;43(12):2228–35.
Fifer KM, Qadir S, Subramanian S, Vijayakumar J, Figueroa AL, Truong QA, et al. Positron emission tomography measurement of periodontal 18F-fluorodeoxyglucose uptake is associated with histologically determined carotid plaque inflammation. J Am Coll Cardiol. 2011;57(8):971–6.
Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48(9):1818–24.
Kim CJ, Han EJ, Chu E-H, Hwang B-H, Kim J-J, Seung K-B, et al. Effect of moderate-intensity statin therapy on plaque inflammation in patients with acute coronary syndrome: a prospective interventional study evaluated by 18F-FDG PET/CT of the carotid artery. Cardiol J. 2018.
Oh M, Lee CW, Lee HS, Chang M, Ahn JM, Park DW, et al. Similar impact of clopidogrel or ticagrelor on carotid atherosclerotic plaque inflammation. Clin Cardiol. 2016;39(11):646–52.
Mizoguchi M, Tahara N, Tahara A, Nitta Y, Kodama N, Oba T, et al. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes: a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imaging. 2011;4(10):1110–8.
Derlin T, Wisotzki C, Richter U, Apostolova I, Bannas P, Weber C, et al. In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: correlation with atherogenic risk factors. J Nucl Med. 2011;52(3):362–8. https://doi.org/10.2967/jnumed.110.081208.
Figueroa AL, Subramanian SS, Cury RC, Truong QA, Gardecki JA, Tearney GJ, et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging. 2012;5(1):69–77.
Liu J, Kerwin WS, Caldwell JH, Ferguson MS, Hippe DS, Alessio AM, et al. High resolution FDG-microPET of carotid atherosclerosis: plaque components underlying enhanced FDG uptake. Int Journal Cardiovasc Imaging. 2016;32(1):145–52.
Johnsrud K, Skagen K, Seierstad T, Skjelland M, Russell D, Revheim M-E. 18 F-FDG PET/CT for the quantification of inflammation in large carotid artery plaques. J Nucl Cardiol. 2017:1–11.
Saito H, Kuroda S, Hirata K, Magota K, Shiga T, Tamaki N, et al. Validity of dual MRI and 18F-FDG PET imaging in predicting vulnerable and inflamed carotid plaque. Cerebrovasc Dis. 2013;35(4):370–7.
Graebe M, Pedersen SF, Borgwardt L, Højgaard L, Sillesen H, Kjær A. Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET). Eur J Vasc Endovasc Surg. 2009;37(6):714–21.
Pedersen SF, Graebe M, Hag AMF, Hoejgaard L, Sillesen H, Kjaer A. Microvessel density but not neoangiogenesis is associated with 18 F-FDG uptake in human atherosclerotic carotid plaques. Mol Imaging Biol. 2012;14(3):384–92.
Pedersen SF, Græbe M, Hag AMF, Højgaard L, Sillesen H, Kjær A. 18F-FDG imaging of human atherosclerotic carotid plaques reflects gene expression of the key hypoxia marker HIF-1α. Am J Nucl Med Mol Imaging. 2013;3(5):384.
Pedersen SF, Graebe M, Hag AMF, Højgaard L, Sillesen H, Kjaer A. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun. 2010;31(5):423–9.
Moustafa RR, Izquierdo-Garcia D, Jones PS, Graves MJ, Fryer TD, Gillard JH, et al. Watershed infarcts in transient ischemic attack/minor stroke with ≥50% carotid stenosis: hemodynamic or embolic? Stroke. 2010;41(7):1410–6.
Moustafa RR, Izquierdo-Garcia D, Fryer TD, Graves MJ, Rudd JH, Gillard JH, et al. Carotid plaque inflammation is associated with cerebral microembolism in patients with recent transient ischemic attack or stroke: a pilot study. Circ Cardiovasc Imaging. 2010;3(5):536–41.
Hyafil F, Schindler A, Sepp D, Obenhuber T, Bayer-Karpinska A, Boeckh-Behrens T, et al. High-risk plaque features can be detected in non-stenotic carotid plaques of patients with ischaemic stroke classified as cryptogenic using combined 18 F-FDG PET/MR imaging. Eur J Nucl Med Mol Imaging. 2016;43(2):270–9.
Calcagno C, Ramachandran S, Izquierdo-Garcia D, Mani V, Millon A, Rosenbaum D, et al. The complementary roles of dynamic contrast-enhanced MRI and 18 F-fluorodeoxyglucose PET/CT for imaging of carotid atherosclerosis. Eur J Nucl Med Mol Imaging. 2013;40(12):1884–93.
Wang J, Liu H, Sun J, Xue H, Xie L, Yu S, et al. Varying correlation between 18F-fluorodeoxyglucose positron emission tomography and dynamic contrast-enhanced MRI in carotid atherosclerosis: implications for plaque inflammation. Stroke. 2014;45(6):1842–5.
Bucerius J, Vijgen GH, Brans B, Bouvy ND, Bauwens M, Rudd JH, et al. Impact of bariatric surgery on carotid artery inflammation and the metabolic activity in different adipose tissues. Medicine. 2015;94(20).
Virmani R, Kolodgie Frank D, Burke Allen P, Finn Aloke V, Gold Herman K, Tulenko Thomas N, et al. Atherosclerotic plaque progression and vulnerability to rupture. Arterioscler Thromb Vasc Biol. 2005;25(10):2054–61. https://doi.org/10.1161/01.ATV.0000178991.71605.18.
Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50(10):1611–20. https://doi.org/10.2967/jnumed.109.065151.
Chen NX, Moe SM. Pathophysiology of vascular calcification. Current osteoporosis reports. 2015;13(6):372–80.
Fiz F, Morbelli S, Piccardo A, Bauckneht M, Ferrarazzo G, Pestarino E, et al. 18F-NaF uptake by atherosclerotic plaque on PET/CT imaging: inverse correlation between calcification density and mineral metabolic activity. J Nucl Med. 2015;56(7):1019–23.
Irkle A, Vesey A, Lewis D, Skepper J, Bird J, Dweck M. Identifying active vascular microcalcification by (18) F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495.
Butcovan D, Mocanu V, Baran D, Ciurescu D, Tinica G. Assessment of vulnerable and unstable carotid atherosclerotic plaques on endarterectomy specimens. Exp Ther Med. 2016;11(5):2028–32. https://doi.org/10.3892/etm.2016.3096.
Stoll G, Bendszus M. Inflammation and atherosclerosis. Stroke. 2006;37(7):1923–32. https://doi.org/10.1161/01.STR.0000226901.34927.10.
McColl B, Allan S, Rothwell N. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. 2009;158(3):1049–61.
Calvert PA, Liew T-V, Gorenne I, Clarke M, Costopoulos C, Obaid DR, et al. Leukocyte telomere length is associated with high-risk plaques on virtual histology intravascular ultrasound and increased proinflammatory activity. Arterioscler Thromb Vasc Biol. 2011;31(9):2157–64.
McVeigh GE, Allen PB, Morgan DR, Hanratty CG, Silke B. Nitric oxide modulation of blood vessel tone identified by arterial waveform analysis. Clin Sci. 2001;100(4):387–93. https://doi.org/10.1042/cs1000387.
Hong YM. Atherosclerotic cardiovascular disease beginning in childhood. Korean Circ J. 2010;40(1):1–9. https://doi.org/10.4070/kcj.2010.40.1.1.
Sader MA, Celermajer DS. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53(3):597–604. https://doi.org/10.1016/s0008-6363(01)00473-4.
Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–74. https://doi.org/10.1210/er.2007-0001.
Gao F, Lucke-Wold BP, Li X, Logsdon AF, Xu L-C, Xu S, et al. Reduction of endothelial nitric oxide increases the adhesiveness of constitutive endothelial membrane ICAM-1 through Src-mediated phosphorylation. Front Physiol. 2018;8(1124). https://doi.org/10.3389/fphys.2017.01124.
Shimbo D, Muntner P, Mann D, Viera AJ, Homma S, Polak JF, et al. Endothelial dysfunction and the risk of hypertension: the multi-ethnic study of atherosclerosis. Hypertension. 2010;55(5):1210–6.
Halcox Julian PJ, Donald Ann E, Ellins E, Witte Daniel R, Shipley Martin J, Brunner Eric J, et al. Endothelial function predicts progression of carotid intima-media thickness. Circulation. 2009;119(7):1005–12. https://doi.org/10.1161/CIRCULATIONAHA.108.765701.
Høilund-Carlsen PF, Moghbel MC, Gerke O, Alavi A. Evolving role of PET in detecting and characterizing atherosclerosis. PET Clinics. 2019;14(2):197–209. https://doi.org/10.1016/j.cpet.2018.12.001.
Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–6.
Qiao J-H, Mishra V, Fishbein MC, Sinha SK, Rajavashisth TB. Multinucleated giant cells in atherosclerotic plaques of human carotid arteries: identification of osteoclast-like cells and their specific proteins in artery wall. Exp Mol Pathol. 2015;99(3):654–62.
Grønholdt M-LM, Nordestgaard BG, Bentzon J, Wiebe BM, Zhou J, Falk E, et al. Macrophages are associated with lipid-rich carotid artery plaques, echolucency on B-mode imaging, and elevated plasma lipid levels. J Vasc Surg. 2002;35(1):137–45. https://doi.org/10.1067/mva.2002.119042.
Prabhakaran S, Singh R, Zhou X, Ramas R, Sacco RL, Rundek T. Presence of calcified carotid plaque predicts vascular events: the Northern Manhattan Study. Atherosclerosis. 2007;195(1):e197–201. https://doi.org/10.1016/j.atherosclerosis.2007.03.044.
Riccio SA, House AA, Spence JD, Fenster A, Parraga G. Carotid ultrasound phenotypes in vulnerable populations. Cardiovasc Ultrasound. 2006;4(1):44. https://doi.org/10.1186/1476-7120-4-44.
Kwee RM, Teule GJ, van Oostenbrugge RJ, Mess WH, Prins MH, van der Geest RJ, et al. Multimodality imaging of carotid artery plaques: 18 F-fluoro-2-deoxyglucose positron emission tomography, computed tomography, and magnetic resonance imaging. Stroke. 2009;40(12):3718–24.
Shaalan WE, Cheng H, Gewertz B, McKinsey JF, Schwartz LB, Katz D, et al. Degree of carotid plaque calcification in relation to symptomatic outcome and plaque inflammation. J Vasc Surg. 2004;40(2):262–9. https://doi.org/10.1016/j.jvs.2004.04.025.
Silvera SS, Aidi HE, Rudd JHF, Mani V, Yang L, Farkouh M, et al. Multimodality imaging of atherosclerotic plaque activity and composition using FDG-PET/CT and MRI in carotid and femoral arteries. Atherosclerosis. 2009;207(1):139–43. https://doi.org/10.1016/j.atherosclerosis.2009.04.023.
Kerwin WS, O’Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology. 2006;241(2):459–68. https://doi.org/10.1148/radiol.2412051336.
Nandalur KR, Baskurt E, Hagspiel KD, Phillips CD, Kramer CM. Calcified carotid atherosclerotic plaque is associated less with ischemic symptoms than is noncalcified plaque on MDCT. Am J Roentgenol. 2005;184(1):295–8. https://doi.org/10.2214/ajr.184.1.01840295.
Waters DD, Ho JE, Boekholdt SM, DeMicco DA, Kastelein JJ, Messig M, et al. Cardiovascular event reduction versus new-onset diabetes during atorvastatin therapy: effect of baseline risk factors for diabetes. J Am Coll Cardiol. 2013;61(2):148–52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology
Rights and permissions
About this article
Cite this article
Piri, R., Gerke, O. & Høilund-Carlsen, P.F. Molecular imaging of carotid artery atherosclerosis with PET: a systematic review. Eur J Nucl Med Mol Imaging 47, 2016–2025 (2020). https://doi.org/10.1007/s00259-019-04622-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04622-y