Abstract
Purpose
The present study is based on a retrospective analysis of Gallium-68 (68Ga)-labelled prostate-specific membrane antigen (68Ga-PSMA I&T) PET/CT performed in newly diagnosed, treatment-naïve prostate cancer (PCa) patients prior to definitive treatment.
Methods
A total of 82 men were included in the study and were imaged with 68Ga-PSMA I&T PET/CT to assess the distribution of PSMA-avid disease for staging purposes (11 with low-risk, 32 with intermediate-risk, and 39 with high-risk PCa). Forty patients (20 with intermediate- and 20 with high-risk disease) underwent subsequent radical prostatectomy with extended pelvic lymph node dissection which allowed for correlation of imaging findings with histopathologic data.
Results
PSMA-positive disease was detected in 83% of patients with 66/82 (80.5%) primary tumours being visualized. PSMA-avid lymph nodes were recorded in 17/82 patients (20.7%, 3 with intermediate-risk and 14 with high-risk PCa); distant disease was found in 14/82 subjects (17.1%, 2 with intermediate-risk and 12 with high-risk PCa). No extraprostatic disease was found in low-risk PCa. SUVmax of primary tumours showed a weak but significant correlation with serum PSA values (r = 0.51, p < 0.001) and Gleason scores (GSC; r = 0.35, p = 0.001), respectively. In correlation with histopathology, calculated per-region sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for detection of lymph node metastases were 35.0%, 98.4%, 63.6%, 95.0%, and 93.0%, respectively.
Conclusions
In patients with initial diagnosis of intermediate- and high-risk prostate cancer, 68Ga-PSMA I&T PET/CT emerges as a relevant staging procedure by identifying nodal and/or distant metastases. Due to the low prevalence of extraprostatic disease, its value seems to be limited in low-risk disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the second most common malignancy in men worldwide with 1,300,000 estimated new cases in 2018 [1]. Despite a decrease in the proportion of patients with metastatic disease, up to 7 per 100,000 individuals still present with distant metastases at the time of initial diagnosis [2]. In addition, up to 30% of patients will experience tumour recurrence despite curatively intended treatment such as radical prostatectomy (RP) or external beam radiation therapy (EBRT) [3]. Thus, one of the key issues for optimal patient management is accurate pre-operative staging. Intraoperatively, extended pelvic lymph node dissection (ePLND) is recommended during RP in intermediate- and high-risk PCa patients [4], as it provides information about the nodal status (with an estimated risk of LN metastases > 5%) and can help prolong patient survival [5, 6]. Non-invasive staging using standard imaging modalities including computed tomography (CT), magnetic resonance imaging (MRI), and bone scintigraphy yield unsatisfactory results with insufficient sensitivity in detecting lymph node metastases (LNMs), thus leading to a significant underestimation of disease [4].
Recently, positron emission tomography/CT (PET/CT) using various gallium-68-labelled ligands of prostate-specific membrane antigen (PSMA) has been successfully introduced in PCa staging. PSMA, also known as glutamate carboxypeptidase II, N-acetyl-α-linked acidic dipeptidase I or folate hydrolase, is a membrane-bound protein which is highly expressed in prostatic carcinoma with only low-level expression in normal prostate cells and organs such as the brain, kidneys, salivary glands, and small intestine. PSMA expression in PCa patients has been demonstrated to increase with tumour de-differentiation, the Gleason score (GSC), and in metastatic and hormone-refractory cancers, which makes it an excellent target for imaging and therapy [7].
PSMA-directed PET/CT has demonstrated a high sensitivity and an excellent specificity for detecting PCa, mainly in recurrent disease, even at very low (0.2–0.5 ng/ml) levels of prostate-specific antigen (PSA) [8,9,10,11]. In the context of primary PCa staging, first results are equally promising, although the body of evidence is not as vast as for the setting of biochemical relapse and tumour recurrence [12]. Currently, there is an ongoing multi-centre clinical trial to assess the diagnostic accuracy and management impact of PSMA PET/CT in men considered for RP or EBRT [13].
While most of the published studies concern PSMA-11 (PSMA HBED-CC)—a gallium-68 labelled compound introduced by the Heidelberg group [14], studies on the diagnostic performance of 68Ga-PSMA I&T which had been developed by Wester and co-workers at the Technical University of Munich [15], are still limited.
We retrospectively analysed and mapped the PSMA-avid distribution of disease in newly diagnosed, treatment-naïve PCa patients prior to definitive treatment. Additionally, in a sub-cohort of patients undergoing RP with subsequent ePLND, a correlative analysis of imaging findings with histopathology was performed.
Material and methods
Patient selection
Between September 2016 and August 2018, one hundred and five consecutive patients with newly diagnosed, biopsy-proven, treatment-naïve PCa underwent 68Ga-PSMA PET/CT (PSMA I&T, i.e. DOTAGA-(I-y)fk (Sub-KuE), Scintomics GmbH, Fürstenfeldbruck, Germany) [16] for primary staging of the disease. Patients with concomitant malignancies (n = 5), recent prior initiation of systemic treatment such as androgen deprivation therapy (ADT; n = 14), or subjects in whom the Gleason score was not available (n = 4) were excluded from the study. Hence, 82 patients were included in this retrospective analysis, of which forty individuals also underwent radical prostatectomy with ePLND according to the guidelines of the European Association of Urology (EAU) [4]. Patients were categorized into three different risk groups (low-, intermediate-, and high-risk groups) according to the D’Amico classification taking clinical primary tumour stage, serum PSA levels, and the Gleason score into account [17].
68Ga-PSMA I&T was administered for clinical work-up in compliance with §37 of the Declaration of Helsinki and The German Medicinal Products Act, AMG §13 2b, and in accordance with the responsible regulatory body (Regierung von Oberfranken, Bavaria, Germany). All patients gave written informed consent to undergo PSMA PET/CT imaging.
Imaging
Preparation of 68Ga-PSMA I&T
68Ga-PSMA I&T was prepared using a cassette-based radiotracer synthesis module (Scintomics, Fürstenfeldbruck, Germany) according to the methods previously described [18]. Briefly, the eluate (68Ga3+ in 0.1 M HCl) of a 68Ge/68Ga-generator (GalliaPharm®, Eckert & Ziegler AG, Berlin, Germany) was transferred to a cation-exchange cartridge, eluted with 5 N NaCl, added to a solution of 20 μg PSMA I&T (Scintomics, Fürstenfeldbruck, Germany) in HEPES buffer, and heated for 6 min at 125 °C. The product was immobilized on a Sep-Pak C18 cartridge, washed with water, and eluted with ethanol/water 50/50. The eluate was passed through a sterile filter (0.22 μm) into a sterile vial and diluted with phosphate buffer solution to a total volume of 15 ml. Radiochemical purity was determined by gradient high-performance liquid chromatography and thin-layer chromatography. Additionally, the product was also tested for ethanol content, pH, radionuclide purity, sterility, and endotoxins.
Acquisition parameters
The patients were injected with a mean activity of 68Ga-PSMA I&T of 132 ± 22 MBq (range, 75–175 MBq). Imaging was performed using a 64-detector PET/CT scanner (Siemens Biograph mCT 64, Siemens Healthineers, Erlangen, Germany) after a mean uptake period of 66 ± 7 min. PET emission data were acquired using 6–8 bed positions (depending on the patient’s height) from the base of the skull to the proximal thighs (2–3-min emission time per bed position). Subsequently, a monophasic full-dose CT scan was performed after i.v. contrast injection (Imeron 350, weight-adapted, 1 ml/kg body weight) and oral contrast ingestion (30 ml Peritrast in 1 l of water) (CARE Dose 4D, 160 mAs, 120 kV, 512 × 512 matrix, 5 mm slice thickness, slice collimation 64 × 0.6 mm, pitch index 1.4). All PET emission data were reconstructed with an iterative algorithm (HD-PET, 24 subsets, 3 iterations, Gaussian filtering 5 mm, 171 × 200 × 200 matrix, axial resolution 5 mm, in-plane resolution 4.07 × 4.07 mm2) using dedicated manufacturer software (syngo MI.PET/CT, Siemens Healthineers, Erlangen, Germany). No adverse events including allergic reactions were observed after administration of radiotracer and contrast media.
Image analysis
All scans were independently analysed by three board certified nuclear medicine specialists with more than 9 years of experience (W.C, K.F., C.L.). Readers were careful to consider physiology and avoid pitfalls of PSMA PET/CT imaging [19]. In case of disagreement, mutual re-evaluation of images was performed to achieve consensus.
Images were interpreted according to recently published PSMA-RADS Version 1.0 criteria [20]. In brief, for evaluation of the malignant primary, focal activity within the prostate gland significantly above the surrounding background level (apart from physiological activity in urethra) was considered positive. Within the pelvic lymphatic drainage areas, LNs with focally increased uptake were reported as positive (N1, according to PROMISE criteria [21]) and attributed to anatomic regions: sacral, peri-rectal, peri-vesical, right/left obturator, right/left internal iliac, right/left external iliac, right/left common iliac. Retroperitoneally or higher located positive LNs were considered (extrapelvic) M1a disease. PSMA-avid skeletal lesions suggesting bone metastases (BMs, M1b) were categorized into 4 regions: pelvis, vertebral column, ribs, and extremities. PSMA-positive visceral lesions suspicious of metastases (M1c) were reported when present.
Maximum standardized uptake values (SUVmax) of all lesions were measured, without pre-defined threshold values to distinguish between positive and negative lesions.
Data analysis
The distribution of PSMA-avid disease was described as percentage of patients with positive lesions in various localizations: primary tumour + local extension (T+ disease); pelvic LNs (N+ disease); retroperitoneal (or higher) LNs, bones, and visceral organs (M+ disease) [21].
In patients who underwent surgery, 68Ga-PSMA I&T PET/CT–positive lesions were compared with post-operative histopathological findings. Sensitivity (SN), specificity (SP), positive predictive value (PPV), and negative predictive value (NPV) were calculated for the detection of LNMs with regard to LN region–based analysis. The same template approach for imaging and surgery was applied in order to allow for matching the surgically removed LN regions with the regions visualized in PET/CT.
The statistical analysis was performed with Statistica 13.1 Software (StatSoft Polska, Copyright 2016). The normal distribution of the variables (age, PSA levels, GSC, radioisotope activity, duration since PCa diagnosis) was verified by the Shapiro-Wilk W test. Normally distributed values are described as mean ± standard deviation (SD) (range); values without normal distribution are described as median (range). SN, SP, PPV, and NPV were calculated according to standard definitions. The names of appropriate statistical tests used in the analysis were given when necessary. In all cases, a p value < 0.05 was considered statistically significant.
Results
Patient characteristics (entire cohort)
Eighty-two patients met the inclusion criteria and were evaluated for assessment of PSMA-avid distribution of the disease. The mean age was 66.7 ± 7.3 years (range 53–83); median GSC (as derived from biopsy in patients not undergoing surgery or post-operative histopathology, respectively) was 7 (range 6–10); median PSA level was 11.0 ng/ml (range, 0.7–872 ng/ml). According to the D’Amico classification [17], low-risk disease was present in 11/82 (13.4%), intermediate-risk in 32/82 (39.0%), and high-risk in 39/82 (47.6%) patients. Detailed patient characteristics are summarized in Table 1.
Imaging findings
Primary tumour
Sixty-six (80.5%) patients presented with focal PSMA uptake of the primary tumour. Most patients with missing uptake in primary tumours were GSC 6 (n = 3) and 7 (n = 10), but also GSC 8 (n = 1) and 9 (n = 2), respectively. SUVmax of the primary tumour demonstrated a weak but significant correlation with the PSA value (r = 0.51, p < 0.001) and GSC (r = 0.35, p = 0.001), respectively (Figs. 1 and 2a, respectively).
Moreover, primary tumour SUVmax was significantly higher in patients with PSMA-positive pelvic LNs (mean SUVmax 24.9 ± 16.0) as compared with that in patients without positive LNs (mean SUVmax 14.1 ± 12.6, p = 0.006) (Fig. 2b). No significant differences in primary tumour uptake were recorded in individuals with or without BMs, respectively.
PSMA-avid lymph nodes
Overall, PSMA-positive pelvic LNs were reported in 17/82 patients (20.7%); 3 of them had intermediate-risk and 14 had high-risk PCa. Presence of N+ disease correlated considerably with GSC and PSA values, and hence with the disease-risk group: positive LNs were reported in 3/32 (9%) patients with intermediate-risk and 14/39 (36%) patients with high-risk disease. No positive LNs were found in low-risk PCa patients. The pattern of PSMA-avid LN distribution is presented in Fig. 3 a.
PSMA-avid distant lesions
PSMA-positive distant lesions were found in 14/82 (17.1%) patients, predominantly in bones (12 patients), but also in retroperitoneal LNs (5 patients). Six out of 14 (42.9%) M1 patients presented with oligometastatic (≤ 5 lesions) disease. Similar to distribution of positive LNs, distant disease increased with the disease-risk group: in low-risk PCa patients, no positive distant lesions were reported, while 2/32 (6%) patients with intermediate-risk and 12/39 (31%) individuals with high-risk disease presented with PSMA-positive retroperitoneal LN and/or bone foci. Distribution of PSMA-avid lesions depending on GSC and PSA levels is presented in Figs. 4 and 5, respectively.
Among the 12 patients with PSMA-avid bone lesions (Table 2), the majority (10/12, 83.3%) had pelvic lesions, but more than a half (7/12, 58.3%) had (additional) lesions outside the pelvis in vertebral column and ribs, whereas the least frequently involved parts of the skeleton were extremities (3/12, 25%; Fig. 3b). All patients with positive bone foci were GSC 7 to 9. Eight out of 12 patients with PSMA-avid bone lesions had PSA values > 20 ng/ml; however, 2 patients were in the group with PSA 10.1 ≤ 20 ng/ml (GSC 8 and 9, respectively) and 2 in the group with PSA ≤ 10 ng/ml (one with GSC 7; one with GSC 8).
SUVmax values for all types of PSMA-PET-positive lesions are presented in Table 3.
Cohort of operated patients
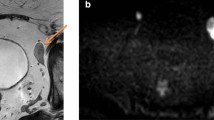
Post-operative histology confirmed PCa in all 40 patients who underwent radical prostatectomy (20 with intermediate-risk and 20 with high-risk PCa). During ePLND, a total of 1022 lymph nodes from 270 regions were removed. The calculated per-region sensitivity, specificity, PPV, NPV, and accuracy for detection of LNMs were 35.0%, 98.4%, 63.6%, 95.0%, and 93.0%, respectively (Table 4). Overall, 7/20 histopathologically confirmed LNMs were found in PSMA-PET/CT. The median intranodal size of LNMs in 13 PSMA-negative lymph nodes was 4.0 mm (range 1.0–9.0), while it was 10 mm (range 1.5–17) in 7 PSMA-positive lymph nodes (p = 0.008, the Mann-Whitney U test; example in Fig. 6). The missed (false negative) LNMs were most commonly located next to the internal iliac arteries and in the obturator fossae (10/13), and were found in high- (n = 5) and intermediate-risk (n = 4) PCa.
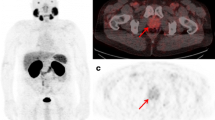
Example of N+/− disease detected by 68Ga-PSMA-PET/CT. 68Ga-PSMA I&T PET/CT of a 72-year-old patient with high-risk prostate cancer (Gleason score 9, PSA 24.08 ng/ml), performed for primary staging. The imaging (fused PET/CT) revealed a pathological uptake of 68Ga-PSMA I&T in the primary tumour of the prostate gland (a; 3D reconstruction of the whole-body PET/CT scan, blue arrow, SUVmax 24.0) and in the right external iliac lymph node (b–d; red arrows, SUVmax 13.0) harbouring 7-mm metastasis of prostate cancer cells (e; dashed red arrows). Post-operative, histopathological staining performed with haematoxylin and eosin (H&E)
Discussion
The present study investigated the performance of 68Ga-PSMA I&T PET/CT in primary staging of biopsy-proven PCa. About 80% of patients presented with PSMA-positive primary tumours, with increasing uptake of 68Ga-PSMA I&T at higher GSC and PSA levels. While our data are generally in line with previously published experience with PSMA-avid ligands [22,23,24] and histopathologic studies [25], we also identified subjects with GSC 8 and 9 PCa that were PSMA-PET-negative. Since these patients did not undergo subsequent radical prostatectomy, immunohistochemical validation of PSMA expression or assessment of potential neuroendocrine de-differentiation (as a reason for PSMA negativity) could not be performed. However, presence of PSMA-negative, high-grade disease as well as its underlying biology should be further investigated in future studies.
In our cohort, extraprostatic disease was present in 28% of the total cohort. Noteworthy, PSMA-avid metastases were exclusively detected in intermediate-risk and high-risk PCa, while all patients with low-risk disease had local disease confined to the prostate. Thus, elaborating whole-body staging with 68Ga-PSMA PET/CT might be of limited use in low-risk disease, given the low prevalence of metastases and therefore limited impact on management of imaging. In contrast, pre-operative PSMA-PET/CT revealed N+ and M+ diseases in 9% and 6% of intermediate-risk PCa and in 36% and 31% of high-risk disease, respectively.
Regarding N+ disease (in the sub-cohort that underwent radical prostatectomy and in whom histological work-up was available), 68Ga-PSMA-PET/CT exhibited almost perfect specificity as reported in the majority of papers [12]. However, pre-operative imaging detected only 7/20 histologically confirmed lymph node metastases, resulting in a sensitivity of 35%, which is lower than in most papers exploring 68Ga-PSMA-11 [12]. This relatively low detection rate could be due to high occurrence of micrometastases (even as small as 1 mm) in the majority of missed LNMs (median intranodal size of 4.0 mm), emphasizing the influence of LNM size on diagnostic accuracy. Another explanation of the lower diagnostic efficacy of 68Ga-PSMA I&T could be the weaker biochemical affinity of the ligand to the PSMA receptor. McCarthy et al. compared directly the two compounds—PSMA I&T and PSMA-HBED (in a small cohort of 19 patients, in whom two PET scans were performed within 2 weeks for primary staging of PCa), and reported a slightly higher diagnostic accuracy of the latter compound (two nodal lesions < 4 mm were missed in 68Ga-PSMA I&T PET/CT) [26]. Still, in our cohort, most of the LNMs were located at the internal iliac and in the obturator fossa areas and thus covered by extensive pelvic lymph node dissection which is per se recommended in all men with high-risk PCa and for intermediate-risk PCa, if the estimated risk of lymph node metastases exceeds 5% with EAU guidelines or 2% with NCCN guideline nomograms [27,28,29]. Whereas the additional value of 68Ga-PSMA-PET/CT might be considered in the detection of nodal disease at unusual sites (including sacral or peri-rectal regions) not routinely covered by standard surgical templates, no definitive conclusion on its role in the guidance of lymph node dissection at the time of radical prostatectomy can be drawn yet.
Beyond pelvic LNMs, identification of M1 disease is crucial for clinical decision-making. PSMA-avid distant metastases (i.e. non-pelvic LNM and/or hematologic metastases) were identified in 6% of individuals with intermediate-risk and more than 30% of patients with high-risk PCa, leading to a change in management in a substantial proportion of subjects. As expected, the most common site of hematologic spread was bone. Although presence of metastatic osseous disease was mainly encountered in patients with high serologic PSA values exceeding 20 ng/ml (8/12 cases), recorded PSA values were < 10 ng/ml in an individual with GSC 7 (Fig. 7) and thus below the recommended threshold for dedicated bone assessment, for example by bone scintigraphy [30].
Example of (oligometastatic) M+/− disease detected by 68Ga-PSMA-PET/CT. 68Ga-PSMA I&T PET/CT (maximum intensity projection, a) of a 58-year-old patient with biopsy-proven intermediate-risk prostate cancer (f, g) (GSC 7, initial PSA 9 ng/ml), with single PSMA-avid focal lesions in bones: the right fifth rib (b, c) and right acetabulum (d, e), highly suspicious for metastases (due to the lack of an anatomical correlate, a false-positive result in the rib, e.g. a benign fibroosseous lesion, cannot be completely excluded)
Notably, given the presence of oligometastatic disease (≤ 5 sites) in 6/14 M1a or M1b cases, 68Ga-PSMA PET/CT enabled the option of metastasis-directed therapy in this sub-cohort. Prospective trials to investigate the impact of initial PSMA-directed PET imaging in intermediate- and high-risk PCa on progression-free and overall survival are highly warranted. The results of an ongoing multi-centre prospective study to ‘assess the diagnostic accuracy and management impact of PSMA-PET scanning in men with prostate cancer being considered for surgery or radiotherapy’ should be published in the near future [13].
This study has several limitations. First, as for most of the papers exploring 68Ga-PSMA PET/CT in primary staging of PCa, it is of retrospective nature and the actual impact on patient outcome cannot be explored. Second, lesions, especially bone metastases, could not be histologically verified in all cases and false-positive findings such as inflammatory processes in lymph nodes or fibrous dysplasia or Paget disease [31] cannot be fully excluded. However, reading physicians were experienced with 68Ga-PSMA I&T, carefully examined both PET and CT images, and avoided known pitfalls [32].
The future perspective of PSMA PET imaging is highly promising. For example, 18F-labelled ligands are increasingly being developed, offering several key advantages such as flexibility in the study design with delayed imaging protocols (due to the longer half-life of 18F), low positron energy along with high spatial resolution due to shortest positron range in tissue, or more widespread distribution from central cyclotron facilities [33]. A recent study already reported on favourable imaging characteristics of the 18F-labelled compound 18F-DCFPyL as compared with 68Ga-PSMA-HBED-CC agents in high-risk patients suffering from biochemically relapsed PCa [34]. As another agent, 18F-PSMA-1007 offers reduced renal excretion that may further increase the diagnostic accuracy for local recurrence or small lymph node metastases in the pelvis [35]. Additionally, novel 18F-radiotracers such as 18F-CTT1057 or 18F-JK-PSMA-7 are being evaluated [36, 37]. Recently, technetium-99m-labelled PSMA ligands (99mTc-MIP-1404 or 99mTc-PSMA-I&S) were clinically established [38, 39], which not only paves the way to the radio-guided prostate surgery, but also enables PSMA-directed diagnostics beyond PET centres.
To conclude, 68Ga-PSMA I&T PET/CT is a useful tool in primary staging of intermediate-risk and high-risk PCa. Due to the low prevalence of extraprostatic disease, its value seems to be limited in low-risk disease.
References
Latest global cancer data: cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. IARC, WHO. 2018. https://www.who.int/cancer/PRGlobocanFinal.pdf. Accessed 6/12/2018
Cetin K, Beebe-Dimmer JL, Fryzek JP, Markus R, Carducci MA. Recent time trends in the epidemiology of stage IV prostate cancer in the United States: analysis of data from the surveillance, epidemiology, and end results program. Urology. 2010:1396–404.
SEER Cancer Stat Facts: Prostate cancer. National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/statfacts/html/prost.html. Accessed 21 Nov 2018
Mottet N, Bellmunt J, Representative EBP, Bolla M, Bourke L, Vice-chair PC, et al. Prostate Cancer EAU Guidelines 2017. Eur Urol. 2017;17:618–29.
Seiler R, Studer UE, Tschan K, Bader P, Burkhard FC. Removal of limited nodal disease in patients undergoing radical prostatectomy: long-term results confirm a chance for cure. J Urol. 2014;191:1280–5.
Abdollah F, Gandaglia G, Suardi N, Capitanio U, Salonia A, Nini A, et al. More extensive pelvic lymph node dissection improves survival in patients with node-positive prostate cancer. Eur Urol. 2015;67:212–9.
Afshar-Oromieh A, Babich JW, Kratochwil C, Giesel FL, Eisenhut M, Kopka K, et al. The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J Nucl Med. 2016;57:79S–89S.
Afshar-Oromieh A, Haberkorn U, Hadaschik B, Habl G, Eder M, Eisenhut M, et al. PET/MRI with a 68Ga-PSMA ligand for the detection of prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40:1629–30.
Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20.
Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of 68Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016;69:393–6.
Rauscher I, Duwel C, Haller B, Rischpler C, Heck MM, Gschwend JE, et al. Efficacy, predictive factors, and prediction nomograms for (68)Ga-labeled prostate-specific membrane antigen-ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol. 2018;73:656–61.
Corfield J, Perera M, Bolton D, Lawrentschuk N. (68)Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: a systematic review. World J Urol. 2018;36:519–27.
A prospective study to assess the diagnostic accuracy and management impact of prostate specific membrane antigen (PSMA) PET scanning in men with prostate cancer being considered for surgery or radiotherapy. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371669. Accessed 19 March 2018
Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95.
Berliner C, Tienken M, Frenzel T, Kobayashi Y, Helberg A, Kirchner U, et al. Detection rate of PET/CT in patients with biochemical relapse of prostate cancer using [68 Ga] PSMA I & T and comparison with published data of [68 Ga] PSMA HBED-CC. Eur J Nucl Med Mol Imaging. 2017:670–7.
Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56:1169–76. https://doi.org/10.2967/jnumed.115.158550.
D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74.
Schmuck S, von Klot CA, Henkenberens C, Sohns JM, Christiansen H, Wester H-J, et al. Initial experience with volumetric (68)Ga-PSMA I&T PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer. J Nucl Med. 2017;58:1962–8.
Sheikhbahaei S, Afshar-Oromieh A, Eiber M, Solnes LB, Javadi MS, Ross AE, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017;44:2117–36.
Rowe SP, Pienta KJ, Pomper MG, Gorin MA. Proposal for a structured reporting system for prostate-specific membrane antigen-targeted PET imaging: PSMA-RADS version 1.0. J Nucl Med. 2018;59:479–85.
Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–79.
Meyrick DP, Asokendaran M, Skelly LA, Lenzo NP, Henderson A. The role of 68Ga-PSMA-I&T PET/CT in the pretreatment staging of primary prostate cancer. Nucl Med Commun. 2017;38:956–63.
Gupta SK, Watson T, Denham J, Shakespeare TP, Rutherford N, McLeod N, et al. Prostate-specific membrane antigen positron emission tomography–computed tomography for prostate cancer: distribution of disease and implications for radiation therapy planning. Int J Radiat Oncol Biol Phys. 2017;99:701–9.
Kabasakal L, Demirci E, Ocak M, Akyel R, Nematyazar J, Aygun A, et al. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl Med Commun. 2015;36:582–7.
Ross JS, Sheehan CE, Fisher HAG, Kaufman RPJ, Kaur P, Gray K, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–62.
McCarthy M, Langton T, Kumar D, Campbell A. Comparison of PSMA-HBED and PSMA-I&T as diagnostic agents in prostate carcinoma. Eur J Nucl Med Mol Imaging. 2017;44:1455–62.
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49.
Cagiannos I, Karakiewicz P, Eastham JA, Ohori M, Rabbani F, Gerigk C, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798–803.
Abuzallouf S, Dayes I, Lukka H. Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J Urol. 2004;171:2122–7.
Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38:200–17.
Werner RA, Sheikhbahaei S, Jones KM, Javadi MS, Solnes LB, Ross AE, et al. Patterns of uptake of prostate-specific membrane antigen (PSMA)-targeted (18)F-DCFPyL in peripheral ganglia. Ann Nucl Med. 2017;31:696–702.
Sanchez-Crespo A. Comparison of Gallium-68 and Fluorine-18 imaging characteristics in positron emission tomography. Appl Radiat Isot. 2013;76:55–62.
Dietlein M, Kobe C, Kuhnert G, Stockter S, Fischer T, Schomacker K, et al. Comparison of [(18)F]DCFPyL and [ (68)Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015;17:575–84.
Giesel FL, Knorr K, Spohn F, Will L, Maurer T, Flechsig P, et al. Detection efficacy of [(18)F]PSMA-1007 PET/CT in 251 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2019;60:362–8.
Behr SC, Aggarwal R, Van Brocklin HF, Flavell RR, Geo K, Small EJ, et al. First-in-human phase I study of CTT1057, a novel (18)F labeled imaging agent with phosphoramidate core targeting prostate specific membrane antigen in prostate cancer. J Nucl Med J Nucl Med. 2018. https://doi.org/10.2967/jnumed.118.220715.
Zlatopolskiy BD, Endepols H, Krapf P, Guliyev M, Urusova EA, Richarz R, et al. Discovery of (18)F-JK-PSMA-7, a novel PET-probe for the detection of small PSMA positive lesions. J Nucl Med. 2018. https://doi.org/10.2967/jnumed.118.218495.
Schmidkonz C, Cordes M, Beck M, Goetz TI, Schmidt D, Prante O, et al. SPECT/CT with the PSMA ligand 99m Tc-MIP-1404 for whole-body primary staging of patients with prostate cancer. Clin Nucl Med. 2018;43:225–31.
Robu S, Schottelius M, Eiber M, Maurer T, Gschwend J, Schwaiger M, et al. Preclinical evaluation and first patient application of 99mTc-PSMA-I&S for SPECT imaging and radioguided surgery in prostate cancer. J Nucl Med. 2017;58:235–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
Hans-Jürgen Wester is founder and shareholder of Scintomics. All other authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Genitourinary
Rights and permissions
About this article
Cite this article
Cytawa, W., Seitz, A.K., Kircher, S. et al. 68Ga-PSMA I&T PET/CT for primary staging of prostate cancer. Eur J Nucl Med Mol Imaging 47, 168–177 (2020). https://doi.org/10.1007/s00259-019-04524-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04524-z