Abstract
Purpose
To evaluate the prognostic value of volumetric parameters calculated from 68Ga-1,4,7,10-tetraazacyclododecane-1, 4, 7, 10-tetraacetic acid (DOTA)-Thr3-octreotate (68Ga-DOTATATE) positron emission tomography/computed tomography (PET/CT) in patients with well-differentiated neuroendocrine tumor (WD-NET).
Methods
Ninety-two patients (44 men and 48 women, mean age of 59.5-year-old) with pathologically confirmed WD-NET (grades 1 or 2) were enrolled in a prospective expanded access protocol. Selected data was analyzed retrospectively for this project. Maximum standardized uptake value (SUVmax) in the lesion with the highest 68Ga-DOTATATE uptake was measured and recorded for each patient. In addition, two volumetric parameters, namely, somatostatin receptor expressing tumor volume (SRETV) and total lesion somatostatin receptor expression (TLSRE), were calculated in each 68Ga-DOTATATE-avid lesion. SRETV was defined as tumor volume with higher 68Ga-DOTATATE uptake than the 50% of SUVmax within the volume of interest (VOI) for each lesion. TLSRE was calculated by multiplying SRETV and mean SUV within the same VOI. Thereafter, the sum of SRETV (ΣSRETV) and TLSRE (ΣTLSRE) for all detected lesions per patient were calculated. Progression-free survival (PFS) was set as primary endpoint. Kaplan-Meier survival analysis, log-rank test, and Cox’s proportional hazard model were used for statistical analysis.
Results
Univariate analyses revealed significant difference of PFS for WHO tumor grade and ΣSRETV (P < 0.05), while there were no significant differences in age, sex, SUVmax, and ΣTLSRE (P > 0.05). Multivariate analysis identified WHO tumor grade and ΣSRETV as independent predictors of PFS.
Conclusion
ΣSRETV calculated from 68Ga-DOTATATE PET/CT may have prognostic value of PFS in WD-NET patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine tumors (NETs) are rare neoplasms originating from neuroendocrine cells in various organs. NETs have an incidence of approximately 7.0 in 100,000 in the USA [1]. According to 2010 WHO classification, gastroenteropancreatic NETs were classified to three groups based on pathological findings (mitotic count and Ki-67 labelling index): well-differentiated NET (WD-NET) grades 1 and 2, and poorly differentiated neuroendocrine carcinoma grade 3 (NEC) [2]. In addition, the most recent WHO classification in 2017 includes a category of WD-NET grade 3 for pancreatic NET [2].
Most WD-NET cells express somatostatin receptors (SSTRs), particularly type 2, which can be targets of imaging and therapy [3]. Positron emission tomography/computed tomography (PET/CT) using 68Ga-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-conjugated peptides enables specific evaluation of NETs by targeting SSTRs on the cell surface. A previous meta-analysis revealed high pooled sensitivity (93%) and specificity (96%) of diagnosing NET by PET/CT using 68Ga-DOTA-conjugated peptides [4]. Recent guidelines from both the USA [5] and Europe [6] recommend the use of 68Ga-DOTA-conjugated peptides PET/CT for diagnosis, staging, restaging, and determination of SSTR status of patients with NETs.
Generally, uptake of 68Ga-DOTA-conjugated peptides in NETs decreases as tumor grade becomes higher. High-grade neuroendocrine neoplasms (NENs) often have high uptake of 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) rather than 68Ga-DOTA-conjugated peptides [7,8,9,10,11]. Although some previous studies have shown a relationship between patients’ prognosis and 18F-FDG PET/CT [12,13,14,15,16,17], most of them included some patients with high-grade NEN. WD-NETs does not usually show high 18F-FDG uptake; therefore, PET/CT using 68Ga-DOTA-conjugated peptides might be more suitable for predicting prognosis of patients with WD-NET.
Previous studies evaluated the relationship between 68Ga-DOTA-conjugated peptide uptake and patients’ prognosis [18,19,20,21]. However, 68Ga-DOTA-conjugated peptides uptake was analyzed based on maximum standardized uptake value (SUVmax) measurements in the lesion with the highest uptake. SUVmax reflects SSTR expression in only the pixel with the highest uptake in a lesion. To take account of the entire tumor, volumetric parameters, similar to metabolic tumor volume (MTV) and total lesion glycolysis (TLG) used in 18F-FDG PET/CT, should be evaluated.
Recently, Abdulrezzak et al. introduced volumetric parameters that can be applied in 68Ga-DOTA-Thr3-octreotate (68Ga-DOTATATE) PET/CT to reflect both SSTR expression and tumor volume in patients with NETs [22]. To our knowledge, there are few studies that assessed the prognostic value of 68Ga-DOTATATE PET/CT using volumetric parameters [23]. Here we evaluated the prognostic value of volumetric parameters calculated from 68Ga-DOTATATE PET/CT in patients with WD-NET.
Materials and methods
Patients
The local institutional review board (Stanford University Research Compliance Office) approved this prospective expanded access protocol. Informed consent was obtained from all individual participants included in the study. Selected data was analyzed retrospectively for this project.
Two-hundred thirty patients had undergone 68Ga-DOTATATE PET/CT in our hospital between January 2014 and December 2016. One patient, whose clinical and radiological data were not accessible, was excluded from our study. We excluded 137 patients according to the following criteria: (1) unknown or insufficient pathological information, (2) diagnosed as NEC or grade 3 WD-NET, (3) NET related to multiple endocrine neoplasia (MEN), (4) lung carcinoid tumor, and (5) insufficient follow-up period after 68Ga-DOTATATE PET/CT (< 6 months) except patients with early tumor progression. In addition, we excluded one patient with coexistence of WD-NET and peritoneal mesothelioma because differentiation between the two types of neoplasm in each lesion was impossible. Finally, 92 patients (44 men and 48 women; mean age ± SD 59.5 ± 12.7 years old; range 25–84 years old) were enrolled in this retrospective study (Fig. 1). Of these 92 patients, 55 and 37 patients were pathologically diagnosed as WD-NET grades 1 and 2, respectively. Characteristics of the enrolled patients are shown in Table 1. The mean follow-up period ranged 59–1250 days (mean ± SD 551.4 ± 296.1 days).
PET/CT protocol
Patients were not required to follow specific preparation prior to the scanning. The scans were performed using Discovery 600 or Discovery 690 PET/CT scanners (GE Healthcare, Waukesha, WI, USA). The details about the preparation of 68Ga-DOTATATE had been presented in the previous report [24]. Approximately 45–60 min after intravenous injection of 68Ga-DOTATATE (range 129.5–262.7 MBq, mean ± SD 224.2 ± 28.1 MBq), CT was acquired for attenuation correction and anatomical localization using the following parameters: 120 kV, 10 mAs, matrix size of 512 × 512, field of view of 867 mm, in 22.5 s. PET data acquisition followed from vertex to mid-thighs, with an acquisition time of 3 min per bed position. The PET datasets were reconstructed with an ordered subsets expectation maximization (OSEM) method with 2 iterations and 32 subsets for the Discovery 600 scanner and 2 iterations and 24 subsets for the Discovery 690 scanner. A previous study has shown that these two scanners can be used without clinical compromise to quantitative measurements [25].
Image analysis
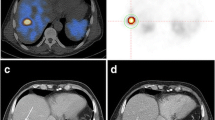
68Ga-DOTATATE PET/CT images were evaluated using a commercially available workstation (the MIM Vista workstation, version 6.7.11, MIM Software Inc.). Two nuclear medicine physicians evaluated all PET/CT images by consensus. 68Ga-DOTATATE uptakes above the background which did not correspond to physiological uptakes were considered to be significant. All 68Ga-DOTATATE-avid lesions were identified in each patient. In addition to SUVmax measurements, two volumetric parameters, namely, somatostatin receptor expressing tumor volume (SRETV) and total lesion somatostatin receptor expression (TLSRE), were calculated for each 68Ga-DOTATATE-avid lesion. These volumetric parameters were defined as in the previous study by Abdulrezzak et al. [22]: SRETV is the tumor volume with higher 68Ga-DOTATATE uptake than the 50% of SUVmax within the volume of interest (VOI) for each lesion, while TLSRE is calculated by multiplying SRETV and mean SUV within the same VOI. VOI for each lesion was manually placed so as to exclude both surrounding physiological uptake and adjacent lesions’ uptake (Fig. 2). To minimize overestimation of volumetric parameters, overlap between adjacent VOIs was strictly avoided.
Illustration of the placement of volume of interest (VOI) on 68Ga-DOTATATE-avid lesion. Maximum intensity projection image (a), fused axial (b, e), coronal (c, f), and sagittal (d, g) PET/CT images are shown. A manual VOI on the liver metastasis was placed so as to exclude surrounding physiological uptake as far as possible (b–d). Thereafter, irregular VOI inside containing voxels with higher SUV than 50% of SUVmax was automatically extracted to calculate volumetric parameters (e–g)
SUVmax in the lesion with the highest 68Ga-DOTATATE uptake in each patient was used in the statistical analysis. The sum of SRETV (ΣSRETV) and TLSRE (ΣTLSRE) for all detected lesions per patient was calculated and used in the analysis.
Statistical analysis
We used EZR software for the statistical analyses [26]. Progression-free survival (PFS), defined as days from 68Ga-DOTATATE PET/CT to tumor progression or patient’s death, was set as a primary endpoint in this study. Tumor progression was defined as significant increase of tumor size or appearance of new metastatic lesions based on RECIST 1.1 criteria [27]. Patients’ cohort was divided in separate groups based on the following parameters: age, sex, tumor grade according to 2010 WHO classification, SUVmax, ΣSRETV, and ΣTLSRE. Cutoff values of age and PET/CT parameters were set on median values. PFS was estimated by Kaplan-Meier survival analysis. As a univariate analysis, the log-rank test was used to compare PFS between two subgroups. Cox’s proportional hazard model was used for multivariate analysis to find independent predictor of PFS. A P < 0.05 was considered to be statistically significant.
Results
Seventy-six of the enrolled 92 patients had one or more 68Ga-DOTATATE-avid lesions, while the other 16 patients had no 68Ga-DOTATATE-avid lesions. All were included in the PFS analyses. Mean SUVmax, ΣSRETV, and ΣTLSRE calculated from PET/CT data of 76 patients with 68Ga-DOTATATE-avid lesions were 37.2 ± 25.8 (range 3.0–120.6), 48.5 ± 87.9 ml (range 0.6–620.0 ml), and 949.4 ± 1687.7 (range 4.0–10778.7), respectively.
Tumor progression based on RECIST 1.1 criteria was confirmed in 36 patients (39.1%) in the follow-up period. Their median PFS was 373.5 days (95% confidence interval 303–457 days). The results of univariate and multivariate analyses are shown in Tables 2 and 3. According to univariate analyses, patients with grade 2 WD-NET and higher ΣSRETV (≥ 11.29 ml) showed significantly shorter PFS compared to the others (P < 0.05). SUVmax and ΣTLSRE did not show significant difference of PFS between the two groups (P = 0.174 and 0.056, respectively). A multivariate analysis found WHO tumor grade and ΣSRETV to be independent predictors of PFS (P < 0.05). Kaplan-Meier curves drawn according to tumor grade and ΣSRETV are shown in Fig. 3.
Discussion
Our study revealed that tumor grade and ΣSRETV calculated from 68Ga-DOTATATE PET/CT have an important potential for prediction of PFS in patients with WD-NET. This may expand the value of these scans in the management of such patients.
A recent study by Tirosh et al. demonstrated similar results for the prognostic utility of 68Ga-DOTATATE-avid tumor volume in terms of PFS [23]. In our study, ΣSRETV showed a prognostic value as well in both univariate and multivariate analyses. However, ΣTLSRE did not show significant difference in PFS by a univariate analysis, and it was not determined to be an independent predictor of PFS by a multivariate analysis. TLSRE is a parameter reflecting SSTR expression in lesions, in addition to volume. SSTR affinity is essential information for patients with NET to select a treatment option, particularly peptide receptor radionuclide therapy (PRRT) [5, 6, 28, 29]. On the other hand, our study suggests that it might not be mandatory to consider the degree of SSTR expression in each lesion for prognostication.
Previous studies have shown significant correlation between lower SUVmax calculated from 68Ga-DOTA-conjugated peptides PET/CT and poorer prognosis in patients with WD-NET [18,19,20,21]. Here we did not find any significant differences in PFS based on SUVmax. The reason for this discrepancy is unclear, and patients’ selection criteria might have affected to some extent. Our results suggest that tumor volume may be better suited PET/CT-based parameter than SUVmax for prediction of PFS in patients with WD-NET. For patients with greater tumor volume, modifying treatment such as dose escalation or additional medication might be effective to prolong survival. Further studies are needed to assess an actual impact of PET/CT-based volumetric parameters on treatment strategy.
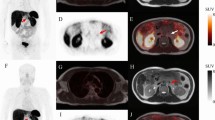
There are shortcomings to be considered when using 68Ga-DOTATATE PET/CT for patients’ prognostication. As previous studies had shown, NET lesions with different tumor grade can often coexist within a single patient [30,31,32]. It is known that some patients with grade 2 WD-NET do not show elevated 68Ga-DOTA-conjugated peptides uptake compared to 18F-FDG [8, 11]. In such patients, high-grade components of NET, which have higher affinity for 18F-FDG rather than 68Ga-DOTA-conjugated peptides, can result in underestimation of actual tumor volumes by 68Ga-DOTATATE PET/CT (Fig. 4). These characteristics of NETs can lead to pitfalls when considering only the SUVmax in a single lesion as it was performed in some studies [18,19,20,21]. A combination of 68Ga-DOTATATE PET/CT and 18F-FDG PET/CT may address this issue in such cases [7]. In one of our participants without DOTATATE-avid lesions, small liver metastases (< 10 mm) were detected by MRI performed 4 months after DOTATATE PET/CT. Such small lesions may not have conspicuous radiotracer uptake due to the partial volume effect and respiratory motion artefacts, leading to underestimation of tumor volumes.
A 50-year-old man with biopsy-proven liver metastasis of well-differentiated neuroendocrine tumor (WHO grade 2). 68Ga-DOTATATE PET/CT was performed 3 months after liver biopsy. A maximum intensity projection image suggested multiple lymph node metastases with elevated 68Ga-DOTATATE uptake in bilateral hilum and mediastinum (a, black circle). Although multiple liver metastases with partial calcification were detected in non-contrast CT images (b, white arrows), these lesions did not show significant 68Ga-DOTATATE uptake (c, fused PET/CT image). Even though pathological assessments had not been performed for the thoracic lymph nodes, size increase both in hepatic and nodal lesions was confirmed on the follow-up CT study (39.8% increase in the sum of diameters based on RECIST 1.1 criteria). Thus, in this case, tumor progression was confirmed 136 days later. Volumetric parameters calculated from 68Ga-DOTATATE PET/CT can be underestimated in such a case
We did not evaluate the change in the 68Ga-DOTATATE PET/CT findings before and after treatment in this study. Haug et al. reported that percentage change in tumor-to-spleen SUV ratio after the first cycle of PRRT predicted time to progression and clinical improvement [33]. However, their analyses had been performed based on SUVmax measured in up to 3 tumors. ΣSRETV and ΣTLSRE can reflect not only SSTR expression but also tumor volumes in DOTATATE-avid lesions. Their change after PRRT therapy might be more useful parameters for assessment of response to PRRT and predicting outcome in patients with WD-NET.
Our study has some limitations. First, we did not control the treatment options used in these patients given the retrospective analysis of data. Although therapeutic strategy prior to and following the 68Ga-DOTATATE PET/CT should have some impacts on the patients’ prognosis, we could not include treatment options in statistical analyses due to quite heterogeneous strategies between enrolled patients. We believe that results of a previous study [23] and ours could indicate prognostic value of volumetric parameters calculated from 68Ga-DOTATATE PET/CT. However, further studies which focus on more specific situations (e.g., patients who will undergo PRRT for multiple metastatic WD-NETs) may be needed before applying prognostication by 68Ga-DOTATATE PET/CT in clinical settings. Second, we could not obtain tumor markers such as chromogranin-A (Cg-A) in about half the patients in this study cohort. A good correlation between volumetric parameters calculated from 68Ga-DOTATATE PET/CT and Cg-A level has been shown in previous studies [19, 34]. Whether calculating ΣSRETV is superior to Cg-A remains unclear.
In conclusion, ΣSRETV calculated from 68Ga-DOTATATE PET/CT may have prognostic value for PFS in WD-NET patients, as larger tumor volumes showed a correlation with shorter PFS.
References
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42.
Chai SM, Brown IS, Kumarasinghe MP. Gastroenteropancreatic neuroendocrine neoplasms: selected pathology review and molecular updates. Histopathology. 2018;72:153–67.
Binderup T, Knigge U, Mellon Mogensen A, Palnaes Hansen C, Kjaer A. Quantitative gene expression of somatostatin receptors and noradrenaline transporter underlying scintigraphic results in patients with neuroendocrine tumors. Neuroendocrinology. 2008;87:223–32.
Geijer H, Breimer LH. Somatostatin receptor PET/CT in neuroendocrine tumours: update on systemic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2013;40:1770–80.
Hope TA, Bergsland EK, Bozkurt MF, Graham M, Heaney AP, Herrmann K, et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J Nucl Med. 2018;59:66–74.
Bozkurt MF, Virgolini I, Balogova S, Beheshti M, Rubello D, Decristoforo C, et al. Guideline for PET/CT imaging of neuoroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur J Nucl Med Mol Imaging. 2017;44:1588–601.
Chan DL, Pavlakis N, Schembri GP, Bernard EJ, Hsiao E, Hayes A, et al. Dual somatostatin receptor/FDG PET/CT imaging in metastatic neuroendocrine tumours: proposal for a novel grading scheme with prognostic significance. Theranostics. 2017;7:1149–58.
Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (dota-DPhe1,Tyr3-octreotate) and 18FDG. Cancer. 2008;112:2447–55.
Lococo F, Perotti G, Cardillo G, De Waure C, Filice A, Graziano P, et al. Multicenter comparison of 18F-FDG and 68Ga-DOTA-peptide PET/CT for pulmonary carcinoid. Clin Nucl Med. 2015;40:e183–9.
Panagiotidis E, Alshammari A, Michopoulou S, Skoura E, Naik K, Maragkoudakis E, et al. Comparison of the impact of 68Ga-DOTATATE and 18F-FDG PET/CT on clinical management in patients with neuroendocrine tumors. J Nucl Med. 2017;58:91–6.
Sampathirao N, Basu S. MIB-1 index-stratified assessment of dual-tracer PET/CT with 68Ga-DOTATATE and 18F-FDG and multimodality anatomic imaging in metastatic neuroendocrine tumors of unknown primary in a PRRT workup setting. J Nucl Med Technol. 2017;45:34–41.
Bahri H, Laurence L, Edeline J, Leghzali H, Devillers A, Raoul JL, et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long-term evaluation. J Nucl Med. 2014;55:1786–90.
Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978–85.
Ezziddin S, Adler L, Sabet A, Poppel TD, Grabellus F, Yuce A, et al. Prognostic stratification of metastatic gastroenteropancreatic neuroendocrine neoplasms by 18F-FDG PET: feasibility of a metabolic grading system. J Nucl Med. 2014;55:1260–6.
Garin E, Le Jeune F, Devillers A, Cuggia M, de Lajarte-Thirouard AS, Bouriel C, et al. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med. 2009;50:858–64.
Johnbeck CB, Knigge U, Langer SW, Loft A, Berthelsen AK, Federspiel B, et al. Prognostic value of 18F-FLT PET in patients with neuroendocrine neoplasms: a prospective head-to-head comparison with 18F-FDG PET and Ki-67 in 100 patients. J Nucl Med. 2016;57:1851–7.
Kim HS, Choi JY, Choi DW, Lim HY, Lee JH, Hong SP, et al. Prognostic value of volume-based metabolic parameters measured by 18F-FDG PET/CT of pancreatic neuroendocrine tumors. Nucl Med Mol Imaging. 2014;48:180–6.
Koch W, Auernhammer CJ, Geisler J, Spitzweg C, Cyran CC, Ilhan H, et al. Treatment with octreotide in patients with well-differentiated neuroendocrine tumors of the ileum: prognostic stratification with Ga-68-DOTA-TATE positron emission tomography. Mol Imaging. 2014;13:1–10.
Campana D, Ambrosini V, Pezzilli R, Fanti S, Labate AM, Santini D, et al. Standardized uptake values of 68Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med. 2010;51:353–9.
Sharma P, Naswa N, Kc SS, Alvarado LA, Dwivedi AK, Yadav Y, et al. Comparison of the prognostic values of 68Ga-DOTANOC PET/CT and 18F-FDG PET/CT in patients with well-differentiated neuroendocrine tumor. Eur J Nucl Med Mol Imaging. 2014;41:2194–202.
Ambrosini V, Campana D, Polverari G, Peterle C, Diodato S, Ricci C, et al. Prognostic value of 68Ga-DOTANOC PET/CT SUVmax in patients with neuroendocrine tumors of the pancreas. J Nucl Med. 2015;56:1843–8.
Abdulrezzak U, Kurt YK, Kula M, Tutus A. Combined imaging with 68Ga-DOTA-TATE and 18F-FDG PET/CT on the basis of volumetric parameters in neuroendocrine tumors. Nucl Med Commun. 2016;37:874–81.
Tirosh A, Papadakis GZ, Millo C, Hammoud D, Sadowski SM, Herscovitch P, et al. Prognostic utility of total 68Ga-DOTATATE-avid tumor volume in patients with neuroendocrine tumors. Gastroenterology. 2018;154:998–1008.
Moradi F, Jamali M, Barkhodari A, Schneider B, Chin F, Quon A, et al. Spectrum of 68Ga-DOTA TATE uptake in patients with neuroendocrine tumors. Clin Nucl Med. 2016;41:e281–7.
Thompson HM, Minamimoto R, Jamali M, Barkhodari A, von Eyben R, Iagaru A. A prospective, matched comparison study of SUV measurements from time-of-flight versus non-time-of-flight PET/CT scanners. Clin Nucl Med. 2016;41:e323–6.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Kratochwil C, Stefanova M, Mavriopoulou E, Holland-Letz T, Dimitrakopoulou-Strauss A, Afshar-Oromieh A, et al. SUV of [68Ga]DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol Imaging Biol. 2014;17:313–8.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35.
Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol. 2011;35:853–60.
Adesoye T, Daleo MA, Loeffler AG, Winslow ER, Weber SM, Cho CS. Discordance of histologic grade between primary and metastatic neuroendocrine carcinomas. Ann Sur Oncol. 2015;22(suppl 3):S817–21.
Richards-Taylor S, Tilley C, Jaynes E, Hu H, Armstrong T, Pearce NW, et al. Clinically significant differences in Ki-67 proliferation index between primary and metastases in resected pancreatic neuroendocrine tumors. Pancreas. 2017;46:1354–8.
Haug AR, Auernhammer CJ, Wangler B, Schmidt GP, Uebleis C, Goke B, et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med. 2010;51:1349–56.
Tirosh A, Papadakis GZ, Millo C, Sadowski SM, Herscovitch P, Pacak K, et al. Association between neuroendocrine tumors biomarkers and primary tumor site and disease type based on total 68Ga-DOTATATE-avid tumor volume measurements. Eur J Endocrinol. 2017;176:575–82.
Acknowledgments
We thank all the patients who participated and their families. We also thank our research coordinators, radiochemists, and technologists.
Funding
The expanded access protocol was partially supported by an anonymous donation provided though Carcinoid Cancer Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Andrei Iagaru receives institutional research support from GE Healthcare and Advanced Accelerator Applications, unrelated to this work. The other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – General
Rights and permissions
About this article
Cite this article
Toriihara, A., Baratto, L., Nobashi, T. et al. Prognostic value of somatostatin receptor expressing tumor volume calculated from 68Ga-DOTATATE PET/CT in patients with well-differentiated neuroendocrine tumors. Eur J Nucl Med Mol Imaging 46, 2244–2251 (2019). https://doi.org/10.1007/s00259-019-04455-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04455-9