Abstract
Introduction
Bacteremia is associated with high mortality, especially when the site of infection is unknown. While conventional imaging usually focus on specific body parts, FDG-PET/CT visualizes hypermetabolic foci throughout the body.
Purpose
To investigate the ability of FDG/PET-CT to detect the site of infection and its clinical impact in bacteremia of unknown origin with catalase-negative Gram-positive cocci (excluding pneumococci and enterococci) or Staphylococcus aureus (BUOCSA).
Methods
We retrospectively identified 157 patients with 165 episodes of BUOCSA, who subsequently underwent FDG-PET/CT. Data were collected from medical records. Decision regarding important sites of infection in patients with bacteremia was based on the entire patient course and served as reference diagnosis for comparison with FDG-PET/CT findings. FDG-PET/CT was considered to have high clinical impact if it correctly revealed site(s) of infection in areas not assessed by other imaging modalities or if other imaging modalities were negative/equivocal in these areas, or if it established a new clinically relevant diagnosis, and/or led to change in antimicrobial treatment.
Results
FDG-PET/CT detected sites of infection in 56.4% of cases and had high clinical impact in 47.3%. It was the first imaging modality to identify sites of infection in 41.1% bacteremia cases, led to change of antimicrobial therapy in 14.7%, and established a new diagnosis unrelated to bacteremia in 9.8%. Detection rate and clinical impact were not significantly influenced by duration of antimicrobial treatment preceding FDG-PET/CT, days from suspicion of bacteremia to FDG-PET/CT-scan, type of bacteremia, or cancer.
Conclusion
FDG-PET/CT appears clinically useful in BUOCSA. Prospective studies are warranted for confirmation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacteremia is associated with high mortality and morbidity and is one of the primary causes of death in the western world [1, 2]. In a Danish population-based cohort study, 30-day mortality was 20.6%, and the reported mortality for nosocomial bacteremia was even higher [1, 3, 4]. Furthermore, bacteremia is associated with increased long-term mortality [5], exceeding that of common conditions such as breast cancer, colorectal cancer, diabetes mellitus, and heart failure [2].
Bacteremia can be divided into Gram-positive and Gram-negative bacteremia. Gram-negative bacteremia is often caused by Escherichia coli and other enterobacteriaceae [6] and is usually associated with gastrointestinal or urinary tract infections [3]. In the Gram-positive group, there are some characteristic associations between bacterial species and site of infection, i.e. pneumococci (pneumonia), enterococci (endocarditis, gastrointestinal and urinary tract infections), and coagulase-negative staphylococci (foreign body infections). On the other hand, it is often more difficult to detect sites of infection in bacteremia with other streptococci/streptococcus-like bacteria (here defined as catalase-negative Gram-positive cocci) and Staphylococcus aureus [7]. For this reason, we chose to focus on these species.
Only few studies have investigated bacteremia of unknown origin (BUO) [8] despite its frequent occurrence at an estimated 8–22% of bacteremia cases [9,10,11]. Studies have pointed towards increased mortality when the origin of bacteremia is unknown/undetermined [12,13,14], underlining the importance of locating the primary site of infection in BUO.
While conventional imaging techniques usually focus on a specific part of the body, fluorine-18-labeled fluordeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) visualizes hypermetabolic foci (including infection) in the entire body, in one single procedure [15]. Despite increased application in infectious/inflammatory diseases and an obvious potential in BUO [15], no larger studies have investigated the use of FDG-PET/CT in BUO [8].
The aim of this retrospective study was to investigate the detection rate and the clinical impact of FDG-PET/CT in patients with BUO with catalase-negative Gram-positive cocci (except pneumococci and enterococci) or Staphylococcus aureus (BUOCSA). Secondary objectives were to correlate these outcomes with duration of antimicrobial treatment prior to FDG-PET/CT scan, time span from suspicion of bacteremia to FDG-PET/CT scan, the type of bacteremia (hospital/healthcare-associated or community-acquired), and known malignancy.
Materials and methods
The study was performed at Odense University Hospital, a 990-bed regional hospital in Denmark. Patients were eligible for inclusion, if they were diagnosed with BUOCSA from 1st January 2009 through 31st December 2013, and had a subsequent FDG-PET/CT scan performed during the same admission. Patients could be included more than once if inclusion criteria were fulfilled again during a later admission. Data regarding positive blood cultures were retrieved from the local laboratory information system (MADS, Aarhus University Hospital, Aarhus, Denmark), and data on FDG-PET/CT scans were obtained from a local data base containing information on all patients examined with FDG-PET/CT.
Patients were identified using two separate lists. One list included all patients with blood cultures positive for catalase-negative Gram-positive cocci (except pneumococci and enterococci) or Staphylococcus aureus in the study period. The second list included all patients undergoing FDG-PET/CT during the same period. Patients appearing on both lists were eligible for inclusion.
Blood cultures were obtained as part of routine diagnostic work-up of suspected infection or sepsis. FDG-PET/CT was performed at the discretion of the treating physician.
We collected the following information from the medical records: patient age, sex, duration of antimicrobial treatment preceding FDG-PET/CT, changes in antimicrobial treatment, clinical findings, discharge summaries, and number and results of diagnostic procedures related to bacteremia before and after FDG-PET/CT (i.e. conventional x-ray, computed tomography, magnetic resonance imaging, ultrasound, echocardiography, bone scintigraphy, odontological examination, repeated FDG-PET/CT, and results of laboratory tests). The date of suspected bacteremia (i.e. the date the blood culture was obtained) and the date of FDG-PET/CT were obtained from the local database.
Patients were excluded if they had bacteremia of known origin and/or active cancer [except non-melanoma skin cancer (NMSC)] without a baseline FDG-PET/CT. The latter was done to prevent false-positive lesions, in regard to infection, in patients with known cancer. However, since NMSC rarely metastasize and FDG-PET has low usefulness in these cases [16], it seemed justified to exempt these.
FDG-PET/CT
FDG-PET/CT was performed with one of four available PET/CT scanners (General Electrics, Milwaukee, WI, USA). Prior to FDG injection, patients fasted for at least 6 h without glucose-containing infusions. Patients with diabetes were managed according to EANM guidelines [17]. A weight-adjusted dose of 4 MBq/kg FDG (maximum 400 MBq) was administered and after 1 h, a low-dose CT without contrast enhancement was obtained from the base of the skull to the proximal femora for anatomic correlation and attenuation correction of PET images. Subsequently, a PET scan of the same area was acquired with 2.5 min per bed position. The PET and CT images were expanded to include the entire skull or part or all of the lower extremities, if the patient history indicated a need for this. Images were reconstructed using iterative reconstruction and displayed in coronal, axial, and sagittal planes. FDG-PET/CT images were evaluated by a nuclear medicine physician as part of the daily routine, i.e. with full access to medical records. Results were reported to the treating physician.
Reference diagnosis and interpretation of scan results
The reference diagnosis was defined as the conclusion on the relevant site(s) of infection in patients with bacteremia and was based on the authors’ thorough evaluation of the patient’s medical records, including clinical findings, image diagnostics (excluding FDG-PET/CT unless this was repeated later in the course of the disease), laboratory results (biochemical, microbiological, and histopathology), and discharge summary. The reference diagnosis could deviate from the discharge summary if the evidence present led to a different conclusion. In cases without an obvious diagnosis, emphasis was placed on the conclusion made in the discharge summery. The gold standard was thus a composite reference diagnosis on a per patient basis.
For each episode of BUOCSA, the reference diagnosis was compared to the result of FDG-PET/CT scan. The detection rate was defined as the proportion of cases where FDG-PET/CT was able to detect the site(s) of infection. If the focus/foci of infection, defined by the reference diagnosis, was mentioned in the interpretation of the scan, FDG-PET/CT was regarded able to detect the focus/foci of bacteremia. Where no conclusion about the origin of bacteremia, and therefore no reference diagnosis, could be made, FDG-PET/CT was regarded as unable to detect the relevant sites of infection, even though there could have been a possible true focus in the scan result. In cases with endocarditis combined with one or more extra-cardiac focus/foci, FDG-PET/CT was considered able to detect the sites of infection if it localized all additional foci although failing to detect endocarditis. In cases where endocarditis without any extra-cardia focus/foci was considered the infection site and FDG-PET/CT failed to detect the endocarditis, FDG-PET/CT was considered unable to identify the site of infection. We found this approach justified in view of the limited sensitivity of FDG-PET/CT to detect endocarditis, when no specific preparation is used, to reduce the physiological myocardial uptake.
Finally, FDG-PET/CT was regarded as true negative if the reference diagnosis indicated a focus outside the scanned area or if the focus of infection was concluded as being fully eradicated (stated in the medical record at the end of antibiotic treatment) by the time of the scan. True negative results were not included in the calculation of detection rate or clinical impact.
FDG-PET/CT was considered of high clinical impact if it a) correctly revealed the sites of infection in bacteremia (in agreement with the reference diagnosis) in areas not assessed by other imaging modalities or if other imaging modalities were negative or equivocal in these areas, or b) if it established a new clinically relevant diagnosis, and/or changed the antimicrobial treatment (extended the duration, changed the administration route, or altered the type of antibiotic). All other cases were considered of low clinical impact.
Definitions regarding bacteremia
BUO was defined as the presence of viable bacteria in blood cultures [1] and uncertainty regarding site(s) of infection after the initial work-up (i.e. physical examination, basic blood tests, and chest x-ray) initiated shortly after suspicion of bacteremia [18]. Positive blood cultures interpreted as contamination by a clinical microbiologist were not included. A complete list of all bacteria species considered for inclusion is shown in Supplemental Table 1. Polymicrobial episodes of bacteremia (≥2 species in the blood culture [12]) were included if at least one of the species was catalase-negative Gram-positive cocci (except pneumococci and enterococci) or Staphylococcus aureus. Bacteremia was considered ‘hospital-acquired’ if the first positive blood culture was obtained >48 h after hospital admission and, ‘healthcare-associated’ if the patient had been admitted to or attended an outpatient clinic in hematology, oncology, or nephrology within 30 days prior to the hospital admission [19]. All other cases were considered ‘community-acquired’.
Statistical analysis
SPSS (version 23.0; SPSS, Inc.) was used for analysis. Confidence intervals (CIs) for proportions were calculated using the Wilson score method. Correlation between detection rate/clinical impact and type of bacteremia, and correlation between detection rate/clinical impact, non-cancer patients, and cancer patients were explored with the Χ2 test. The Χ2 test was also used to test for differences between proportions. The Wilcoxon rank sum test was used to test correlation between detection rate/clinical impact and number of days with antibiotic treatment prior to FDG-PET/CT and the number of days from suspected BUOCSA to FDG-PET/CT. A p value ˂ 0.05 was considered statistically significant, and CIs were reported at the 95% level.
Results
From the local databases, we identified 744 Gram-positive catalase-negative bacteremias (excluding pneumococci and enterococci), 847 bacteremias with Staphylococcus aureus (S. aureus), and 2173 FDG-PET/CT scans. From these, we found 260 patients with a total of 270 episodes of bacteremia and a matching FDG-PET/CT scan. After excluding 105 episodes in 103 patients (Fig. 1), we had 165 episodes in 157 patients for inclusion (8 of the included patients had more than one episode of BUOCSA). Baseline characteristics for these 157 patients are displayed in Table 1. S. aureus was the most common cause of bacteremia (64.2%), and most episodes were community-acquired (62.4%). The most common final diagnosis was endocarditis (38.2%). An overview of reference diagnoses is displayed in Table 2. A median of 4.0 (range 0–12) diagnostic tests/imaging procedures were performed prior to FDG-PET/CT, and a median of 1.0 (range 0–9) diagnostic tests/imaging procedures were performed afterwards. FDG-PET/CT was performed within a median of 7.0 days (range 0–29) after the first positive blood culture.
FDG-PET/CT had an overall detection rate of 56.4% (CI: 48.8–63.8). Table 3 shows the detection rate in different subgroups. There was no significant difference in the ability of FDG-PET/CT to detect sites of infection in patients with bacteremia, in relation to duration of prior antimicrobial treatment, number of days from first positive culture to FDG-PET/CT (p = 0.89), or type of bacteremia (p = 0.19). Compared to endocarditis, FDG-PET/CT detected sites of infection in a significantly higher proportion of patients with spondylodiscitis/spondylitis (p < 0.001), pneumonia (p = 0.002), and arthritis (p = 0.01).
FDG-PET/CT had a high clinical impact in 47.3% (CI: 39.8–54.9) of all episodes of BUOCSA (Table 4). FDG-PET/CT was the first imaging modality to identify the sites of infection in bacteremia in 41.1% of episodes, led to changes in antimicrobial treatment in 14.7%, and resulted in a new diagnosis unrelated to bacteremia in 9.8% of episodes (Fig. 2). The clinical impact was not significantly related to the duration of preceding antimicrobial treatment (p = 0.77), days from diagnosis of bacteremia to FDG-PET/CT (p = 0.42), or the type of bacteremia (p = 0.21).
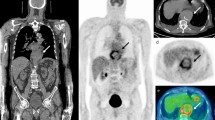
Examples of true positive scans in patients with bacteremia. a Eighty-year-old woman with thoracic spondylodiscitis (blue arrow). An initial (pre-PET/CT) magnetic resonance imaging scan was negative, but subsequent scan confirmed spondylodiscitis. b Eighty-nine-year-old male with an infected biologic aortic valve (blue arrow). c Sixty-three-year-old male with infection related to a central venous catheter (blue arrow)
A total of 7.9% of the patients had current cancer. The FDG-PET/CT detection rate was similar in cancer and non-cancer patients, i.e. 61.5% (CI: 35.5–82.3) versus 55.9% (CI: 48.0–63.6); p = 0.70. The clinical impact was similar in both the cancer group and the non-cancer group, i.e. 53.8% (CI: 29.1–76.8) and 46.7% (CI: 39.0–54.6); p = 0.62.
Discussion
This study is the first to examine the clinical value of FDG-PET/CT in BUO with catalase-negative Gram-positive cocci (excluding pneumococci and enterococci) or Staphylococcus aureus. FDG-PET/CT detected sites of infection in patients with bacteremia in 56.4% of the patients. In 41.1% of cases, FDG-PET/CT was the first imaging modality to identify these sites, even if it was used within a median of 7 days after the first positive blood culture and following a median of four other diagnostic tests or imaging procedures. FDG-PET/CT had a high clinical impact in almost half the cases (47.3%), and this was not related to type of bacteremia, number of days on antibiotic medication, or time elapsed until FDG-PET/CT was performed. Even if FDG is a non-specific tracer of both inflammation/infection and cancer, we found no significant difference in detection rate or clinical impact in patients with and without cancer.
The retrospective nature of the study made it a challenge to decide the exact focus of the bacteremia, especially in cases of endocarditis. We defined FDG-PET/CT as being able to detect relevant sites of infection in bacteremia in cases with endocarditis and additional foci, only if all the additional foci were identified by FDG-PET/CT. This may have introduced a bias, but in the view of the difficulties concerning the endocarditis diagnosis [20], we found this approach justified. The high number of patients and the few exclusion criteria add strength to our results. Also, no significant changes in routine work-up or treatment were instituted during the study period. There were no specific criteria for referring patients to FDG-PET/CT, and the study population probably represents a subset of patients with bacteremia—likely those with a focus/foci of bacteremia most difficult to detect. Transient bacteremia without a clinically significant site of infection may have occurred in some cases, underestimating the detection rate and the clinical impact.
In our study, FDG-PET/CT led to a new clinically relevant diagnosis in 9.8% of cases (Fig. 2). These findings included undiagnosed infections and malignant foci needing further management. While FDG-PET/CT is known to present false-positive lesions in bacteremia, we did not evaluate the occurrence of these and believe that prospective studies are needed to evaluate this properly. The cost of FDG-PET/CT compared to other imaging modalities may also be considered a limitation in a hospital setting. However, early use of FDG-PET/CT may spare patients futile tests and procedures and thereby save substantial expenses. Our study was not designed to draw firm conclusions about this particular matter, but we found that first-line FDG-PET/CT could potentially save a median of four (0–12) futile diagnostic procedures in 56.4% of patients, and a median of 1 (0–9) procedure after FDG-PET/CT, indicating that early and rational use of FDG-PET/CT might significantly reduce the number of diagnostic procedures applied. In a study on FDG-PET/CT and patients with Gram-positive bacteremia and risk of metastatic infectious foci, the cost-effectiveness was calculated, and FDG-PET/CT was found to be cost-effective [21]. It seems fair to assume that the use of FDG-PET/CT in patients with BUOCSA could also be cost-effective. The clinical impact of FDG-PET/CT in our study was not limited to the early stages of BUOCSA, suggesting that unresolved BUOCSA patients may benefit from FDG-PET/CT, even if quite some time has passed.
Concerning the most frequent final diagnoses, FDG-PET/CT detected sites of infection significantly more often in patients with spondylodiscitis/spondylitis, pneumonia, and arthritis than in patients with a final diagnosis of endocarditis. Several factors may explain the low yield of FDG-PET/CT in cases with endocarditis. The heart remains a challenge for the diagnostic sensitivity and specificity in FDG-PET/CT due to the high physiological uptake of FDG [22], although this may be alleviated to some degree by prolonged fasting or delayed imaging [23, 24]. Our results clearly show that FDG-PET/CT can not be used as a substitute for echocardiography in the diagnosis of endocarditis, but FDG-PET/CT may supplement echocardiography by detecting other (extracardiac) relevant infectious foci in patients with endocarditis [20].
Although no prior studies on BUOCSA and FDG-PET/CT exist, two previous studies have focused on FDG-PET/CT in patients with bacteremia and risk of metastatic infectious foci [25, 26]. In both studies, FDG-PET/CT contributed to the diagnosis of infectious lesions. The first study was a prospective study of 115 patients [25] that reported sensitivity of 100% and specificity of 87% where 30% of foci were first recognized with FDG-PET/CT. The second study retrospectively investigated 40 patients, and FDG-PET/CT found 31 true positives, 6 true negatives, 3 false positives, and no false negatives [26]. Although these results may seem more positive than ours, the included patients were more selected, as they all had risk factors for metastatic infectious foci and were thus more likely to present with FDG-avid lesions.
FDG-PET/CT is a well-established option for infection and inflammation in general [15], but less so for infectious diseases in cancer patients. A study from 2012 investigated incidental FDG-PET/CT findings suggestive of infections when staging malignancies [27]: 47 of 60 abnormal scans were highly suggestive of infection with a positive predictive value of 89%. Other studies have focused on cancer patients with febrile neutropenia, a common side-effect of chemotherapy [28]. FDG-PET/CT significantly improved the diagnostic yield and influenced patient management and treatment [29,30,31]. However, it should be noted that in our study, a lack of baseline FDG-PET/CT for reference was an exclusion criterion.
In retrospect, it would have been interesting to report separately on the methicillin-resistant cases among the Staphylococcus aureus-positive patients and on patients who were immunosuppressed (induced neutropenia, steroids, biotherapies, splenectomy, etc.) to elucidate whether PET performed equally well in methicillin-resistant vs. methicillin-sensitive infections and in immunosuppressed vs. non-immunosuppressed patients. Unfortunately, however, these data were not collected originally, and due to the present Danish legislation on patient data protection, we are not allowed to gather this information retrospectively.
Conclusion
FDG-PET/CT appears to be clinically useful in BUOCSA as it can identify clinically relevant sites of infection. FDG-PET/CT influenced clinical management in approximately half of the cases, and it can be used throughout the disease course, even in patients with underlying cancer. FDG-PET/CT provided the highest diagnostic yield in patients with spondylodiscitis/spondylitis, pneumonia, and arthritis, and the poorest in patients with endocarditis. Prospective studies are needed to more precisely define the clinical usefulness and cost-effectiveness of FDG-PET/CT in BUOCSA.
References
Nielsen SL. The incidence and prognosis of patients with bacteremia. Dan Med J. 2015;62:B5128.
Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19:501–9.
Sogaard M, Norgaard M, Dethlefsen C, Schonheyder HC. Temporal changes in the incidence and 30-day mortality associated with bacteremia in hospitalized patients from 1992 through 2006: a population-based cohort study. Clin Infect Dis. 2011;52:61–9.
Diekema DJ, Beekmann SE, Chapin KC, Morel KA, Munson E, Doern GV. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol. 2003;41:3655–60.
Nielsen SL, Lassen AT, Gradel KO, Jensen TG, Kolmos HJ, Hallas J, et al. Bacteremia is associated with excess long-term mortality: a 12-year population-based cohort study. J Inf Secur. 2015;70:111–26.
Skogberg K, Lyytikainen O, Ollgren J, Nuorti JP, Ruutu P. Population-based burden of bloodstream infections in Finland. Clin Microbiol Infect. 2012;18:E170–6.
Jensen AG. Importance of focus identification in the treatment of Staphylococcus aureus bacteraemia. J Hosp Infect. 2002;52:29–36.
Hess S, Jacobsen N, Brøndserud MB, Segtnan EA, Schifter S. FDG-PET/CT for systemic infections. Curr Mol Imaging. 2015;3:182–90.
Larsen IK, Pedersen G, Schonheyder HC. Bacteraemia with an unknown focus: is the focus de facto absent or merely unreported? A one-year hospital-based cohort study. APMIS. 2011;119:275–9.
Ruiz-Giardin JM, Jimenez BC, Martin RM, Ortiz J, Condori Arenas MH, Sanmartin JV, et al. Clinical diagnostic accuracy of suspected sources of bacteremia and its effect on mortality. Eur J Intern Med. 2013;24:541–5.
Hernandez C, Feher C, Soriano A, Marco F, Almela M, Cobos-Trigueros N, et al. Clinical characteristics and outcome of elderly patients with community-onset bacteremia. J Inf Secur. 2015;70:135–43.
Pedersen G, Schonheyder HC, Sorensen HT. Source of infection and other factors associated with case fatality in community-acquired bacteremia--a Danish population-based cohort study from 1992 to 1997. Clin Microbiol Infect. 2003;9:793–802.
Retamar P, Lopez-Prieto MD, Natera C, de Cueto M, Nuno E, Herrero M, et al. Reappraisal of the outcome of healthcare-associated and community-acquired bacteramia: a prospective cohort study. BMC Infect Dis. 2013;13:344.
Leibovici L, Konisberger H, Pitlik SD, Samra Z, Drucker M. Bacteremia and fungemia of unknown origin in adults. Clin Infect Dis. 1992;14:436–43.
Vaidyanathan S, Patel CN, Scarsbrook AF, Chowdhury FU. FDG PET/CT in infection and inflammation-current and emerging clinical applications. Clin Radiol. 2015;70:787–800.
Juan YH, Saboo SS, Tirumani SH, Khandelwal A, Shinagare AB, Ramaiya N, et al. Malignant skin and subcutaneous neoplasms in adults: multimodality imaging with CT, MRI, and 18F-FDG PET/CT. Am J Roentgenol. 2014;202:W422–38.
Buscombe J. Guidelines for the use of 18F-FDG in infection and inflammation: a new step in cooperation between the EANM and SNMMI. Eur J Nucl Med Mol Imaging. 2013;40:1120–1.
Saginur R, Suh KN. Staphylococcus aureus bacteraemia of unknown primary source: where do we stand? Int J Antimicrob Agents. 2008;32(Suppl 1):S21–5.
Gradel KO, Nielsen SL, Pedersen C, Knudsen JD, Ostergaard C, Arpi M, et al. No specific time window distinguishes between community-, healthcare-, and hospital-acquired bacteremia, but they are prognostically robust. Infect Control Hosp Epidemiol. 2014;35:1474–82.
Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8.
Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med. 2011;52:1673–8.
Hess S, Blomberg BA, Zhu HJ, Hoilund-Carlsen PF, Alavi A. The pivotal role of FDG-PET/CT in modern medicine. Acad Radiol. 2014;21:232–49.
Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol. 2009;16:801–10.
Leccisotti L, Perna F, Lago M, Leo M, Stefanelli A, Calcagni ML, et al. Cardiovascular implantable electronic device infection: delayed vs standard FDG PET-CT imaging. J Nucl Cardiol. 2014;21:622–32.
Vos FJ, Bleeker-Rovers CP, Sturm PD, Krabbe PF, van Dijk AP, Cuijpers ML, et al. 18F-FDG PET/CT for detection of metastatic infection in gram-positive bacteremia. J Nucl Med. 2010;51:1234–40.
Bleeker-Rovers CP, Vos FJ, Wanten GJA, Van Der Meer JWM, Corstens FHM, Kullberg BJ, et al. 18F-FDG PET in detecting metastatic infectious disease. J Nucl Med. 2005;46:2014–9.
Wong PS, Lau WF, Worth LJ, Thursky KA, Drummond E, Slavin MA, et al. Clinically important detection of infection as an ‘incidental’ finding during cancer staging using FDG-PET/CT. Intern Med J. 2012;42:176–83.
Raghavendra M, Hoeg RT, Bottner WA, Agger WA. Management of neutropenic fever during a transition from traditional hematology/oncology service to hospitalist care. WMJ. 2014;113:53–8.
Koh KC, Slavin MA, Thursky KA, Lau E, Hicks RJ, Drummond E, et al. Impact of fluorine-18 fluorodeoxyglucose positron emission tomography on diagnosis and antimicrobial utilization in patients with high-risk febrile neutropenia. Leuk Lymphoma. 2012;53:1889–95.
Guy SD, Tramontana AR, Worth LJ, Lau E, Hicks RJ, Seymour JF, et al. Use of FDG PET/CT for investigation of febrile neutropenia: evaluation in high-risk cancer patients. Eur J Nucl Med Mol Imaging. 2012;39:1348–55.
Gafter-Gvili A, Paul M, Bernstine H, Vidal L, Ram R, Raanani P, et al. The role of (1)(8)F-FDG PET/CT for the diagnosis of infections in patients with hematological malignancies and persistent febrile neutropenia. Leuk Res. 2013;37:1057–62. https://doi.org/10.1016/j.leukres.2013.06.025.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
The study was approved by the Danish Data Protection Agency (no. 14/28781, 2008-58-003) and The Danish Health Authority (no. 3-3013-675/1/). For this type of study, formal consent is not required.
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Table 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Brøndserud, M.B., Pedersen, C., Rosenvinge, F.S. et al. Clinical value of FDG-PET/CT in bacteremia of unknown origin with catalase-negative gram-positive cocci or Staphylococcus aureus. Eur J Nucl Med Mol Imaging 46, 1351–1358 (2019). https://doi.org/10.1007/s00259-019-04289-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04289-5