Abstract
Purpose
This study sought to provide preliminary results on the biodistribution and dosimetry following intra-arterial liver injection of 188Re-SSS Lipiodol on hepatocellular carcinoma patients included in the Phase I Lip-Re 1 study.
Methods
Results of the first six patients included are reported. Analysis of the 188Re-SSS Lipiodol biodistribution was based on planar scintigraphic and tomoscintigraphic (SPECT) studies performed at 1, 6, 24, 48, and 72 h post-administration. Quantification in blood, urine, and stool samples was performed. Determination of the tumour to non-tumour uptake ratio (T/NT) was calculated. Absorbed doses to target organs and tumours were evaluated using the MIRD formalism.
Results
The mean injected activity of 188Re-SSS Lipiodol was 1645 ± 361 MBq. Uptakes were seen in the liver (tumour and healthy liver) and the lungs only. All these uptakes were stable over time. A mean 1.4 ± 0.7% of 188Re-SSS Lipiodol administered was detected in serum samples at 6 h, declining rapidly thereafter. On average, 1.5 ± 1.6% of administered activity was eliminated in urine and feces over 72 h. Overall, 90.7 ± 1.6% of detected activity on SPECT studies was found in the liver (74.9 ± 1.8% in tumours and 19.1 ± 1.7% in the healthy liver) and 9.3 ± 1.6% in the lungs (5.7 ± 1.1% in right and 3.7 ± 0.5% in left lungs). Mean doses absorbed were 7.9 ± 3.7Gy to the whole liver, 42.7 ± 34.0Gy to the tumours, 10.2 ± 3.7Gy to the healthy liver, and 1.5 ± 1.2Gy to the lungs. Four patients had stable disease on CT scans at 2 months. The first patient with rapidly progressive disease died at 1 month, most probably of massive tumour progression. Due to this early death and using a conservative approach, the trial independent evaluation committee decided to consider this event as a treatment-related toxicity.
Conclusion
188Re-SSS Lipiodol has a favorable biodistribution profile concerning radioembolization, with the highest in-vivo stability among all radiolabeled Lipiodol compounds reported to date. These preliminary results must be further confirmed while completing this Phase I Lip Re1 study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide and the second most common cause of cancer-related death [1,2,3,4,5]. In spite of using sorafenib and 90Y-loaded microsphere radioembolization, the treatment of advanced Barcelona Clinic Liver Cancer (BCLC) classification B patients and those with portal vein thrombosis (PVT) is still highly challenging. In this setting, overall survival is only 10.7 months under sorafenib [2]. In addition, the two recently published randomized studies comparing 90Y-loaded resin microsphere therapy versus sorafenib did not demonstrate any increase in overall survival when using radioembolization [3,4,5].
131I-Lipiodol has, meanwhile, been applied for many years since a randomized study demonstrated a superior overall survival in HCC patients with PVT treated with this novel therapeutic modality in comparison with best supportive care [6]. However, 131I-Lipiodol marketing was discontinued in 2010 by the manufacturer for several reasons, including sorafenib approval, 90Y-loaded microsphere development, and radioprotection constraints, as well as lung toxicities in several cases. Furthermore, 131I has long half-life (8 days) and an abundant high-energy photon emission (364 keV, 81.7%) resulting in major radioprotection constraints. These observations highlight the necessity of developing new therapeutic agents for managing advanced HCC.
With this objective in mind, Lipiodol labeling with a new radiolabeled stable complex of rhenium 188 (188Re) has been developed over the last years, namely 188Re-SSS Lipiodol [7,8,9,10,11,12,13,14]. 188Re exhibits a short half-life (17 h), with only a small amount of lower-energy gamma radiation compared to 131I-Lipiodol (155 keV, abundance 14%), resulting in more favorable radioprotection constraints. Using 188Re-SSS Lipiodol in HCC patients is presently being evaluated in the Phase I Lip-Re 1 study that is still ongoing (ClinicalTrials.gov, number NCT01126463 [15]). The main objective of this report is to provide preliminary results on 188Re-SSS Lipiodol biodistribution and dosimetry assessments in humans, from the first six patients treated in this Phase 1 trial.
Methods
Patients
Patients with unresectable HCC were enrolled in this prospective, interventional, mono-center Phase I Lip Re 1 activity-escalation study of intra-arterial 188Re-SSS Lipiodol. A classical 3 ± 3 patients scheme was used: three patients included by activity step, plus 3 patients if a limiting toxicity occurred in the first three patients, the higher activity step allowed only if ≤ 1 limiting toxicity occurred in the evaluated step. The maximal tolerated dose (MTD) is defined by the highest activity level producing no more than one limiting toxicity. A maximum of four steps was a priori defined (1850, 3700, 5550, and 7400 MBq).
For study inclusion, patients had to comply with the following inclusion criteria: age ≥ 18 years; WHO performance status score 0–2; histologically- or cytologically-proven HCC, or liver tumour associated with chronic hepatopathy and alpha-fetoprotein (aFP) > 400 ng/ml, or tumorous hepatic formation considered as hypervascularized by at least two imaging methods in cirrhotic patients considered non-operable, non-resecable, non-transplantable, non-accessible to percutaneous treatment tumour; measurable tumour, either uninodular or multinodular, taking up less than 50% of hepatic volume, Stage A to C of BCLC classification (or Stage 0 to 4 of CLIP) with intolerance causing sorafenib treatment discontinuation, contraindication to sorafenib, or therapeutic escape to sorafenib. Patients with Stage ≥ 3 toxicity of the CTCAE Version 4.03, Stage D of BCLC classification, acute hepatic functions impairment (Child–Pugh B9 or C), Grade III HCC of Okuda classification, encephalopathy with even moderate cognitive impairment, advanced chronic respiratory insufficiency, creatinine clearance < 55 ml/min, polynuclear neutrophils < 1500 G/l, platelets < 50 G/l, prothrombin < 40% (INR > 2.3), contraindication to intra-arterial administration, patients unable to undergo follow-up for psychological or geographical reasons, patients dependent on another person for daily care, urinary incontinence, progressive cancer, pregnant or breastfeeding women, as well as women not employing effective contraception were excluded from participating to the study.
All patients provided written informed consent before enrolment. Ethical approval for this study was obtained from the institutional review board. This study was registered on ClinicalTrials.gov, number NCT01126463 [15].
Synthesis of 188Re-SSS lipiodol
188Re-super six sulfur Lipiodol (188Re-(PhCS2)(PhCS3)2, abbreviated as 188Re-SSS Lipiodol) was prepared, as previously described [9, 11, 12]. 188Re as carrier-free Na[188ReO4] in physiological solution was obtained by saline elution and concentration of 188W/188Re generator (Institut des RadioEléments, Fleurus, Belgium). Automation of Lipiodol radiolabeling was conducted on a remotely controlled TADDEO module (COMECER, Italy). Radiochemical purity (RCP) of 188Re compounds was assessed using an HPLC system (Dionex U3000).
A lyophilized reducing kit (Vial A) was reconstituted with Na[188ReO4] in 0.5–1 ml saline. After 5 min at room temperature, vial B containing the dithiobenzoate ligand was reconstituted in 0.5–1 ml EtOH and 0.5 ml Lipiodol. This solution was then transferred into reaction vessel R. The content of vial A was subsequently transferred into reaction vessel R, which was then heated at 100° C. After 15 min of heating, reaction vessel R was cooled down by an air flux for 7 min. Next, the reactor’s content was purified on a Sep-Pak column (C8), with the 188Re-SSS complex washed with 10 ml of water followed by 2 ml of a water:EtOH (1:1) mixture, and finally eluted with 2.5 ml EtOH into empty sterile vial C, using a sterile 0.2 mm filter. After EtOH evaporation, the residue was resuspended with 2–3 ml Lipiodol (Lipiodol ultra-fluide, Guerbet, France).
Treatment
Intra-arterial injection of 188Re-SSS Lipiodol into the hepatic artery was performed under local anesthesia using the classical Seldinger technique. The activity targeted in this first step of the trial was 1850 MBq.
Biodistribution analysis
Following 188Re-SSS Lipiodol administration, patients were hospitalized in a dedicated radionuclide therapy room for 3 days for biodistribution analysis.
Images acquisition
Whole body planar scintigraphic studies (256 × 1054 matrix), thoraco-abdominal planar scintigraphic studies (256 × 256 matrix), and thoraco-abdominal single-photon emission computed tomography (SPECT) studies (ordered-subset expectation maximization, 32 projections, 180°, 128 × 128 matrix, five iterations, eight subsets with a Gauss filter, 4.8 mm/pixel) were acquired for each patient at 1, 6, 24, 48, and 72 h post-administration, using a double-headed gamma camera (Symbia T2, Siemens Healthcare) equipped with high-energy parallel-hole collimators (due to the emission in low abundance of high-energy gamma rays, i.e., 633 keV and higher energies). The imaging window was set at 155 keV (20%). All SPECT/CT images were reconstructed using corrections for attenuation (low-dose CT-based attenuation), dead time, and scatter (dual-energy-window-based scatter correction [16]). No correction for partial volume effect was performed, due to the large-sized lesions.
Quantitative analysis
For quantitative purposes, the geometric mean of anterior and posterior measurements on planar scintigraphic studies was computed.
On each geometric mean image, in whole-body planar scintigraphic studies, regions of interest (ROIs) were drawn around the liver (including tumour), tumour, lungs, and a background region in order to calculate the total amount of activity in these areas. Area and activity in the healthy liver were calculated by subtraction of the liver and tumour parameters. Tumour to non-tumour (T/NT) uptake ratio was likewise calculated on planar scintigraphic studies (using a 1 cm2 ROI positioned on the higher-uptake area of the tumour and surrounding healthy liver). The lung shunt fraction (%) was calculated as the ratio of lung activity to total activity detected on a geometric mean planar scintigraphy.
On each SPECT study, volume of interests (VOIs) were drawn around the liver (including tumour), tumour, lungs, and a background region in order to calculate the total activity amount in these volumes. Volume and activity in the healthy liver were calculated by subtraction of the liver and tumour parameters. T/NT uptake ratio was likewise calculated on abdominal SPECT studies (using a 3 cm3 VOI positioned on the higher-uptake area of the tumour and on the surrounding healthy liver).
Full organ segmentation on planar and SPECT studies was performed by a single experienced nuclear physician (syngo Volumetric Analysis®, Siemens®). All these segmentations were reproduced as faithfully as possible at each analysis timepoint.
Dosimetric studies
Dosimetric steps were schematically as follows: 1) identify all source organs, 2) calculate time-integrated activity in each source organ, 3) calculate personalized S factor for each source organ, and 4) sum all source organ contributions to target organ irradiation.
MIRD formalism
From the distribution percentages determined on scintigraphic studies (planar scintigraphy and SPECT), the absorbed doses (in Gray [Gy]) to the various organs that concentrate 188Re-SSS Lipiodol were calculated according to the medical internal radiation dose (MIRD) formalism [17, 18] and based on Zanzonico [19], while adjusting for the personalized S factors. The personalized S factor was calculated by adjusting for the difference in mass between the patient and reference-man organ from the MIRD abaq [17,18,19] (Table 1). Patient’s organ volume was defined manually on CT scans (in cm3, Simplicit90Y, BTG). The volume of the healthy liver was calculated by subtraction of the whole liver and tumour volume. Time-integrated activities in source organ (in h) were calculated according to Zanzonico [19], with the biological elimination considered negligible (effective half-life and physical half-life were identical, equal to 60,840 s) and calculated from the SPECT images as a percent of injected activity corrected for biological elimination (urines and feces). The absorbed dose to the red marrow was calculated as well, employing the Sgouros methods [20].
Blood, urine, and feces collecting
Total urine and feces emissions were collected during hospitalization. Blood was sampled at 1, 6, 12, 24, 48, and 72 h post-treatment for 188Re content measurements. The total activity (in % of administrated activity, % AI) was then extrapolated by considering a blood volume of 6 l and a hematocrit of 40%. Samples were analyzed using a gamma counter calibrated for 188Re (Packard Bioscience Cobra II model 5002).
Exposure analysis
The patient’s dose rate was regularly measured at 1 m, 50 cm, 30 cm distance and in contact with the liver region with an ionizing chamber (Babyline, Eurisys Mesures) at 1, 6, 12, 24, 48, and 72 h post-treatment.
Follow up
Follow-up consisted of physical examinations, clinical chemistry assessments (including electrolytes, renal and liver function tests, and alpha-fetoprotein [aFP]) hematological tests at 24, 48, 72 h post administration, which were performed every month for 4 months, with a triphasic contrast-enhanced abdominal CT carried out at 4, 8, 16 weeks post-treatment.
Toxicity assessment and limiting toxicity definition
Any clinical or laboratory adverse event was scored according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03. For each parameter, the highest CTCAE grade was recorded upon follow-up. Imputability of treatment for the suspected toxicity was defined according to ICH E2B (R3), meaning that for patients with both liver toxicity and evidence of largely progressive disease, toxicity was attributed to disease progression (rather than to the treatment). A limiting toxicity was defined as a permanent Grade ≥ 3 toxicity not compatible with a retreatment at the same activity occurring within the 2 months after the treatment and still present at 2 months with regard to liver toxicity.
Response evaluation
Tumour response assessment on triphasic contrast-enhanced abdominal CT was evaluated at 2 months according to the response evaluation criteria in Solid Tumours Version 1.1. The aFP reduction was measured as well. Concerning αFP, patients were classified into: 1) partial biochemical responders when αFP reduction was > 50%, 2) stable disease when αFP change was between −50% and + 50%, and 3) progression when αFP increase was > 50%.
Statistical analysis
Quantitative values were expressed as means ± standard deviation. Statistical analyses were performed using R software (R Foundation for Statistical Computing, version 3.2.4, Vienna, Austria).
Results
Patients
Between May 19, 2010 and September 1, 2017, six patients (five males and one female) were administered 1645 ± 361 MBq of 188Re-SSS Lipiodol (Table 2). All six had multifocal disease, and three PVT.
Visual analysis
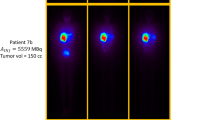
Uptakes were seen in only two organs on whole-body and abdominal scintigraphic studies and SPECT studies, namely the liver (tumour and healthy liver) and lungs (Fig. 1). No gastro-intestinal, bladder, or thyroid activity was observed on whole-body scintigraphic studies and abdominal SPECT. These uptakes were stable over time.
Biodistribution and relative quantification assessment
The hepatic uptake on planar scintigraphic studies (geometric mean) proved to be high (Fig. 2a): 79.9 ± 0.3% detected activity was quantified in the whole liver; 59.1 ± 1.5% of detected activity in the tumour, 20.8 ± 5.2% of detected activity in the healthy liver, and 9.7 ± 0.6% of detected activity in the lungs, with 11.3 ± 0.3% and 8.3 ± 0.3% in the right and left lungs respectively.
The hepatic uptake on SPECT studies was likewise high (Fig. 2b): 90.7 ± 1.6% of activity was detected in the liver, with 74.9 ± 1.8% of detected activity in the tumour. On SPECT studies, 19.1 ± 1.7% of detected activity was in the healthy liver and 9.3 ± 1.6% in the lungs, with 5.7 ± 1.1% in the right lung and 3.7 ± 0.5% in the left lung.
Average T/NT uptake ratio was high, measured at 5.4 ± 0.4 on planar scintigraphic studies and at 42.7 ± 7.8 on SPECT studies. These T/NT uptake ratios were stable over time (Fig. 3).
Blood samples, and urinary and feces excretion
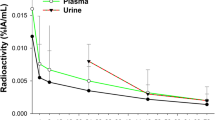
The average amount of 188Re-SSS Lipiodol excreted in blood samples was 1.4 ± 0.7% (min: 0.6%; max: 2.1%) of administrated activity at 6 h post-administration, declining rapidly thereafter. The activity was insignificant at 72 h post-administration, 0.2% ± 0.2% of administered activity (min: 0.1%; max: 0.7%).
A mean 1.4 ± 1.6% (min: 0.3%; max: 4.5%) of administered activity was excreted in urine within 72 h post-administration. The largest fraction was excreted within the first 24 h (mean: 0.9 ± 1.1%; min: 0.1%; max 2.9%), declining rapidly thereafter. The total activity excreted in feces was very low, assessed at 564 ± 767 kBq at 96 h post-administration. On average, 1.5 ± 1.6% (min: 0.3%; max: 4.5%) of administered activity was biologically eliminated in urine and feces. Consequently, biological elimination was considered negligible.
Dosimetric assessment
Absorbed doses to whole liver, tumour, healthy liver, and lungs were calculated based on planar scintigraphic studies and SPECT studies, individually for each patient (Table 3). Based on planar scintigraphic biodistribution studies, the mean absorbed doses were as follows: 7.0 ± 3.3Gy to whole liver, 42.4 ± 37.0Gy to tumour, 9.1 ± 3.5Gy to healthy liver, and 3.2 ± 1.5Gy to lungs. Based on SPECT studies, mean absorbed doses were as follows: 7.9 ± 3.7Gy to whole liver, 42.7 ± 34.0Gy to tumour, 10.2 ± 3.7Gy to healthy liver, and 1.5 ± 1.2Gy to lungs. Based on blood activities, the mean absorbed dose to red marrow was 7.0 ± 4.7 mGy.
Dose rate
The average dose rate at 1 m was 11.9 ± 6.0 μSv/h (0.007 ± 0.002 μSv/h/MBq injected) at 1 h post-administration. At 72 h, dose rate at 1 m was 2.0 ± 1.8 μSv/h (0.001 ± 0.001 μSv/h/MBq injected) (Fig. 4). At 50 cm distance, the average dose rate was 38.5 ± 12.3 μSv/h (0.02± 0.01 μSv/h/MBq injected) at 1 h and 3.4 ± 2.3 μSv/h (0.0± 0.0 μSv/h/MBq injected) at 72 h. At 30 cm distance, the average dose rate was 68.5± 44.5 μSv/h (0.04± 0.00 μSv/h/MBq injected) at 1 h and 5.2 ± 3.0 μSv/h (0.0± 0.0 μSv/h/MBq injected) at 72 h. In contact with the liver region, the average dose rate was 275.0± 85.8 μSv/h (0.15± 0.03 μSv/h/MBq injected) at 1 h and 26.7 ± 13.3 μSv/h (0.01± 0.01 μSv/h/MBq injected) at 72 h.
Toxicity
All events attributable to 188Re-SSS Lipiodol were Grade 1 or 2, transitory, asymptomatic, and non-limiting. No Grade 3 or 4 events definitely attributable to 188Re-SSS Lipiodol were observed. However, the first patient with a large (12 cm) and rapidly progressive HCC with portal vein thrombosis died of liver decompensation at 1 month post-treatment, most probably in relation with a massive tumour progression (progression of aFP from 46,046 UI at baseline to 298,594 UI at week 3). The trial independent evaluation committee agreed with the fact that tumour progression was the most probable cause of death but, due to this early death (occurring before the time point of toxicity evaluation of 2 months) and also considering that it was not possible to formally exclude a treatment-related toxicity, decided to use a conservative approach and considered this death as a treatment-related toxicity.
The most remarkable biologic event was lymphopenia (83%, in five patients). Increases in bilirubin, ASAT, and gamma GT levels were recorded as well, although these increases were only transitory.
Response
Response on CT-scan was as follows: during the first month post-treatment, four patients experienced stable disease, and two patients progressive disease. The time to progression was 152 ± 123 days (min: 1; max: 297). The survival was 236 ± 155 days (min: 27; max: 424).
Discussion
In general, anti-tumour radiopharmaceuticals (RP) must display the following characteristics: (1) a high T/NT uptake ratio, rapid, intense, and selective tumour biodistribution, (2) long intratumor retention in order to maximize the tumoricidal effect, and (3) extratumor retention as reasonable as possible so as to limit toxicity, evaluated by residence time. In-vivo stability and pharmacokinetics are determinants of RP efficacy. The RP must be stable post-administration, while the tumour retention (measured by the residence time) should be high enough to ensure selective irradiation of the tumour compartment. In contrast, RP exposure to non-target tissues should be minimal.
Our preliminary results have clearly shown that 188Re-SSS Lipiodol displays remarkable biodistribution characteristics and in-vivo stability, as well as tumour targeting and retention, in addition to favorable dosimetric features in humans. The results of biodistribution and in-vivo stability revealed in this study are perfectly in line with our previously described findings in animals [9, 11].
Only three different 188Re Lipiodol radiolabeled complexes have been tested in humans to date, namely188Re-SSS Lipiodol, 188Re-HDD Lipiodol, and 188ReN-DEDC Lipiodol (Table 4).
188Re-HDD Lipiodol is the most extensively studied compound and is still in use only in Asia. The complex displays, in fact, major disadvantages: its in-vivo stability does prove to be not optimal, and the RP shows high urinary excretion. Based on clinical trial results with 188Re-HDD Lipiodol [21, 22], 36.2% ± 5.7% [22] to 44.1 ± 11.7% [21] of injected activity was excreted in urine within 52 to 76 h post-injection. In comparison, with 188Re-SSS Lipiodol only 1.4 ± 1.6% (min: 0.3%; max: 4.5%) of administered activity was excreted in urine within 72 h post-administration.
Consequently, dosimetric assessments obtained with 188Re-HDD Lipiodol proved to be less favorable than those acquired with 188Re-SSS Lipiodol. When employing 188Re-SSS Lipiodol, we have achieved an absorbed dose to the whole liver (based on planar scintigraphic biodistribution studies) of 7.0Gy for 1.645 GBq injected, resulting in an absorbed dose of 4.2Gy/GBq injected versus only 1.3 to 2.3Gy/GBq injected with 188Re-HDD Lipiodol [21,22,23,24]. For tumours, we have achieved a mean dose of 25.8 Gy/GBq injected with188Re-SSS Lipiodol. Only one single study conducted by Bernal et al. assessed dose to tumour. For 188Re-HDD Lipiodol, with 3.9GBq injected in this study, the authors achieved a mean 63.4Gy to tumour, with an absorbed dose of 15.9Gy/GBq administered [25, 26]. Nevertheless, no dose to critical organs was evaluated by Bernal et al., with no dose to tumour assessed by Lambert et al. Therefore, due to their unavailability, these data could not be employed for comparison with our results [21,22,23].
The absorbed doses to the lungs were likewise shown to be lower with 188Re-SSS Lipiodol. In our study, we have achieved a mean absorbed dose (based on SPECT biodistribution studies) of 1.5Gy for 1.645GBq administered, resulting in an absorbed dose of 0.9Gy/GBq administered, as compared to 1.1 [23] to 1.3 [23,24,25] Gy/GBq administered with 188Re-HDD Lipiodol.
In these trials, dosimetric assessments were quite approximated with regard to several points. In the Lambert et al. publication, only whole-body images but no tomoscintigraphic studies were performed. In the Bernal et al. paper, dosimetric assessments were carried out using a scout dose of 188Re-HDD Lipiodol, with whole-body scintigraphic studies conducted. In all cases, dose to target organs or critic organs were calculated considering that there was no biological elimination, as recommended by Zanzonico [19]. This approximation, however, is not accurate. Overall, 188Re-HDD Lipiodol displays elevated urinary excretion (36.2% to 44.1% within 52 to 76 h [21, 22]), and in these studies, doses to the whole liver or to tumour are therefore overestimated. In our study, we have calculated the dose to both target and critic organs, integrating biological elimination in both urine and feces.
Another complex, the 188ReN-DEDC Lipiodol, has been tested in both animals and humans [27], though no dosimetric measurements were conducted. The authors only assessed biodistribution in animals and humans, and concluded that when applying 188ReN-DEDC Lipiodol, there was no significant release of this complex after in-vivo administration, with excellent retention in tumour. It should be noted that biodistribution was not quantified, particularly in the human studies.
This superior in-vivo stability of 188Re-SSS Lipiodol compared to other radiolabeling complexes, such as 188Re-HDD Lipiodol and 188ReN-DEDC Lipiodol, is probably due to the lower oxidation degree of the rhenium complex, with an oxidation degree + III for 188Re-SSS complex versus an oxidation degree + V for the others. Overall, + III is an oxidation level that is chemically more stable than the + V level [8, 14]. Consequently, 188Re-HDD Lipiodol exhibits an increased risk of re-oxidation, and thus of 188Re release in serum than 188Re-SSS Lipiodol. Owing to its superior in-vivo stability, the dosimetric profile and tolerance profile obtained with 188Re-SSS Lipiodol appear more favorable than those acquired with 188Re-HDD Lipiodol and 188ReN-DEDC Lipiodol.
One clinical interest of dosimetric analysis is the potential ability to demonstrate a dose/response correlation (and an eventual impact of tumour dose on survival). Even if the two patients with progressive disease in our preliminary results received a quite low tumour dose (patients 2 and 4, TD of respectively 12,7 and 4,3 Gy based on SPECT studies) such dose/response correlation is not evaluable in tis study due to the patients selected (end-stage patients with huge lesions refractory to any previous therapy) and the small number of cases required in a phase 1 study. This interesting point will have to be evaluated further in a phase 2 study, including more and better selected patients.
At present, the most common compounds used for radioembolization are 90Y-labeled resin or glass microspheres. However, 90Y-labeled microspheres display several disadvantages. Their high costs (around €12,000 per treatment) limit their accessibility in many countries, especially in developing countries. 188Re is an interesting candidate for nuclear therapy, emitting β− particles of 2.12 MeV (80%) and 1.96 MeV (18%). As comparison β− particle energy is of 2.28 Mev (99.9%) for 90Y and only 0.606 MeV (89%) and 0.303 MeV (7%) for 131I. Furthermore, 188Re is generator-produced. The 188W/188Re generator has a long useful shelf-life of several months and is of reasonable cost, resulting in a quite low cost for one therapetic vial of 188Re [28, 29].
Moreover, Lipiodol, currently used for chemoembolization, is a vector that completely differs from microspheres for liver therapies. Lipiodol has the ability to penetrate peritumoral sinusoidal capillaries, the interstitium, and tumour cells themselves [30, 31], which does not apply to microspheres. Therefore, as a vector, Lipiodol may prove to be more suitable than microspheres. This point underlines the specific usefulness of radiolabeled Lipiodol for radioembolization of liver tumours. Lastly, two recently performed Phase III studies failed to demonstrate any increase in overall survival in comparison with sorafenib in advanced HCC patients [3,4,5].
One limitation of our study is the absence of absolute 188Re quantification for dosimetric analysis. This must be accounted for by the fact that quantification of 188Re is not very easy, with no one single absolute quantification method clearly described to date. That is the reason why in this study, we have applied relative quantification on planar and SPECT studies (in % of detected activity) with attenuation, scatter, and dead-time correction. As an example, there are no generalized recommendations for tomographic reconstruction with correct scatter correction for 188Re available. 188Re emits many gamma rays: a 155 keV gamma ray (15%), 478 and 633 keV high gamma rays (2.3%), and bremsstrahlung gamma rays (generated by the interaction of beta particles with tissues) which result in contaminated images and 188Re activity overestimations in organs. Several methods have meanwhile been proposed for quantitative purposes. Zanzonico recommended DEW scatter correction [19] whereas several studies based on phantom experiments recommended triple-energy-window (TEW) scatter correction [32, 33]. Moreover, to acquire truly absolute quantitative results, corrections for scatter, dead time, and attenuation should be recommended. As a result, absolute quantification appears very difficult to perform, requiring further development.
Guidelines for calculating dose to tumour and to critical organs likewise proved to be contradictory. Zanzonico did not integrate biological elimination when calculating dose to tumour or to critical organs [19], while for 188Re-HDD Lipiodol, approximately half of the administered activity is eliminated in urine. Though biological elimination of 188Re-SSS Lipiodol proved to be rather low in our study, we have integrated biological elimination in our dose calculations. Consequently, our dose measurements appear to be more rigorous.
Conclusion
188Re-SSS Lipiodol displays favorable biodistribution features for radioembolization, exhibiting the highest in-vivo stability of any radiolabeled Lipiodol compound described to date. These preliminary results must be further confirmed while completing this phase I Lip Re1 study.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Vilgrain V, Bouattour M, Sibert A, Lebtahi R, Ronot M, Pageaux G-P, et al. SARAH: a randomised controlled trial comparing efficacy and safety of selective internal radiation therapy (with yttrium-90 microspheres) and sorafenib in patients with locally advanced hepatocellular carcinoma. J Hepatol. 2017;66:S85–6.
Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux G-P, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624–36.
Chow PKH, Gandhi M, Tan S-B, Khin MW, Khasbazar A, Ong J, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36(19):1913–21. https://doi.org/10.1200/JCO.2017.76.0892.
Raoul JL, Guyader D, Bretagne JF, Duvauferrier R, Bourguet P, Bekhechi D, et al. Randomized controlled trial for hepatocellular carcinoma with portal vein thrombosis: intra-arterial iodine-131-iodized oil versus medical support. J Nucl Med. 1994;35:1782–7.
Noiret N, Garin E, Lepareur N, Ardisson V. Composition for treating liver cancer in humans based on rhenium-188 and method for preparing such a composition [Internet]. 2011 [cited 2017 Oct 11]. Available from: https://www.google.com/patents/EP2536438A1?hl=fr&cl=en
Lepareur N. Vectorisations active et passive de radiopharmaceutiques du technetium-99m et du rhénium-188 pour l’imagerie médicale et la thérapie [Internet]. Rennes 1; 2003 [cited 2017 Jul 10]. Available from: http://www.theses.fr/2003REN10110
Garin E, Noiret N, Malbert C, Lepareur N, Roucoux A, Caulet-Maugendre S, et al. Development and biodistribution of 188Re-SSS lipiodol following injection into the hepatic artery of healthy pigs. Eur J Nucl Med Mol Imaging. 2004;31:542–6.
Garin E, Noiret N, Malbert C-H, Lepareur N, Roucoux A, Dazord L, et al. Development of 99mTc labelled lipiodol: biodistribution following injection into the hepatic artery of the healthy pig. Nucl Med Commun. 2004;25:291–7.
Garin E, Denizot B, Noiret N, Lepareur N, Roux J, Moreau M, et al. 188Re-SSS lipiodol: radiolabelling and biodistribution following injection into the hepatic artery of rats bearing hepatoma. Nucl Med Commun. 2004;25:1007–13.
Garin E, Rakotonirina H, Lejeune F, Denizot B, Roux J, Noiret N, et al. Effect of a 188 re-sss lipiodol/131i-lipiodol mixture, 188 re-sss lipiodol alone or 131i-lipiodol alone on the survival of rats with hepatocellular carcinoma. Nucl Med Commun. 2006;27:363–9.
Lepareur N, Ardisson V, Noiret N, Boucher E, Raoul J-L, Clément B, et al. Automation of labelling of Lipiodol with high-activity generator-produced 188Re. Appl Radiat Isot. 2011;69:426–30.
Lepareur N, Ardisson V, Noiret N, Garin E. (188)re-SSS/Lipiodol: development of a potential treatment for HCC from bench to bedside. Int J Mol Imaging. 2012;2012:278306.
188RE-SSS lipiodol to treat hepatocellular carcinomas — full text view.ClinicalTrials.gov [Internet]. 2016 [cited 2016 Jun 9]. Available from: https://clinicaltrials.gov/ct2/show/NCT01126463
Jaszczak RJ, Greer KL, Floyd CE Jr, Harris CC, Coleman RE. Improved SPECT quantification using compensation for scattered photons. J Nucl Med. 1984;25:893–900.
Snyder WS, Ford MR, Warner GG, Watson SB. MIRD Pamphlet #11: S, Absorbed dose per unit cumulated activity for selected radionuclides and organs. 1975 [cited 2017 Oct 10]; Available from: https://www.scienceopen.com/document?vid=a0909e6e-4b0b-469b-b9c7-09c8dac3fc37
Siegel JA, Thomas SR, Stubbs JB, Stabin MG, Hays MT, Koral KF, et al. MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med. 1999;40:37S–61S.
Zanzonico PB, Divgi C. Patient-specific radiation dosimetry for radionuclide therapy of liver tumours with intrahepatic artery rhenium-188 lipiodol. Semin Nucl Med. 2008;38:S30–9.
Sgouros G. Bone marrow dosimetry for radioimmunotherapy: theoretical considerations. J Nucl Med. 1993;34:689–94.
Lambert B, Bacher K, Defreyne L, Gemmel F, Van Vlierberghe H, Jeong JM, et al. 188Re-HDD/lipiodol therapy for hepatocellular carcinoma: a phase I clinical trial. J Nucl Med. 2005;46:60–6.
Lambert B, Bacher K, De Keukeleire K, Smeets P, Colle I, Jeong JM, et al. 188Re-HDD/lipiodol for treatment of hepatocellular carcinoma: a feasibility study in patients with advanced cirrhosis. J Nucl Med. 2005;46:1326–32.
Lambert B, Bacher K, Defreyne L, Van Vlierberghe H, Jeong JM, Wang RF, et al. (188)re-HDD/lipiodol therapy for hepatocellular carcinoma: an activity escalation study. Eur J Nucl Med Mol Imaging. 2006;33:344–52.
Lambert B, Bacher K, Defreyne L. Rhenium-188 based radiopharmaceuticals for treatment of liver tumours. Q J Nucl Med Mol Imaging. 2009;53:305–10.
Bernal P, Raoul J-L, Stare J, Sereegotov E, Sundram FX, Kumar A, et al. International Atomic Energy Agency-sponsored multination study of intra-arterial rhenium-188-labeled lipiodol in the treatment of inoperable hepatocellular carcinoma: results with special emphasis on prognostic value of dosimetric study. Semin Nucl Med. 2008;38:S40–5.
Bernal P, Raoul J-L, Vidmar G, Sereegotov E, Sundram FX, Kumar A, et al. Intra-arterial Rhenium-188 Lipiodol in the treatment of inoperable hepatocellular carcinoma: results of an IAEA-sponsored multination study. Int J Radiat Oncol. 2007;69:1448–55.
Boschi A, Uccelli L, Duatti A, Colamussi P, Cittanti C, Filice A, et al. A kit formulation for the preparation of 188Re-lipiodol: preclinical studies and preliminary therapeutic evaluation in patients with unresectable hepatocellular carcinoma. Nucl Med Commun. 2004;25:691–9.
Knapp FF. Continued availability of the tungsten-188/rhenium-188 generator to enhance therapeutic utility of 188Re. Int J Nucl Med Res [Internet]. 2017 [cited 2018 Dec 7]; Available from: http://www.cosmosscholars.com/special-issues-ijnmr/46-abstracts/ijnmr/724-abstract-continued-availability-of-the-tungsten-188-rhenium-188-generator-to-enhance-therapeutic-utility-of-188re
Pillai MR, Dash A, Knapp FF. Rhenium-188: availability from the 188W/188Re generator and status of current applications. Curr Radiopharm. 2012;5:228–43.
Kan Z, Ivancev K, Hägerstrand I, Chuang VP, Lunderquist A. In vivo microscopy of the liver after injection of lipiodol into the hepatic artery and portal vein in the rat. Acta Radiol. 1989;30:419–25.
Park C, Choi SI, Kim H, Yoo HS, Lee YB. Distribution of Lipiodol in hepatocellular carcinoma. Liver. 1990;10:72–8.
Celler A, Esquinas PL. Personalized dosimetry for 188 Re radionuclide therapies based on post-treatment SPECT/CT scans. 2017 [cited 2017 Aug 24]; Available from: http://cosmosscholars.com/phms/index.php/ijnmr/article/view/776
Fernández E, Luis P. Quantitative measurements of Rhenium-188 for radionuclide therapies [Internet]. University of British Columbia; 2017 [cited 2017 Aug 24]. Available from: https://open.library.ubc.ca/cIRcle/collections/ubctheses/24/items/1.0348703
Acknowledgements
This work has been supported in part by a grant from the French National Agency for Research called “Investissements d’Avenir” Labex IRON n°ANR-11-LABX-0018-01.
We are grateful to Prof Mario Marengo, from Bologna University, for his help with the calibration settings for 188Re.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
Yan Rolland, Julien Edeline, and Etienne Garin are consultants for BTG UK Ltd.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Delaunay, K., Edeline, J., Rolland, Y. et al. Preliminary results of the Phase 1 Lip-Re I clinical trial: biodistribution and dosimetry assessments in hepatocellular carcinoma patients treated with 188Re-SSS Lipiodol radioembolization. Eur J Nucl Med Mol Imaging 46, 1506–1517 (2019). https://doi.org/10.1007/s00259-019-04277-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04277-9