Abstract
Purpose
The fast-increasing use of positron emission tomography (PET) with prostate-specific membrane antigen (PSMA) ligand for the imaging of prostate cancer (PCA) biochemical recurrence has led to a rapid change in treatment concepts. Since the superiority of 68Ga-PSMA-11 PET in detecting recurrent PCA is well established, the aim of our study was to assess its effect on management and outcome in all patients imaged during the first year after its introduction into clinical routine.
Methods
Of 327 patients imaged, 223 were referred for recurrent PCA and gave written informed consent for further analysis of their data for this retrospective consecutive cohort analysis. Twenty patients were lost to further follow-up. The rate of detection of recurrence by 68Ga-PSMA-11 PET was based on the clinical reports. Management before the availability of PET diagnostic information was assessed according to guidelines (therapy option without 68Ga-PSMA-11 PET). In the 203 patients with follow-up 6 months after 68Ga-PSMA-11 PET, the therapies effectively implemented as well as follow-up PSA levels were evaluated, with a PSA value of <0.2 ng/ml representing a complete response and a decrease in PSA value of at least 50% from baseline representing a partial response.
Results
68Ga-PSMA-11 PET was positive and identified recurrence in 166 of the 223 patients (74%), with a detection rate of 50% for recurrent disease at low PSA values of <0.5 ng/ml. 68Ga-PSMA-11 PET led to a change in management in 122 of the 203 patients (60%). A substantial increase in the use of metastasis-targeted treatment and a reduction in the use of systemic treatment were observed, with 59 of the 203 patients (29%) undergoing targeted radiotherapy (RTXa) only, and 20 patients (10%) undergoing RTXa with hormonal therapy as the two most frequently selected therapy options. The proportion of patients in whom systemic therapy was selected decreased from 60% (133 of 223 patients) to 34% (70 of 203 patients) on the basis of the information provided by the 68Ga-PSMA-11 PET scan. PSMA PET-directed metastasis-targeted treatment led to a complete response after 6 months in 45% of patients.
Conclusion
The high rate of recurrence detection by PSMA PET was confirmed and PSMA PET led to a change in management in 60% of patients. Focal therapy for PSMA-positive lesions is a promising approach with complete responses in 45% of patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of positron emission tomography (PET) with prostate-specific membrane antigen (PSMA) ligand in the setting of biochemical recurrence of prostate cancer after local treatment has increased significantly in the last few years due to its superior detection rate compared with conventional imaging and PET with previously established tracers such as choline [1,2,3,4]. More important than the superior detection rate is the impact on patient management. Therefore, there is a fast-increasing body of literature on the impact of PSMA PET in various settings, and PSMA PET has been reported to lead to a change in management in about 50% of patients [2, 5,6,7,8,9]. This is mostly due to the identification of focal recurrence when conventional imaging has failed [2, 10] or the identification of additional sites of disease [6], that potentially lead to changes in radiotherapy (RT) planning or even to local surgical approaches in selected patients [7]. Data from recent studies demonstrate a change in dose or RT planning target volume and an increased use of stereotactic body RT after PSMA PET [5, 6, 11]. A meta-analysis has also confirmed that after PSMA PET more patients receive targeted RT and surgical resection, while the proportion receiving systemic treatment decreases by more than 50%, mostly due to fewer patients receiving androgen-deprivation therapy (ADT), and fewer patients are followed with watchful waiting [8].
Only a few studies have assessed outcomes in patients who have undergone PSMA PET for biochemical recurrence by measurement of prostate-specific antigen (PSA) levels after treatment, and have shown promising results [11,12,13,14,15,16]. Several authors have observed a significant decrease in PSA levels after PSMA PET-guided RT (RTXa). Henkenberens et al. found that RTXa for isolated lymph node metastases after primary therapy provides effective local control and improved outcomes, and delays the initiation of systemic treatment (chemotherapy or ADT) [16]. Schmidt-Hegemann et al. investigated the value of RTXa in 129 patients and found that nearly 90% of the patients treated with RTXa without additional ADT were free of biochemical recurrence after a median follow-up of 20 months [15]. These promising results suggest that PSMA PET-guided therapies could be an effective strategy for treating recurrent disease and for postponing the introduction of systemic therapies.
The progress towards personalized therapies is desirable in all disease contexts but is even more important in the context of cancer, since treatments can cause severe side effects. Furthermore, avoiding, unnecessary and costly therapies is of increasing importance, especially with the increasing cancer burden in western societies [17, 18]. Although many studies have assessed the impact of PSMA PET in patient management there is less data on its impact on patient outcomes and most importantly on overall survival [19].
The aim of this study was to assess the impact of 68Ga-PSMA-11 PET on the management and outcome in all patients examined in the first year after its clinical introduction for prostate cancer imaging in Switzerland.
Materials and methods
Study population
This retrospective study included all patients who underwent 68Ga-PSMA-11 PET for prostate cancer restaging at the Department of Nuclear Medicine, University Hospital Zürich, in the first year after its clinical introduction for prostate cancer imaging in Switzerland, from April 2016 to April 2017. The local ethics committee approved the study protocol and all patients gave general written informed consent for the retrospective use of their data (BASEC no. 2018-01284).
Study design
We collected all relevant clinical information from patient charts at the time of the 68Ga-PSMA-11 PET scan including TNM stage, Gleason score, resection margins for prostatectomy, RT and systemic treatments, and PSA levels. For follow-up evaluation, information on the management implemented and PSA levels 6 months after 68Ga-PSMA-11 PET was obtained.
Changes in management were assessed by comparing the potential therapy options before 68Ga-PSMA-11 PET based on PSA levels, initial tumour stage, resection margins and previous treatment according to guidelines [20, 21], with the therapy implemented after 68Ga-PSMA-11 PET. Dose escalation to local recurrences for prostate bed salvage RT (sRTX) was not considered a change in management. However, if a lesion outside the prostate bed RT field was targeted, a change in management was recorded. To compare our results with those reported in the literature, we defined two therapy groups. The first group comprised patients with metastasis-targeted treatment based on a positive 68Ga-PSMA-11 PET scan including RTXa or surgery with our without ADT and excluding PSMA-targeted internal RT (IRT), and the second group comprised patients with only local treatment including: RTXa, surgery or sRTX after a positive or a negative 68Ga-PSMA-11 PET scan, without systemic therapy (with ADT also considered as systemic therapy). An experienced urologist evaluated the management initially intended and the management implemented in all patients in-house (80 patients), and, for external patients, the referring physicians were contacted and asked to determine whether the 68Ga-PSMA-11 PET scan had had an impact on management.
Outcomes were assessed by analysis of PSA levels after 6 months. According to previous studies, a PSA value of ≤0.03 ng/ml was defined as undetectable, a PSA value of <0.2 ng/ml was considered a complete response and a decrease in PSA level by at least 50% compared with the level at the time of the scan was considered a partial response [15, 22,23,24]. In patients with a baseline PSA value of <0.2 ng/ml, a decrease of at least 50% was considered a partial response, and a PSA value of ≤0.03 ng/ml as undetectable.
Imaging techniques
Patients underwent 68Ga-PSMA-11 PET/CT or 68Ga-PSMA-11 PET/MR after a single injection of 68Ga-PSMA-11 (dose: mean 128.3 MBq, SD 17 MBq, range 86–162 MBq). To reduce tracer activity in the bladder, ureters and kidneys, furosemide (0.13 mg/kg) was injected intravenously 30 min prior to tracer injection, and patients were asked to void prior to the scan. The institutional protocol was in agreement with the EANM and SNMMI procedure guidelines [25].
PET/MR protocol
A clinical routine whole-body PET/MR scan was performed 60 min after injection on a hybrid scanner (SIGNA PET/MR; GE Healthcare, Waukesha, WI, USA) as used in previous studies in our department with the same protocol for prostate imaging as recently described [10]. In brief, six bed positions with 2 min acquisition time per bed position for the whole-body protocol, and additional specific sequences covering the pelvis, including a high-resolution T1-weighted LAVA-FLEX sequence, and a T2-weighted fast recovery fast spin-echo sequence in at least two planes.
PET/CT protocol
In patients who underwent a PET/CT scan, PET was performed with six bed positions with 2.5 min acquisition time per bed position, and an attenuation CT scan was acquired on a Discovery VCT 690 PET/CT scanner (GE Healthcare, Waukesha, WI, USA) 60 min after injection with the following whole-body scan parameters: tube voltage 140 kV, tube current with automated dose modulation with a maximum of 80 mA/slice, collimation 512 × 0.976, pitch 0.984:1, rotation time 0.5 s, coverage speed 78 mm/s, field of view 50 cm, and images with a transverse pixel size of 0.976 and a slice thickness of 1.25 mm reconstructed in the axial plane.
Image analysis
The acquired PET, CT and MR images were transmitted to a dedicated review workstation (Advantage workstation, version 4.6 or 4.7; GE Healthcare), which allows PET and CT or MR images to be reviewed side by side or in fused mode. All scans were analysed by dual board-certified radiologists and nuclear medicine physicians with 4–8 years of experience, incorporating both the MRI or CT and PET information. The readers had access to the clinical information for the readouts. Care was taken to avoid false-positive findings by considering the physiological biodistribution and the known PSMA-positive pitfalls such as neural ganglia, Paget’s disease, sarcoidosis and others [26, 27].
The clinical decisions based on the clinical reports were obtained for patients in our own urology or radiotherapy departments using our hospital patient information system, or assessed by questionnaires sent to the referring physicians for patients referred to our hospital.
Statistical analysis
Descriptive statistics are used to display patient data as medians, means, and ranges and percentages. Previous treatments in the patient cohort are illustrated using a pie chart. The detection rates are plotted against the absolute PSA values at the time of the scan. Changes in management are illustrated using Sankey diagrams that show the selected therapies in relation to previous treatments with and without PET information. Outcomes in terms of relative changes in PSA values are illustrated using waterfall plots. Graphics were generated using R ® version 3.5.1, USA).
Results
Patient characteristics
A total of 327 patients underwent a 68Ga-PSMA-11 PET scan during the study period. Of these, 223 were included in the analysis of detection rates and 203 in the analysis of changes in management and follow-up PSA levels. Patient selection is illustrated in Fig. 1.
The mean age of the patients at the time of the scan was 68 years (SD 6.8 years) and the mean PSA value was 4.3 ng/ml (SD 10.4 ng/ml, range 0.03–99 ng/ml). One patient with a PSA value of 0.03 ng/ml was referred for 68Ga-PSMA-11 PET because of suspected remaining nodal disease after radical prostatectomy (RPE); however, the scan was negative and PSA was undetectable at follow-up.
The initial T stage was available in 200 patients. The majority had pathological staging after surgery and only a few had clinical staging. Most patients were diagnosed with stage T3, 52 had local nodal metastasis at diagnosis and only four had distant metastasis at diagnosis. The most frequent Gleason score at diagnosis was 4 + 3 (33%), followed by 3 + 4 (20%). Patient characteristics are shown in Table 1.
Treatment before 68Ga-PSMA-11 PET
RPE without any additional radiation or systemic treatment was the only previous treatment in 115 of the 223 patients (52%). In 69 patients (31%) a combination of RPE and sRTX due to first PSA relapse was performed before 68Ga-PSMA-11 PET, and in an additional 17 patients ADT was added after RPE and sRTX (8%). Overall, in 35 patients (16%) ADT was given before the scan, alone or together with another treatment modality. An overview of the previous treatments of the patients before they were referred for 68Ga-PSMA-11 PET is given in Fig. 2.
Treatments the patients had received before recurrence. The most common treatment was radical prostatectomy (RPE), followed by RPE and salvage radiotherapy (sRTX). Only two patients underwent definitive radiotherapy (RTX). A total of 15 patients had received androgen deprivation therapy (ADT) and 22 had received surgery, sRTX, ADT and/or chemotherapy combined
Detection rate of 68Ga-PSMA-11 PET
Overall, 68Ga-PSMA-11 PET for restaging was positive in 166 of the 223 patients, resulting in a detection rate of 74%. PSA values at the time of the scan were available in 220 patients. Site of recurrence was detected more frequently in patients with higher PSA values, as illustrated in Fig. 3. Findings were suspicious for local recurrence in 49 patients (30%), lymph node metastasis in 113 patients (68%), bone metastasis in 54 patients (33%) and distant visceral metastasis in 12 patients (7%).
Impact of 68Ga-PSMA-11 PET on management
Overall 68Ga-PSMA-11 PET changed management in 122 of 203 patients (60%). Considering only the changes based on scans with positive findings, management was changed in 114 of 152 patients (75%). Twenty patients were lost to follow-up. The potential therapy options before PET in relation to the effective options after PET are illustrated as Sankey diagrams that show the absolute numbers of selected therapies and the pathways for the individual patients. Figure 4a shows the theoretical therapy options according to previous treatments and guidelines without PET information available, Figure 4b shows the corresponding therapies actually selected after integration of the 68Ga-PSMA-11 PET information in relation to the previous treatments.
Therapy options in all 223 patients in relation to previous treatments: a therapy options according to guidelines without 68Ga-PSMA PET (w/o PSMA PET); b therapies implemented after 68Ga-PSMA PET (with PSMA PET). RPE radical prostatectomy, ADT androgen-deprivation therapy, sRTX salvage radiotherapy, RTX radiotherapy, RTXa targeted radiotherapy, Chemo chemotherapy, IRT internal radiotherapy, Multimodal surgery, sRTX, ADT and/or chemotherapy combined, PSA prostate-specific antigen
In 86 patients (42% of 203) the information from 68Ga-PSMA-11 PET was used for metastasis-targeted treatment. RTXa and RTXa with ADT were the most frequently selected therapy options (59 patients, 69%, and 20 patients, 23%, respectively). In seven patients, surgery was performed as the therapy option, leading to a complete response in two patients without additional therapy. Systemic therapy, including ADT, with or without local treatment, would have been the therapy option according to guidelines before 68Ga-PSMA-11 PET in 133 of the 223 patients (60%). After the 68Ga-PSMA-11 PET this number decreased to 70 of 203 patients (34%). 68Ga-PSMA-11 PET findings led to IRT with 223Ra by exclusion of visceral metastasis in two patients and to IRT with 177Lu-PSMA-617 in two other patients.
Of 115 patients treated with RPE only, 90 were considered for sRTX alone before the scan, 68Ga-PSMA-11 PET was positive in 54 (60%) and changed the therapy in 43 (55%) of 78 patients who had follow-up available. In 42 of 54 patients (78%), 68Ga-PSMA-11 PET showed recurrence outside the prostate/prostate bed. Of these 42 patients, 26 (62%) had RTXa and three underwent surgery. Of the 100 patients for whom a curative treatment approach was intended by the referring physician before the scan, 68Ga-PSMA-11 PET was positive in 13 patients for distant metastasis either in bone or in soft tissue, ruling out a curative approach.
Figure 5 shows the changes from the theoretical treatment options according to previous treatments and guidelines to the actually selected treatments after integration of 68Ga-PSMA-11 PET information.
Changes from theoretical treatment options according to previous treatments and guidelines (w/o PSMA PET) to the treatments implemented in 203 patients after integration of 68Ga-PSMA-11 PET information (with PSMA PET). ADT androgen-deprivation therapy, sRTX salvage radiotherapy, RTXa targeted radiotherapy, Chemo chemotherapy, IRT internal radiotherapy, PSA prostate-specific antigen
Impact of 68Ga-PSMA-11 PET on PSA
In 203 patients, PSA values at the time of 68Ga-PSMA-11 PET and 6 months later were available. The mean PSA value at the time of the scan in the 203 patients was 4.26 ng/ml (SD 9.7 ng/ml) and the median value was 0.97 ng/ml (range 0.03–99 ng/ml). After 6 months the mean PSA value was 2.99 ng/ml (SD 9.7 ng/ml) and the median was 0.4 ng/ml (range 0.001–82.7 ng/ml). Figure 6 shows the relative changes in PSA values in all patients.
Overall relative decreases in PSA values from the time of the 68Ga-PSMA PET scan to follow-up at 6 months in 203 patients. Blue bars represent patients who had any therapy implemented (171 patients); green bars represent those without therapy and PSA follow-up only (32 patients). *Nine patients with an increase of more than 300%
68Ga-PSMA-11 PET showed extensive disease (multiple metastatic lymph nodes or bone metastasis) in three patients after RPE with a mean PSA value of 1.01 ng/ml (range 0.08–2 ng/ml). These patients received ADT only, which resulted in a decrease in PSA level in all three (mean decrease 81%; Fig. 7). 68Ga-PSMA-11 PET was negative in 57 patients despite an elevated PSA level, leading to sRTX with or without ADT in 33 patients, while PSA levels were followed in 18 patients without therapy. PSA progression was seen in only 9 of the 33 patients (27%) treated despite a negative 68Ga-PSMA-11 PET scan, while progression was seen 13 of the 18 patients (72%) selected for PSA level follow-up, with an increase of up to 209% (from 4.65 to 14.39 ng/ml).
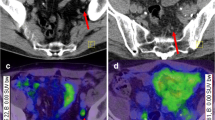
A patient selected for curative salvage radiotherapy before the scan with a PSA value of 0.96 ng/ml. Imaging was positive for lymph node metastasis (not shown) and two bone metastases in the pelvis (yellow arrows). a68Ga-PSMA PET maximum intensity projection. b–g Transaxial PET, T1 MRI and fused PET/MRI images (b–d first lesion, e–g second lesion, respectively). The patient received androgen deprivation therapy instead of salvage radiotherapy with undetectable PSA 6 months later
Follow-up was available in 25 of the 27 patients with a PSA baseline value of <0.2 ng/ml. At follow-up PSA was undetectable in 9 of the 25 patients (36%), and a partial response was seen in 14 of the 25 patients (56%).
Metastasis-targeted treatment
Follow-up PSA levels were available in all 86 patients who underwent treatment on the basis of the 68Ga-PSMA-11 PET findings (59 RTXa, 6 surgery, 20 RTXa and ADT, and 1 surgery and ADT). PSA was undetectable in 22 patients (25%), and a complete response was seen in 40 patients (45%; Fig. 8). A partial response was seen in 54 patients (63%), and the PSA level decreased in 65 patients (76%). Figure 9 shows the relative changes in PSA values in all patients who underwent 68Ga-PSMA-11 PET-guided metastasis-targeted treatment.
A patient selected for androgen-deprivation therapy after radical prostatectomy and salvage radiotherapy before the scan with a PSA value of 0.28 ng/ml. Imaging showed two metastatic pelvic lymph nodes (yellow arrows). a68Ga-PSMA PET maximum intensity projection. b–g Transaxial 68Ga-PSMA PET, T1 MRI and PET/MRI fusion images (b–d first lesion, e–g second lesion, respectively). The two lymph nodes were resected and PSA was undetectable 6 months after the scan, without additional therapy
Overall relative decreases in PSA values from the time of the PSMA PET scan to follow-up at 6 months in 86 patients who underwent targeted treatment with additional ADT (pink bars, 21 patients) and without additional ADT (blue bars, 65 patients). One patient with bone metastases showed an increase in PSA level despite RTXa and ADT. ADT androgen-deprivation therapy, RTXa targeted radiotherapy. *Two patients with an increase of more than 300%
In seven patients surgical resection of 68Ga-PSMA-11-positive lymph nodes was performed. 68Ga-PSMA-11 PET detected a total of 14 suspicious lymph nodes with a median maximum standardized uptake value (SUVmax) of 10.1 (range 2.7–128), in three patients the lymph nodes were rated with low suspicion (SUVmax 2.7–4.2). and in these patients the dissected lymph nodes were negative. In the remaining four patients 68Ga-PSMA-11 PET detected 10 lymph nodes, and in these patients 11 lymph node metastases were confirmed on histopathology.
Local treatment
The 97 patients (48% of 203) who received local treatment without systemic therapy included 65 patients who received metastasis-targeted treatment (59 RTXa, 6 surgery) and 32 who received sRTX. A complete response was seen in 44 patients (45%).
In 28 patients (29%) who received local treatment only, the PSA level had increased after 6 months. Follow-up imaging was available in five patients: in two patients the second 68Ga-PSMA-11 scan was still negative, in two patients the second 68Ga-PSMA-11 PET scan showed a previously negative lymph node to be positive for metastasis, and in one patient distant metastasis remained PSMA-positive after treatment. Details of the responses to the various selected therapies are given in Table 2.
Discussion
68Ga-PSMA-11 PET led to a change in management in 60% of our patients. This is in accordance with the findings of a meta-analysis by Han et al. that showed a change in management in 54% of patients [8]. We observed a decrease in systemic treatment and an increase in the number of local treatments after 68Ga-PSMA-11 PET, which has also been observed by other authors [7, 14, 16, 28]. Based on positive 68Ga-PSMA-11 PET findings, metastasis-targeted treatment was performed in 43% of the patients, leading to a substantial number of complete responses (40 of 88 patients (45%), reached a PSA value of <0.2 ng/ml). Including patients who underwent sRTX, 48% of all patients had local treatment only, and 45% of these patients showed a complete response.
The observed impact on patient management was mainly based on the high 68Ga-PSMA-11 PET detection rate (74% in our cohort), which remained good (around 50%) even in patients with a PSA value of <0.5 ng/ml. This detection rate is consistent with those found in large retrospective studies [1, 3, 29]. Compared with the findings of Afaq et al. [30] and Sterzing et al. [31], who found management changes in 39% and 51% of patients, respectively, the impact on management was slightly higher in our cohort. However, only 47% of the scans in the cohort of Afaq et al. were positive, a significantly lower proportion than our rate (74%), and the cohort of Sterzing et al. differed from ours in that it included patients imaged for initial staging. It is known that PSMA status, and therefore the PSMA PET detection rate, can be altered by ADT. Initial results suggest a downregulation of PSMA expression in patients on long-term androgen deprivation [32]. However, more recent preclinical studies have shown that short-term androgen deprivation can lead to an increase in PSMA expression in prostate cancer cells [33, 34]. Afshar-Oromieh et al. have shown that PSMA uptake decreases in patients on long-term ADT, reducing the sensitivity of PSMA PET for detecting prostate cancer lesions, and these authors suggested that patients should be scanned before the start of ADT [35]. In our study, 35 of 223 patients (15.6%) received ADT at some point before the scan and with again rising PSA levels. The detection rate in this subgroup was higher than in the rest of the cohort (88% vs. 74%), with a positive scan in 31 of the 35 patients.
In 41 (31%) of 133 patients in whom systemic treatment was planned before the 68Ga-PSMA-11 PET, treatment was converted to local treatment (RTXa, surgery or sRTX, without additional ADT). This represents 21% of the whole cohort (41/203), and a complete response was seen in 13 of these 41 patients (32%). Hope et al. found a similar conversion rate to local therapy of 32% in 126 patients with biochemical recurrence [36]. However, they included patients with additional ADT in the group receiving local therapy. Applying the same criteria would increase the conversion rate in our population from 31% to 41%.
Notwithstanding the observed tendency towards an increase in metastasis-targeted treatment and local treatments, we observed many different patient pathways from the intended therapy before PET to the therapy implemented after 68Ga-PSMA-11 (Fig. 5). As also observed by Han et al. [8] in their meta-analysis, in our cohort, 68Ga-PSMA-11 PET likewise contributed to a more personalized approach to patient management, allowing the physician to select and combine therapy modalities based on the location and extension of disease in each patient.
In 90 patients of our cohort, sRTX alone would have been planned before 68Ga-PSMA-11 PET. In 42 patients (47%) 68Ga-PSMA-11 PET showed positive disease outside the prostate bed. Therefore, theoretically, in almost half of the patients sRTX confined to the prostate bed would not have included PSMA-positive recurrence, probably leading to treatment failure. In a cohort of 70 patients, Van Leeuwen et al. found that 28.6% of PSMA-positive lesions were located in either regional lymph nodes or bone and would not have been included in a conventional sRTX field to the prostatic bed [37]. However, their cohort had an overall lower PSA level, with 90% of the patients having a PSA value of <0.5 ng/ml, while our cohort had a mean PSA value of 0.57 ng/ml. Also Schmidt-Hegemann et al. [38] found that a lower proportion of patients (20%) had pelvic lymph node metastasis, but again among patients with a maximum PSA value of 0.5 ng/ml.
The high detection of PSMA-positive lesions outside the prostate bed raises the expectation that outcomes following RTXa might be substantially better than following sRTX. Among the 65 patients who underwent PSMA PET-directed metastasis-targeted treatment without additional ADT, 27 (42%) achieved a complete response. In the subgroup of patients with PSMA PET-directed metastasis-targeted treatment in whom sRTX alone was planned before PET, the proportion of patients achieving a complete response increases to 56%.
The success of sRTX depends on the PSA level before sRTX [39, 40] and the presence of risk factors [41]. In patients with high PSA levels and unfavourable prognostic factors, the 5–7-year progression-free survival after SRT ranges from 32% to 45% [40,41,42,43,44,45,46] and the 10-ear progression-free survival can be as low as 22% [44]. In our cohort of 78 patients in whom sRTX alone was planned before the scan with follow-up data, only 27 patients actually received sRTX, and 34 had metastasis--targeted treatment, and 44 of these 78 patients (56%) had a complete response. In a recent randomized multicentre trial including 374 patients with PSA values between 0.2 ng/ml and 2 ng/ml, Carrie et al. found a 5-year progression-free survival rate of 62% among patients receiving sRTX alone [47]. However, comparing the two cohorts, our patients selected for sRTX before 68Ga-PSMA-11 PET had more aggressive disease: 31% versus 11% had a Gleason score of 8 or above, and 40% versus 18% had a PSA value of ≥0.5 ng/ml.
Although the follow-up in our study was short in comparison with studies assessing progression-free survival, there is evidence that early PSA values after therapy are predictive of outcome [23, 48,49,50]. In addition, long-term disease-free survival in patients undergoing only PET imaging-targeted salvage lymphadenectomy has been reported, with 5 of 11 patients having a PSA value of ≤0.03 ng/ml after a mean follow-up of 41 months [51]. Therefore, the high rate of complete responses in our cohort might reflect the contribution of more assertive therapies after 68Ga-PSMA PET, including metastasis-targeted treatment.
Finally, regarding the question as to whether therapy should be postponed on the basis of a negative PSMA PET scan, our results showed a PSA progression rate of 72% in patients who underwent PSA follow-up only. This is in accordance with recently published data of Emmett et al. who also found a poorer outcome in patients who did not receive sRTX after a negative PSMA PET scan compared with patients who received sRTX [13]. This further strengthens the hypothesis that a negative PSMA PET scan should not be a reason to withhold active treatment in light of the high response rate of sRTX in patients with a low PSA level [47, 52].
Improved laboratory tests now allow more sensitive and accurate detection of PSA recurrence. The use of a PSA value of 0.2 ng/ml as a cut-off value for a complete response is therefore controversial. However, to enable comparison with previously reported results, this study included the PSA cut-off value of 0.2 ng/ml for a complete response, and added the results for undetectable PSA (0.03 ng/ml).
A limitation of this study was that, despite careful consideration of known pitfalls in PSMA PET such as second primary, sarcoidosis, neural ganglia and haemangioma [26, 27], false-positive findings cannot be excluded with certainty, since most lesions were not assessable by histopathology. There are other limitations to this study, such as the already discussed short follow-up of 6 months in our cohort, the heterogeneity of the cohort and the hypothetical assessment of the therapy option before the 68Ga-PSMA-11 PET scan. However, we believe that a sufficient number of patients were included to allow adequate analysis for the purposes of the study, and to show the impact of 68Ga-PSMA-11 PET in clinical routine in different disease contexts. Because many patients were not treated in our institution, changes in RT dose and target volume were not assessed in detail, and therefore changes in target volume and dose modulation were not considered as changes in management in our cohort. As RTX protocols vary widely among institutions, the analysis of these data would be of questionable value.
It is important to point out that this study investigated the impact of PSMA findings in daily practice today, with some insights into treatment response. More prospective evaluations are still needed to prove the overall benefit of these strategy changes in our patients.
Conclusion
The high rate of detection of recurrent prostate cancer with 68Ga-PSMA-11 PET was confirmed in the current study and translated into a change in management in 60% of patients. Metastasis-targeted therapy options, most commonly RTXa, in patients with PSMA-positive lesions resulted in complete responses in 45% of patients, and this may be an interesting new approach to individualized therapy. However, prospective clinical trials demonstrating the long-term benefit of this approach including improvement in overall survival are currently lacking. In the setting of postoperative biochemical recurrence, a negative PSMA PET scan should not be a contraindication to the use of sRTX, which is in accordance with current guidelines.
References
Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–68. https://doi.org/10.1007/s00259-017-3711-7.
Eissa A, El Sherbiny A, Coelho RF, Rassweiler J, Davis JW, Porpiglia F, et al. The role of 68Ga-PSMA PET/CT scan in biochemical recurrence after primary treatment for prostate cancer: a systematic review of literature. Minerva Urol Nefrol. 2018;70:462–78. https://doi.org/10.23736/S0393-2249.18.03081-3.
Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–90. https://doi.org/10.2967/jnumed.115.160382.
Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, et al. Comparison of (68)Ga-labelled PSMA-11 and (11)C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:92–101. https://doi.org/10.1007/s00259-016-3490-6.
Calais J, Czernin J, Cao M, Kishan AU, Hegde JV, Shaverdian N, et al. (68)Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–7. https://doi.org/10.2967/jnumed.117.201749.
Roach PJ, Francis R, Emmett L, Hsiao E, Kneebone A, Hruby G, et al. The impact of (68)Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. J Nucl Med. 2018;59:82–8. https://doi.org/10.2967/jnumed.117.197160.
Calais J, Fendler WP, Eiber M, Gartmann J, Chu FI, Nickols NG, et al. Impact of (68)Ga-PSMA-11 PET/CT on the management of prostate cancer patients with biochemical recurrence. J Nucl Med. 2018;59:434–41. https://doi.org/10.2967/jnumed.117.202945.
Han S, Woo S, Kim YJ, Suh CH. Impact of (68)Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2018;74:179–90. https://doi.org/10.1016/j.eururo.2018.03.030.
Mena E, Lindenberg ML, Shih JH, Adler S, Harmon S, Bergvall E, et al. Clinical impact of PSMA-based (18)F-DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur J Nucl Med Mol Imaging. 2018;45:4–11. https://doi.org/10.1007/s00259-017-3818-x.
Kranzbuhler B, Nagel H, Becker AS, Muller J, Huellner M, Stolzmann P, et al. Clinical performance of (68)Ga-PSMA-11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur J Nucl Med Mol Imaging. 2018;45:20–30. https://doi.org/10.1007/s00259-017-3850-x.
Zschaeck S, Wust P, Beck M, Wlodarczyk W, Kaul D, Rogasch J, et al. Intermediate-term outcome after PSMA-PET guided high-dose radiotherapy of recurrent high-risk prostate cancer patients. Radiat Oncol. 2017;12:140. https://doi.org/10.1186/s13014-017-0877-x.
Bluemel C, Linke F, Herrmann K, Simunovic I, Eiber M, Kestler C, et al. Impact of (68)Ga-PSMA PET/CT on salvage radiotherapy planning in patients with prostate cancer and persisting PSA values or biochemical relapse after prostatectomy. EJNMMI Res. 2016;6:78. https://doi.org/10.1186/s13550-016-0233-4.
Emmett L, van Leeuwen PJ, Nandurkar R, Scheltema MJ, Cusick T, Hruby G, et al. Treatment outcomes from (68)Ga-PSMA PET/CT-informed salvage radiation treatment in men with rising PSA after radical prostatectomy: prognostic value of a negative PSMA PET. J Nucl Med. 2017;58:1972–6. https://doi.org/10.2967/jnumed.117.196683.
Grubmuller B, Baltzer P, D'Andrea D, Korn S, Haug AR, Hacker M, et al. (68)Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy - diagnostic performance and impact on therapeutic decision-making. Eur J Nucl Med Mol Imaging. 2018;45:235–42. https://doi.org/10.1007/s00259-017-3858-2.
Schmidt-Hegemann NS, Fendler WP, Ilhan H, Herlemann A, Buchner A, Stief C, et al. Outcome after PSMA PET/CT based radiotherapy in patients with biochemical persistence or recurrence after radical prostatectomy. Radiat Oncol. 2018;13:37. https://doi.org/10.1186/s13014-018-0983-4.
Henkenberens C, von Klot CA, Ross TL, Bengel FM, Wester HJ, Katja H, et al. (68)Ga-PSMA ligand PET/CT-based radiotherapy for lymph node relapse of prostate cancer after primary therapy delays initiation of systemic therapy. Anticancer Res. 2017;37:1273–9. https://doi.org/10.21873/anticanres.11444.
Wait S, Han D, Muthu V, Oliver K, Chrostowski S, Florindi F, et al. Towards sustainable cancer care: reducing inefficiencies, improving outcomes – a policy report from the All.Can initiative. J Cancer Policy. 2017;13:47–64.
Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–28. https://doi.org/10.1093/jnci/djq495.
Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R, et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2018;73:178–211. https://doi.org/10.1016/j.eururo.2017.06.002.
Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. https://doi.org/10.1016/j.eururo.2016.08.002.
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29. https://doi.org/10.1016/j.eururo.2016.08.003.
Kang J, Reiter R, Steinberg M, King C. Ultra-sensitive PSA following prostatectomy reliably identifies patients requiring postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 2014;90:S130–S1.
Chang JH, Park W, Park JS, Pyo H, Huh SJ, Choi HY, et al. Significance of early prostate-specific antigen values after salvage radiotherapy in recurrent prostate cancer patients treated with surgery. Int J Urol. 2015;22:82–7. https://doi.org/10.1111/iju.12604.
Fendler WP, Rahbar K, Herrmann K, Kratochwil C, Eiber M. (177)Lu-PSMA radioligand therapy for prostate cancer. J Nucl Med. 2017;58:1196–200. https://doi.org/10.2967/jnumed.117.191023.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. Ga-68-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–24. https://doi.org/10.1007/s00259-017-3670-z.
Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38:200–17. https://doi.org/10.1148/rg.2018170108.
Fendler WP, Calais J, Allen-Auerbach M, Bluemel C, Eberhardt N, Emmett L, et al. (68)Ga-PSMA-11 PET/CT Interobserver agreement for prostate cancer assessments: an international multicenter prospective study. J Nucl Med. 2017;58:1617–23. https://doi.org/10.2967/jnumed.117.190827.
Albisinni S, Artigas C, Aoun F, Biaou I, Grosman J, Gil T, et al. Clinical impact of (68)Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach. BJU Int. 2017;120:197–203. https://doi.org/10.1111/bju.13739.
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–74. https://doi.org/10.2967/jnumed.115.154153.
Afaq A, Alahmed S, Chen SH, Lengana T, Haroon A, Payne H, et al. Impact of (68)Ga-prostate-specific membrane antigen PET/CT on prostate cancer management. J Nucl Med. 2018;59:89–92. https://doi.org/10.2967/jnumed.117.192625.
Sterzing F, Kratochwil C, Fiedler H, Katayama S, Habl G, Kopka K, et al. (68)Ga-PSMA-11 PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2016;43:34–41. https://doi.org/10.1007/s00259-015-3188-1.
Liu T, Wu LY, Fulton MD, Johnson JM, Berkman CE. Prolonged androgen deprivation leads to downregulation of androgen receptor and prostate-specific membrane antigen in prostate cancer cells. Int J Oncol. 2012;41:2087–92. https://doi.org/10.3892/ijo.2012.1649.
Meller B, Bremmer F, Sahlmann CO, Hijazi S, Bouter C, Trojan L, et al. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015;5:66. https://doi.org/10.1186/s13550-015-0145-8.
Kranzbuhler B, Salemi S, Umbricht CA, Muller C, Burger IA, Sulser T, et al. Pharmacological upregulation of prostate-specific membrane antigen (PSMA) expression in prostate cancer cells. Prostate. 2018;78:758–65. https://doi.org/10.1002/pros.23522.
Afshar-Oromieh A, Debus N, Uhrig M, Hope TA, Evans MJ, Holland-Letz T, et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:2045–54.. https://doi.org/10.1007/s00259-018-4079-z.
Hope TA, Aggarwal R, Chee B, Tao D, Greene KL, Cooperberg MR, et al. Impact of (68)Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med. 2017;58:1956–61. https://doi.org/10.2967/jnumed.117.192476.
van Leeuwen PJ, Stricker P, Hruby G, Kneebone A, Ting F, Thompson B, et al. (68)Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016;117:732–9. https://doi.org/10.1111/bju.13397.
Schmidt-Hegemann NS, Fendler WP, Buchner A, Stief C, Rogowski P, Niyazi M, et al. Detection level and pattern of positive lesions using PSMA PET/CT for staging prior to radiation therapy. Radiat Oncol. 2017;12:176. https://doi.org/10.1186/s13014-017-0902-0.
King CR. Adjuvant versus salvage radiotherapy for high-risk prostate cancer patients. Semin Radiat Oncol. 2013;23:215–21. https://doi.org/10.1016/j.semradonc.2013.01.009.
Tendulkar RD, Agrawal S, Gao T, Efstathiou JA, Pisansky TM, Michalski JM, et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016;34:3648–54. https://doi.org/10.1200/JCO.2016.67.9647.
Stephenson AJ, Shariat SF, Zelefsky MJ, Kattan MW, Butler EB, The BS, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–32. https://doi.org/10.1001/jama.291.11.1325.
Song DY, Thompson TL, Ramakrishnan V, Harrison R, Bhavsar N, Onaodowan O, et al. Salvage radiotherapy for rising or persistent PSA after radical prostatectomy. Urology. 2002;60:281–7.
Goenka A, Magsanoc JM, Pei X, Schechter M, Kollmeier M, Cox B, et al. Long-term outcomes after high-dose postprostatectomy salvage radiation treatment. Int J Radiat Oncol Biol Phys. 2012;84:112–8. https://doi.org/10.1016/j.ijrobp.2011.10.077.
Pacholke HD, Wajsman Z, Algood CB, Neulander EZ, Morris CG, Zlotecki RA. Postoperative adjuvant and salvage radiotherapy for prostate cancer: impact on freedom from biochemical relapse and survival. Urology. 2004;64:982–6. https://doi.org/10.1016/j.urology.2004.06.020.
Liauw SL, Webster WS, Pistenmaa DA, Roehrborn CG. Salvage radiotherapy for biochemical failure of radical prostatectomy: a single-institution experience. Urology. 2003;61:1204–10.
Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41. https://doi.org/10.1200/JCO.2006.08.9607.
Carrie C, Hasbini A, de Laroche G, Richaud P, Guerif S, Latorzeff I, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17:747–56. https://doi.org/10.1016/S1470-2045(16)00111-X.
Bartkowiak D, Bottke D, Thamm R, Siegmann A, Hinkelbein W, Wiegel T. The PSA-response to salvage radiotherapy after radical prostatectomy correlates with freedom from progression and overall survival. Radiother Oncol. 2016;118:131–5. https://doi.org/10.1016/j.radonc.2015.10.028.
Ray ME, Thames HD, Levy LB, Horwitz EM, Kupelian PA, Martinez AA, et al. PSA nadir predicts biochemical and distant failures after external beam radiotherapy for prostate cancer: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2006;64:1140–50. https://doi.org/10.1016/j.ijrobp.2005.07.006.
Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol. 2006;24:3984–90. https://doi.org/10.1200/Jco.2006.06.4246.
Winter A, Henke RP, Wawroschek F. Targeted salvage lymphadenectomy in patients treated with radical prostatectomy with biochemical recurrence: complete biochemical response without adjuvant therapy in patients with low volume lymph node recurrence over a long-term follow-up. BMC Urol. 2015;15:10. https://doi.org/10.1186/s12894-015-0004-y.
Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376:417–28. https://doi.org/10.1056/NEJMoa1607529.
Acknowledgments
The authors acknowledge the technicians Josephine Trinckauf and Marlena Hofbauer and their team for the excellent work in producing high-quality PET images.
Funding
The Department of Nuclear Medicine holds an institutional Research Contract with GE Healthcare. The authors thank the Sick legat and the Iten-Kohaut Foundation for their financial support.
Author information
Authors and Affiliations
Contributions
Julian Müller – data collection, manuscript editing.
Daniela A. Ferraro – data collection, manuscript editing.
Urs J. Muehlematter – statistics, data analysis.
Helena I. Garcia Schüler – data analysis, manuscript editing.
Sarah Kedzia - data collection, data analysis, manuscript editing.
Daniel Eberli, Matthias Guckenberger, Stephanie G. C. Kroeze, Tullio Sulser, Daniel M. Schmid, Aurelius Omlin, Alexander Müller, Thomas Zilli, Hubert John, Helmut Kranzbuehler – patient assessment, data analysis.
Philipp A. Kaufmann, Gustav K. von Schulthess, Irene A. Burger – study design and manuscript editing.
All authors reviewed and agreed to the manuscript content.
Corresponding author
Ethics declarations
Conflicts of interest
I.A.B., G.K.v.S. and P.A.K. received research grants and speaker honoraria from GE Healthcare. I.A.B. received research grants from Swiss Life and speaker honoraria from Bayer Health Care and Astellas Pharma AG. M.G. received research grants from Varian. A.O. has an advisory role (compensated, institutional): Astellas, Bayer, Sanofi, Roche, Janssen, MSD, Molecular Partners. Research support (institutional): Teva, Janssen. Travel support: Astellas, Bayer, Sanofi, Janssen. Speaker Bureau (compensated, institutional): Astellas, Janssen, Bayer. All other authors declare no conflicts of interest.
Ethical approval and consent to participate
The local ethics committee approved the study protocol and all patients gave general written informed consent for retrospective use of their data (BASEC Nr. 2018–01284).
Consent for publication
Not applicable.
Availability of data and material
Patient imaging was done in the scope of routine clinical diagnostic studies, and the raw data are stored in the hospital archiving system at the Zurich University Hospital, Zurich, Switzerland.
Rights and permissions
About this article
Cite this article
Müller, J., Ferraro, D.A., Muehlematter, U.J. et al. Clinical impact of 68Ga-PSMA-11 PET on patient management and outcome, including all patients referred for an increase in PSA level during the first year after its clinical introduction. Eur J Nucl Med Mol Imaging 46, 889–900 (2019). https://doi.org/10.1007/s00259-018-4203-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4203-0