Abstract
Purpose
We present our first clinical experience with O-(2-18F-fluoroethyl)-l-tyrosine (FET) PET in patients with high-grade glioma treated with various neurooncological therapies including tumour-treating fields (TTFields) for the differentiation of tumour progression from treatment-related changes.
Methods
We retrospectively assessed 12 patients (mean age 51 ± 12 years, range 33–72 years) with high-grade glioma (11 glioblastomas, 1 gliosarcoma) in whom the treatment regimen included TTFields and who had undergone FET PET scans for differentiation of tumour progression from treatment-related changes. Mean and maximum tumour-to-brain ratios (TBRmean, TBRmax) were calculated. The definitive diagnosis (tumour progression or posttherapeutic changes) was confirmed either by histopathology (4 of 12 patients) or on clinical follow-up.
Results
In all nine patients with confirmed tumour progression, the corresponding FET PET showed increased uptake (TBRmax 3.5 ± 0.6, TBRmean 2.7 ± 0.7). In one of these nine patients, FET PET was consistent with treatment-related changes, whereas standard MRI showed a newly diagnosed contrast-enhancing lesion. In two patients treated solely with TTFields without any other concurrent neurooncological therapy, serial FET PET revealed a decrease in metabolic activity over a follow-up of 6 months or no FET uptake without any signs of tumour progression or residual tumour on conventional MRI.
Conclusion
FET PET may add valuable information in monitoring therapy in individual patients with high-grade glioma undergoing neurooncological treatment including TTFields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) is the most common form of glioma and is also one of the most aggressive and lethal primary brain tumours, with a median survival of only 15–20 months despite maximal aggressive and multimodal therapy [1,2,3]. Thus, current therapeutic approaches provide modest improvement in progression-free and overall survival, necessitating the investigation of novel therapies.
Tumour-treating fields (TTFields) deliver low-intensity, alternating electric energy at an intermediate frequency of 200 kHz as a locoregional intervention that inhibits cell division and causes neoplastic cell death with minimal effect on normal quiescent cells [4]. It has been demonstrated that in patients with newly diagnosed GBM who have completed standard chemoradiation therapy, adding TTFields to maintenance (adjuvant) temozolomide chemotherapy significantly prolongs progression-free and overall survival [5, 6]. Furthermore, TTFields treatment is also used in patients with progressive GBM [7].
Standard MRI, including contrast-enhanced T1-weighted and T2-/FLAIR-weighted sequences, is the method of choice for brain tumour diagnostics and follow-up. In particular, changes in the extent of contrast enhancement on MRI are used as an indicator of therapy response or tumour progression [8, 9]. However, treatment-related changes such as pseudoprogression and radiation necrosis can cause disruption of the blood–brain barrier, resulting in nonspecific contrast enhancement on MRI [10,11,12]. Furthermore, blood–brain barrier breakdown may also result from postoperative inflammation, seizures, true tumour recurrence, or other treatment-related effects (e.g. immunotherapy). Thus, contrast enhancement resulting from increased blood–brain barrier permeability is nonspecific and may not always be an accurate surrogate for neoplastic tissue, tumour extent or treatment effect. Most importantly, treatment-related changes are of considerable importance in neurooncology because an effective treatment might be erroneously terminated too early with potentially negative effects on survival.
PET using biologically active molecules labelled with short-lived positron-emitting isotopes at micromolar or nanomolar concentrations is one of the most promising techniques for the imaging of specific molecular processes in vivo. Molecular imaging using PET may provide relevant additional information on tumour metabolism, and may also be helpful in clinical decision-making, especially in patients with equivocal MRI findings following neurooncological treatment [13, 14]. Furthermore, more widespread use of amino acid PET for the management of patients with brain tumours has been strongly recommended by the RANO group [15, 16]. The PET tracer O-(2-18F-fluoroethyl)-l-tyrosine (FET) is a well-established 18F-labelled amino acid (half-life 110 min) that shows logistic advantages over 11C-methyl-l-methionine for clinical practice [17]. The clinical value of FET PET for the identification of tumour relapse has been demonstrated in numerous studies including patients with gliomas as well as patients with brain metastasis [12, 18,19,20,21,22,23,24].
We present our first clinical experience with FET PET in patients with GBM treated with various neurooncological therapies including TTFields for the differentiation of tumour progression from treatment-related changes.
Materials and methods
Patients
We retrospectively assessed 12 patients (mean age 51 ± 12 years, range 33–72 years; four women and eight men) with high-grade glioma (11 GBMs, 1 gliosarcoma) in whom the treatment regimen included TTFields and who had undergone a FET PET scan for the differentiation of tumour progression from treatment-related changes. For FET PET imaging, patients were referred to the Forschungszentrum Juelich (seven patients) or to the Department of Nuclear Medicine, University of Essen (five patients). This retrospective study was approved by the local ethics committee, and all patients gave written informed consent before each FET PET investigation.
PET imaging
As described previously, the amino acid FET was produced via nucleophilic 18F-fluorination with a radiochemical purity of greater than 98%, a specific radioactivity greater than 200 GBq/μmol and a radiochemical yield of about 60% [25]. According to the German guidelines for brain tumour imaging using labelled amino acid analogues [26], all patients fasted for at least 4 h before the PET measurements. At the Forschungszentrum Juelich, patients underwent a dynamic PET scan from 0 to 50 min after injection of 3 MBq of FET per kg of body weight. PET imaging was performed either on an ECAT Exact HR+ PET scanner (11 scans) in three-dimensional mode (Siemens Medical Systems; axial field of view 15.5 cm, spatial resolution 6 mm) or using a BrainPET insert simultaneously with 3-T MR imaging (two scans). The BrainPET is a compact cylinder that fits in the bore of the Magnetom Trio MR scanner (axial field of view 19.2 cm, optimum spatial resolution 3 mm) [27]. Iterative reconstruction parameters were 16 subsets and six iterations using the OSEM algorithm for the ECAT HR+ PET scanner, and two subsets and 32 iterations using the OP-OSEM algorithm provided by the manufacturer of the BrainPET, with correction for random, scattered coincidences, and dead time for both systems. Attenuation correction for the ECAT HR+ PET scan was based on a transmission scan, and for the BrainPET scan on a template-based approach [27]. The reconstructed dynamic dataset consisted of 16 time frames (5 × 1, 5 × 3, 6 × 5 min).
At the Department of Nuclear Medicine, University of Essen, static FET PET imaging (five scans) was performed on a 3-T whole-body hybrid imaging system (Biograph mMR; Siemens Healthcare, Erlangen, Germany). For the evaluation of 18F-FET uptake, summed PET images over the period 20–40 min after injection were used for static data.
PET data analysis
Mean tumoral 18F-FET uptake was determined using a two-dimensional autocontouring process with a tumour-to-brain ratio (TBR) of at least 1.6. This cut-off was based on a biopsy-controlled study in cerebral gliomas and differentiated best between tumoral and peritumoral tissue [28]. In order to exclude any influence of the different resolutions of the HR+ scanner and the BrainPET scanner (Forschungszentrum Juelich), a circular region of interest (ROI) with a diameter of 1.6 cm was centred on the maximal tumour uptake [19] for evaluation of the maximal FET uptake. Mean and maximum TBR (TBRmean and TBRmax) were calculated by dividing the mean and maximum standardized uptake value (SUV) of the tumour ROI by the mean SUV of a larger ROI placed in the semioval centre of the contralateral unaffected hemisphere including the white and grey matter [26]. Tumour volumes on FET PET were calculated using a three-dimensional autocontouring process with a threshold of 1.6 using PMOD (version 3.505; PMOD Technologies Ltd.).
MR imaging
On suspicion of tumour progression, all patients underwent routine MRI (1.5 T or 3 T) with standard coils before and after administration of a gadolinium-based contrast agent (T1- and T2-weighted and FLAIR sequences). Diagnosis of tumour progression or recurrence was based on RANO criteria [8].
FET PET for differentiation of tumour progression from treatment-related changes
Based on the findings of a previous study investigating the potential of FET PET to differentiate tumour recurrence or progression from treatment-induced changes in a large series of patients with pretreated brain tumours [19], tumour progression as evaluated by FET PET was assumed when a TBRmax of ≥2.3 or a TBRmean of ≥2.0 was present. The histological diagnosis was used as the reference to confirm the FET PET-based diagnosis of tumour progression. If histology was not available, the diagnosis was confirmed on follow-up (i.e. clinical course and results of follow-up MRI). The presence of tumour progression was assumed when clinical worsening prompted a change in treatment, if palliative care had been initiated during follow-up. or if the patient died. Treatment-related changes were assumed when a TBRmax of <2.3 or a TBRmean of <2.0 was present. The diagnosis of treatment-related changes was confirmed on follow-up (i.e. clinical course and results of follow-up MRI) and was assumed if lesions showed spontaneous shrinkage or remained stable in size on contrast-enhanced MRI, and/or neurological deficits remained unchanged (i.e. no new neurological symptoms occurred during follow-up).
Results
All 12 patients received neurooncological treatment including TTFields during the course of the disease. Eight of the 12 patients were examined on suspicion of tumour relapse using FET PET. All of these eight patients had had previous tumour relapses prior to TTFields (one or two relapses each; Table 1). In the remaining four patients, TTFields was added to the first-line treatment regimen (patients 2, 7, 10 and 12). Two of these four patients were examined using FET PET on suspicion of tumour relapse, and the remaining two underwent baseline and follow-up FET PET imaging for treatment monitoring, and had no signs of tumour relapse (patients 2 and 7; Table 1).
Histopathological results for a definite diagnosis were available in four of the 12 patients. In the remaining eight patients, diagnosis of treatment-related changes or tumour progression was based on follow-up (clinical course and follow-up MRI). An overview of the patients’ characteristics is presented in Table 1.
In patients in whom the diagnosis confirmed tumour progression (nine patients), all corresponding FET PET scans showed increased uptake (TBRmax 3.5 ± 0.6, range 2.5–4.4; TBRmean 2.7 ± 0.7, range 2.0–4.0). In four of these nine patients, tumour progression was diagnosed histologically. Imaging and histology in a representative patient (patient 11) are presented in Fig. 1. In patients in whom the diagnosis of tumour progression was confirmed clinically (five of nine patients), the median follow-up was 4 months (range 3–6 months). Static data on FET uptake in the lesions are presented in Table 1. Furthermore, in three of these nine patients, a baseline FET PET scan prior to initiation of neurooncological treatment including TTFields was available (patients 3, 4 and 6; Table 1). Compared with baseline, either a significant increase in the metabolically active tumour volume (patient 4; Fig. 2) or a significant increase in TBR (patients 3 and 6) on FET PET were observed. In patient 3, TBRmax increased from 3.2 to 3.9 (22%) and TBRmean from 1.9 to 2.3 (21%). In patient 4, TBRmax increased from 1.9 to 2.8 (47%), and TBRmean from 1.7 to 1.9 (12%). In patient 4, the metabolically active tumour volume increased significantly from 9 ml (at baseline) to 42 ml (at 9 months; Fig. 2).
Contrast-enhanced MRI, FLAIR-weighted MRI and PET-MRI fusion images (top row) in a 55-year-old man with glioblastoma at the time of progression treated with lomustine and TTFields (patient 11). In line with the MRI findings, FET PET shows increased metabolic activity (TBRmax 4.4, TBRmean 4.0). Histology (haematoxylin and eosin stain, bottom) after resection is consistent with progressive glioblastoma
Hybrid PET/MR imaging including contrast-enhanced T1-weighted images and FET PET images in a 49-year-old man with glioblastoma at the time of recurrence and at follow-up 9 months later (patient 4). In spatial correspondence with the newly diagnosed contrast-enhancing lesion (bottom left), the FET PET image shows a metabolically active tumour (top left) with increased tumour-to-brain ratios (TBRmax 4.0, TBRmean 2.3) and a metabolically active tumour volume of 9 ml. Treatment with radiotherapy, bevacizumab and TTFields maintenance therapy was initiated. At 9 months, the MRI image shows signs of tumour progression (bottom right). The FET PET image shows a corresponding increase of almost fivefold in the metabolically active tumour volume to 42 ml (top right). The tumoral FET uptake is almost unchanged (TBRmax 4.0, TBRmean 2.1)
In two patients (patients 2 and 7), TTFields alone without any other concurrent neurooncological therapy was used as maintenance therapy. In patient 2, TTFields was started 5 months after completion of radiotherapy with concomitant and adjuvant temozolomide chemotherapy over six cycles. In this patient, TTFields was initiated at the patient’s personal request. Prior to TTFields and 5 months later, FET PET showed no increased uptake. In patient 7, TTFields was started 4 weeks after completion of radiotherapy with concomitant temozolomide chemotherapy. Due to an immune thrombocytopenia, adjuvant temozolomide chemotherapy could not be administered. Prior to TTFields, baseline FET PET showed slightly increased metabolic activity (TBRmax 2.0, TBRmean 1.6; Fig. 3). Follow-up serial FET PET imaging at 3 months and at 6 months showed a subsequent decrease in metabolic activity as indicated by a reduction in TBR (Fig. 3).
Imaging in a 73-year-old woman with glioblastoma (patient 7) prior to TTFields and during follow-up. Due to an immune thrombocytopenia, adjuvant temozolomide chemotherapy could not be administered. Thus, only TTF was administered and was started 4 weeks after completion of radiotherapy with concomitant temozolomide chemotherapy. Prior to TTFields, the baseline FET PET image shows slightly increased metabolic activity (TBRmax 2.0, TBRmean1.6) without spatially corresponding contrast enhancement. The follow-up serial FET PET images at 3 months and 6 months show a decrease in metabolic activity as indicated by reductions in tumour-to-brain ratios
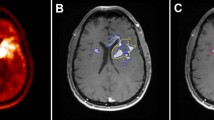
In contrast to standard MRI which suggested tumour recurrence (patient 1), FET PET findings were consistent with posttherapeutic changes (Fig. 4). This diagnosis was confirmed clinically; the follow-up was 6 months.
Hybrid PET/MR imaging including a contrast-enhanced T1-weighted images and FET PET images in a 44-year-old man with glioblastoma treated with temozolomide and TTFields (patient 1). In contrast to standard MRI which suggests tumour recurrence (top), the FET PET images show slightly increased tumour-to-brain ratios (TBRmax 2.2, TBRmean 1.8) are consistent with posttherapeutic changes (bottom)
Discussion
In the present study, we evaluated the use of FET PET for the differentiation of tumour progression from treatment-related changes in patients with high-grade gliomas in whom the treatment regimen included TTFields. The main finding was that in all patients in whom histology or clinical follow-up confirmed disease progression, FET PET showed increased uptake or an increase in metabolically active tumour volume (Table 1). Moreover, in one patient FET PET and MR imaging findings were discordant and consistent with treatment-related changes (Fig. 4). Thus, the combined use of TTFields and other treatment regimens leads to similar results with respect to the ability of FET PET to differentiate tumour progression from treatment-related effects. Furthermore, in two patients treated solely with TTFields (patients 2 and 7), either serial FET PET revealed a decline in metabolic activity over 6 months or FET PET showed no uptake without any signs of tumour progression or residual tumour on conventional MRI. These data support the hypothesis that FET PET can be used to measure response to TTFields.
For decades, in patients with brain tumours, changes in the extent contrast enhancement on MRI have traditionally been used as an indicator of therapy response or tumour relapse [8, 9]. However, contrast enhancement resulting from increased blood–brain barrier permeability is nonspecific and may not always be an accurate surrogate for neoplastic tissue, tumour extent or treatment-related changes [10, 29, 30]. In order to help determine tumour progression, the use of FLAIR or T2 signal hyperintensity as a surrogate marker for nonenhancing tumour has been recommended [8]. However, differential diagnoses such as tumour-related oedema, radiation injury, demyelination, ischaemia, and infection can also result in hyperintense FLAIR or T2 signal hyperintensity, which is difficult to distinguish from nonenhancing tumour [30].
In neurooncology, many treatments may cause benign treatment-related effects that are difficult to differentiate from true tumour progression on conventional MRI. For example, treatment-related changes have been observed during and after various radiotherapy treatments (e.g. external fractionated radiotherapy, radiosurgery), chemoradiation with concurrent temozolomide, antiangiogenic therapy, and immunotherapy by blocking immune checkpoints such as CTLA-4 (cytotoxic T lymphocyte-associated antigen 4) and PD-1 (programmed cell death 1 receptor) [14, 31,32,33,34,35,36].
TTFields is increasingly being used for the treatment of patients with newly diagnosed GBM as well as for the treatment of patients with recurrent high-grade glioma. Additionally, in the USA the FDA has recently approved the use of TTFields for the treatment of newly diagnosed GBM. The postulated mechanism of the antitumoral effect involves the disruption of microtubule assembly during mitosis induced by the low-intensity, alternating electric energy at an intermediate frequency of 200 kHz. This blocks formation of the mitotic spindle apparatus, resulting in inhibition of cell division and neoplastic cell death [4]. Nevertheless, the effects of this postulated antitumoral mechanism on neoplastic cells especially on neuroimaging are still unclear. Additionally, data on therapy monitoring, in particular the assessment of treatment response and the differentiation between tumour progression and treatment-related changes, in patients undergoing neurooncological treatment including TTFields or TTFields therapy alone are scarce.
In order to overcome the limitations of conventional MRI, alternative imaging methods have been used for the evaluation of treatment response in patients undergoing neurooncological treatment including TTFields. In 2016, in a case report, Mohan et al. [37] described the assessment of treatment response in a patient with newly diagnosed GBM using advanced MRI techniques including perfusion-weighted MRI, diffusion tensor imaging and proton MR spectroscopy. In their patient, first-line chemoradiation with concurrent temozolomide was completed 5 months prior to TTFields initiation, and adjuvant treatment consisted a low-dose temozolomide maintenance therapy. The patient underwent serial MRI scans including a baseline scan (prior to TTFields) and two follow-up scans (1 and 2 months after initiation of TTFields). At follow-up, an increase in mean diffusivity, and decreases in fractional anisotropy, relative cerebral blood volume (rCBV) and choline/creatine ratio relative to baseline imaging were observed. The authors suggested that the changes in advanced MRI metrics are of value in the assessment of early treatment response to TTFields in combination with maintenance temozolomide chemotherapy. However, in the patient a correlation between changes in imaging parameters and outcome was not found. Furthermore, poor image quality and artefacts partially hampered advanced evaluation of the MRI data [37].
Molecular imaging using amino acid PET provides relevant additional information on tumour metabolism and is therefore helpful in clinical decision-making, especially if the MRI findings are equivocal following neurooncological treatment [13,14,15]. Usually, amino acid uptake is increased in tumour tissue but low or absent in treatment-related changes [18,19,20, 36, 38]. In view of the described limitations of conventional MRI, molecular imaging can also provide valuable information for the evaluation of treatment response [39,40,41]. Furthermore, the Response Assessment in Neuro-Oncology (RANO) Working Group has recently analysed the clinical role of amino acid PET in the diagnostic assessment of brain tumours, and strongly recommends the additional use of this imaging technique at every stage of brain tumour management [15].
For the evaluation of treatment response in patients with recurrent GBM undergoing TTFields therapy, the amino acid PET tracer α-11C-methyl-l-tryptophan (AMT) has been used [42, 43]. All patients underwent baseline and follow-up AMT PET imaging prior to and 1.5–3 months after initiation of TTFields therapy. In the majority of patients, objective responses in terms of metabolic tumour volume reduction were observed. The authors suggested that this decrease was related to TTFields therapy. However, all patients had other concurrent neurooncological treatment (predominantly temozolomide and bevacizumab) and it cannot be excluded that the observed reduction in metabolic tumour volumes was an effect of these therapies.
Interestingly, in the present study, in a GBM patient treated with TTFields alone we observed a decrease in metabolic activity on serial FET PET imaging (Fig. 3). This could be interpreted as a direct effect of TTFields. However, chemoradiation was completed only 4 weeks prior to initiation of TTFields. Thus, despite the fact that this patient was treated solely with TTFields, it cannot be excluded that the observed decrease in metabolic activity was an effect of chemoradiation. In another patient (patient 2) treated solely with TTFields, maintenance therapy initiated 5 months after completion of radiotherapy with concomitant and adjuvant temozolomide chemotherapy over six cycles, FET PET showed no increased uptake at baseline or on follow-up. Correspondingly, MRI showed no contrast enhancement at either time point.
Besides its retrospective character with a low number of patients, a further limitation of the present study is that the majority of patients were receiving various concurrent treatment regimens (i.e. alkylating chemotherapy, antiangiogenic therapy, radiotherapy, and combinations thereof), impeding the evaluation of the effects of TTFields using amino acid PET. However, the dataset represents a common clinical situation, and to the best of our knowledge, there is to date no larger study in the literature concerning this topic.
In summary, our findings suggest that FET PET is a reliable diagnostic tool in patients undergoing neurooncological treatment including TTFields and may add valuable additional information, particularly in patients with treatment-related changes as well as in patients treated solely with TTFields, i.e. for treatment monitoring. Further studies are warranted to confirm the clinical usefulness of FET PET in patients undergoing neurooncological treatment including TTFields or TTFields therapy alone.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96.
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22.
Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18:1373–85.
Pless M, Weinberg U. Tumor treating fields: concept, evidence and future. Expert Opin Investig Drugs. 2011;20:1099–106.
Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314:2535–43.
Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318:2306–16.
Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–202.
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010;28:1963–72.
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–80.
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217:377–84.
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–61.
Ceccon G, Lohmann P, Stoffels G, Judov N, Filss CP, Rapp M, et al. Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro Oncol. 2017;19:281–8.
Galldiks N, Langen KJ, Pope WB. From the clinician’s point of view – what is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol. 2015;17:1434–44.
Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13:279–89.
Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18:1199–208.
Langen KJ, Watts C. Neuro-oncology: amino acid PET for brain tumours – ready for the clinic? Nat Rev Neurol. 2016;12:375–6.
Langen KJ, Hamacher K, Weckesser M, Floeth F, Stoffels G, Bauer D, et al. O-(2-[18F]fluoroethyl)-L-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol. 2006;33:287–94.
Galldiks N, Dunkl V, Stoffels G, Hutterer M, Rapp M, Sabel M, et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015;42:685–95.
Galldiks N, Stoffels G, Filss C, Rapp M, Blau T, Tscherpel C, et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015;17:1293–300.
Kebir S, Fimmers R, Galldiks N, Schafer N, Mack F, Schaub C, et al. Late Pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin Cancer Res. 2016;22:2190–6.
Lohmann P, Stoffels G, Ceccon G, Rapp M, Sabel M, Filss CP, et al. Radiation injury vs. recurrent brain metastasis: combining textural feature radiomics analysis and standard parameters may increase 18F-FET PET accuracy without dynamic scans. Eur Radiol. 2017;27:2916–27.
Kebir S, Rauschenbach L, Galldiks N, Schlaak M, Hattingen E, Landsberg J, et al. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET imaging for the detection of checkpoint inhibitor-related pseudoprogression in melanoma brain metastases. Neuro Oncol. 2016;18:1462–4.
Romagna A, Unterrainer M, Schmid-Tannwald C, Brendel M, Tonn JC, Nachbichler SB, et al. Suspected recurrence of brain metastases after focused high dose radiotherapy: can [18F]FET-PET overcome diagnostic uncertainties? Radiat Oncol. 2016;11:139.
Pöpperl G, Götz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K. Value of O-(2-[18F]fluoroethyl)-L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging. 2004;31:1464–70.
Hamacher K, Coenen HH. Efficient routine production of the 18F-labelled amino acid O-2-18F fluoroethyl-L-tyrosine. Appl Radiat Isot. 2002;57:853–6.
Langen KJ, Bartenstein P, Boecker H, Brust P, Coenen HH, Drzezga A, et al. German guidelines for brain tumour imaging by PET and SPECT using labelled amino acids. Nuklearmedizin. 2011;50:167–73.
Herzog H, Langen KJ, Weirich C, Rota Kops E, Kaffanke J, Tellmann L, et al. High resolution BrainPET combined with simultaneous MRI. Nuklearmedizin. 2011;50:74–82.
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Müller HW, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128:678–87.
Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9:906–20.
Ahluwalia MS, Wen PY. Antiangiogenic therapy for patients with glioblastoma: current challenges in imaging and future directions. Expert Rev Anticancer Ther. 2011;11:653–6.
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20.
Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16:e534–42.
Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11:504–14.
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–7.
Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22:633–8.
Galldiks N, Kocher M, Langen KJ. Pseudoprogression after glioma therapy: an update. Expert Rev Neurother. 2017;17:1109–15.
Mohan S, Chawla S, Wang S, Verma G, Skolnik A, Brem S, et al. Assessment of early response to tumor-treating fields in newly diagnosed glioblastoma using physiologic and metabolic MRI: initial experience. CNS Oncol. 2016;5:137–44.
Rachinger W, Goetz C, Pöpperl G, Gildehaus FJ, Kreth FW, Holtmannspotter M, et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57:505–11.
Yang I, Aghi MK. New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol. 2009;6:648–57.
Galldiks N, Langen K, Holy R, Pinkawa M, Stoffels G, Nolte K, et al. Assessment of treatment response in patients with glioblastoma using [18F]Fluoroethyl-L-tyrosine PET in comparison to MRI. J Nucl Med. 2012;53:1048–57.
Galldiks N, Rapp M, Stoffels G, Fink GR, Shah NJ, Coenen HH, et al. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40:22–33.
Mittal S, Barger GR, Bosnyák E, Shah VD, Juhász C. Early metabolic response to tumor-treating fields in patients with recurrent glioblastoma. Cancer Res. 2017;77(13 Suppl):3730.
Bosnyak E, Barger GR, Michelhaugh SK, Robinette NL, Amit-Yousif A, Mittal S, et al. Amino acid PET imaging of the early metabolic response during tumor-treating fields (TTFields) therapy in recurrent glioblastoma. Clin Nucl Med. 2018;43:176–9.
Funding
This work was supported by the Wilhelm-Sander Stiftung, Germany (grant number 2016.069.1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed written consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ceccon, G., Lazaridis, L., Stoffels, G. et al. Use of FET PET in glioblastoma patients undergoing neurooncological treatment including tumour-treating fields: initial experience. Eur J Nucl Med Mol Imaging 45, 1626–1635 (2018). https://doi.org/10.1007/s00259-018-3992-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-3992-5