Abstract
Purpose
To evaluate the diagnostic performance of integrated whole-body positron emission tomography (PET)/magnetic resonance (MR) enterography in patients with Crohn’s disease (CD).
Methods
Fifty patients with known CD and recurrent symptoms underwent ileocolonoscopy (reference standard) as well as PET/MR enterography. Seven ileocolonic segments were endoscopically analysed using the Simplified Endoscopic Activity Score for Crohn’s Disease (SES-CD) and additionally classified into three categories of inflammation (none, mild to moderate and severe ulcerative inflammation). A total of 14 PET/MR parameters were applied for the assessment of inflamed segments. Contingency tables and the chi-squared test were used for the analysis of qualitative parameters, and the Mann-Whitney U test and receiver operating characteristic (ROC) curve for the analysis of quantitative parameters. The PET/MR parameters were ranked according to their diagnostic value by random forest classification. Correlations between PET/MR parameters and the severity of inflammation on endoscopy and SES-CD were tested using Spearman’s rank correlation test.
Results
A total of 309 segments could be analysed. Based on multivariate regression analysis, wall thickness and the comb sign were the most important parameters for predicting segments with active inflammation of any type. SUVmax ratio of the bowel segment (relative to SUVmax of the liver) was the most important parameter for detecting segments with severe ulcerative inflammation. Wall thickness was the only parameter that moderately correlated with inflammation severity on endoscopy as well as with SES-CD (ρ = 0.56 and 0.589, both p < 0.001).
Conclusion
PET/MR enterography is an excellent noninvasive diagnostic method, and both MR parameters and PET findings provided high accuracy in detecting inflamed segments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
MR enterography is a well-established and dominant cross-sectional imaging modality in the assessment of Crohn’s disease (CD) because of its superior tissue contrast and lack of ionizing radiation. Various MR parameters and signs of inflammation have been validated and proposed as biomarkers of disease activity [1,2,3,4,5]. The magnetic resonance index of activity (MaRIA) has been proposed as a parameter for use in clinical practice because of its high accuracy in detecting active and severe lesions in CD, providing an objective, quantitative and reproducible measure of activity [1, 2]. Novel MR imaging techniques including diffusion-weighted imaging (DWI), dynamic contrast-enhanced imaging and motility imaging may further enhance diagnostic accuracy in disease assessment [6,7,8].
Recently several studies have evaluated the diagnostic performance of 18F-fluorodeoxyglucose ([18F]FDG) PET and [18F]FDG PET/CT in detecting inflamed bowel segments in CD. They were found to be able to reliably detect segments with moderate to severe inflammation, providing a simple, fast and noninvasive imaging modality [9,10,11,12]. However, the role of PET in the diagnosis and management of patients with inflammatory bowel disease (IBD) remains unclear, since according to the 2013 consensus guidelines of the European Crohn’s and Colitis Organization (ECCO) and the European Society of Gastrointestinal and Abdominal Radiology (ESGAR), there is not enough evidence to support its use in everyday clinical practice [5].
Clinical PET/MR scanners that use either a sequential or a simultaneous approach have been introduced into the market in recent years as a novel imaging technique for a broad range of potential applications, particularly in oncological imaging [13]. The coregistration of PET and MR data in an integrated PET/MR scanner offers the possibility of simultaneous evaluation and comparison of MR and PET findings for detecting inflammatory lesions in CD. The feasibility of PET/MR enterography in the assessment of malignant and inflammatory bowel lesions has been demonstrated in initial investigations that included rather small and heterogeneous patient cohorts [14]. A hybrid biomarker comprising both MR and PET parameters was shown to perform better for stricture evaluation in CD than either modality alone [15].
The aim of this study was to prospectively evaluate and compare the performance of different PET/MR parameters in detecting inflamed segments in relation to the endoscopic reference standard in CD.
Materials and methods
Patients and examinations
Between October 2014 and February 2016, 50 patients with diagnosed CD were enrolled in this prospective study. Inclusion criteria were confirmed CD (based on clinical, endoscopic and histological diagnosis), age over 18 years and recurrent symptoms (e.g. diarrhoea or abdominal pain). Exclusion criteria were contraindications to MR, severe renal failure (glomerular filtration rate <30 ml/min) and primary sclerosing cholangitis (PSC), as patients with PSC may show increased tracer uptake in the liver due to an inflammatory condition, potentially leading to misleading calculated ratios of tracer uptake in the bowel segment in relation to the liver. All patients gave informed consent to participate in the study, which was approved by the local ethics committee.
Ileocolonoscopy
Ileocolonoscopy with biopsy was considered the reference standard for the assessment of disease activity and severity. All patients were asked to ingest 4,000 ml of an electrolyte solution (Klean-Prep®; Helsinn, Switzerland) for bowel cleansing the evening prior to the day of ileocolonoscopy. The examinations were performed by a gastroenterologist (J.L.) with more than 10 years of endoscopic experience, using standard colonoscopy equipment (CF-Q 165 L; Olympus, Shinjuku, Japan). The Simplified Endoscopic Activity Score for Crohn’s Disease (SES-CD), which strongly correlated with the Crohn’s Disease Endoscopic Index of Severity for rapid evaluation in routine clinical practice [16], was used as the endoscopic scoring system. Instead of five segments (ileum, right colon segment, transverse colon segment, left colon segment and rectum) as usually used in SES-CD evaluation, seven ileocolonic segments were analysed separately in this study: (1) terminal ileum, (2) caecum, (3) ascending colon, (4) transverse colon, (5) descending colon, (6) sigmoid and (7) rectum. In addition to the SES-CD, the severity of inflammation in each segment was also categorized as: 0 absence of inflammation, 1 mild to moderate and nonulcerative inflammation (including erythema, oedema, pseudopolyps and aphthae), and 2 severe inflammation with the presence of superficial or deep ulcerations.
PET/MR enterography and imaging protocol
An integrated 3.0-T PET/MR scanner (Biograph mMR; Siemens Healthcare, Erlangen, Germany) for the simultaneous registration of MR signals and PET data was used. Patients stayed on liquid food after the ileocolonoscopy and fasted for at least 6 h prior to the PET/MR scan. About 45 min prior to the start of the PET/MR scan the patients were asked to ingest 2,000 ml iso-osmotic solution containing 2.5% mannitol and 0.2% locust bean gum (Roeper, Hamburg, Germany) for optimal bowel distension, and a body weight-adapted dose of [18F]FDG (mean dose 150 ± 22 MBq, approximately 3.0 mSv) as radioactive tracer was injected intravenously 60 min prior to the scan.
For better separation of bowel loops and to reduce potential motion artefacts and the anterior–posterior scan range, patients were placed in the prone position. For acquisition of the PET data, three bed positions were required to cover the entire abdomen and pelvic area. Scopolamine (20 mg, Buscopan®; Boehringer Ingelheim, Germany) was administered intravenously to minimize motion artefacts before the acquisition of contrast-enhanced T1-weighted (T1w) sequences. The MR sequences included in the PET/MR enterography protocol are summarized in Supplementary Table 1. PET images were reconstructed using ordinary Poisson ordered subset expectation maximization (OP-OSEM, 344 × 344 matrix, gaussian filter with 4.0 mm full-width at half-maximum). PET datasets were automatically attenuation-corrected using a four-compartment model attenuation map, calculated from fat-only and water-only datasets, as obtained with Dixon-based sequences.
Image analysis
The PET/MR enterography dataset was analysed and documented in consensus by two radiologists (Y.L., K.B.) each with 4 years of experience in abdominal MRI as well as nuclear medicine physicians specializing in hybrid imaging. They were blinded to the results of the ileocolonoscopy. The PET data and the PET/MR fusion data were analysed using postprocessing software (Syngo.via, version VB10B; Siemens Healthcare, Erlangen, Germany). The MR quantitative parameters were measured using a diagnostic workstation (Universal Viewer, version 6.0; GE Healthcare, Barrington, IL).
A total of 14 parameters were analysed for each segment. The seven qualitative parameters included: (1) the presence of irregular mucosal surface, (2) the presence of fluid in the mesenterium, (3) positive comb sign, (4) the presence of lymph nodes in the mesenterium (from one to three lymph nodes of diameter >5 mm, or more than three lymph nodes of diameter >5 mm), (5) the presence of stratified mural contrast enhancement, (6) the presence of homogeneous transmural contrast enhancement, and (7) the presence of hyperintensity in the b1,000 DWI dataset. The seven quantitative parameters included: (1) wall thickness, (2) mural T2w ratio (bowel wall to cerebrospinal fluid signal intensity, SI, ratio measured on fat-saturated HASTE images), (3) relative arterial contrast enhancement of the wall (RACE; ratio of the bowel wall SI 20 s after contrast medium administration to the SI before administration measured on fat-saturated T1w 3D VIBE images), (4) relative portal venous contrast enhancement of the wall (RPVCE; ratio of the bowel wall SI 60 s after contrast medium administration to the SI before administration), (5) mean DWI ADC value, (6) maximum standardized uptake value (SUVmax) of the segment, and (7) SUVmax ratio (bowel segment to liver SUVmax ratio). The mural T2w ratio, RACE, RPVCE and mean ADC value were measured and calculated by placing a ring-shaped region of interest on the most thickened part of each bowel segment. The SUVmax of the bowel segment was calculated by placing a spherical volume of interest on the most thickened part and the SUVmax of the liver was measured the same way in the right lobe of the liver using a larger spherical volume.
Statistical analysis
The frequencies of the qualitative parameters in each segment were compared with the severity of inflammation on endoscopy (0, 1 or 2) using contingency tables, and the differences were tested separately using the chi-squared test. The median values of the quantitative parameters from bowel segments with different severities of inflammation on endoscopy (0, 1 or 2) were calculated and compared using the Mann-Whitney U test. The performance of each quantitative parameter in assessing inflamed bowel segments (active inflammation of any type and severe ulcerative inflammation) was also tested by creating receiver operating characteristic (ROC) curves. Optimal cut-off points were determined from the maximum Youden’s index values. To test the performance of all PET/MR parameters in detecting inflamed bowel segments (active inflammation of any type and severe ulcerative inflammation) conditional inference trees with stratified tenfold cross-validation were used [17]. Correlations between quantitative parameters, severity of inflammation on endoscopy and SES-CD scores were tested using Spearman’s rank correlation test.
Using a method based on that of Holm [18], p values were adjusted for multiple testing. A p value <0.05 was considered significant. For the statistical tests, SPSS, version 23 (IBM, Armonk, NY) was used and random forest regression tests were performed separately with the R software environment for statistical computing, version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients’ characteristics
Of the 50 patients, 64% (32) were women and the median age of the patients was 45 years (20–67 years). The average duration of disease was 12 years (standard deviation 11.42 years). More than 90% of the PET/MR examinations were performed within 5 days after ileocolonoscopy (average 3 days, maximum 14 days), while two patients underwent PET/MR enterography 1 day before ileocolonoscopy. One patient did not complete the PET/MR examination because of claustrophobia and was thus excluded from further analysis. Ileocolonoscopy showed that 53% (26) of the 49 patients had active inflammation. Due to previous surgical resections and nonpassable stenosis, a total of 309 ileocolonic segments could be endoscopically evaluated, of which 9.7% (30 segments) showed nonulcerative lesions with mild to moderate inflammation lesions and 4.5% (14 segments) showed severe ulcerative lesions.
PET/MR qualitative parameters and disease activity
The 309 bowel segments were first divided into two groups, 44 with and 265 without any type of inflammatory lesions. The diagnostic performance of the seven qualitative PET/MR parameters in terms of accuracy, specificity and sensitivity are summarized in Table 1. The comb sign and a stratified pattern of mural enhancement showed the best diagnostic accuracy (91% and 90%, respectively), yet markedly lower sensitivity (57% and 50%, respectively). Except for DWI, all the qualitative parameters showed similar diagnostic performance with high specificity and low sensitivity. The differences between the two groups with regard to all of the quantitative parameters were highly significant (p < 0.001) in the chi-squared test.
With increasing severity of inflammation, more MR findings were observed (Supplementary Table 2), except for the presence of one to three lymph nodes. The prevalences of all qualitative parameters were significantly different between segments without inflammation and segments with mild to moderate inflammation (p < 0.05) in the chi-squared test. Only the comb sign and hyperintensity on DWI showed significant differences between segments with mild to moderate inflammation and segments with severe inflammation (p = 0.009 and p = 0.032, respectively).
PET/MR quantitative parameters and disease activity
Six of the seven PET/MR quantitative parameters showed highly significant differences (p < 0.001) between segments with and without active inflammation in the Mann-Whitney U test (Table 2). The exception was the mean ADC value (p = 0.688).
The ROC curves with the calculated areas under the curve (AUC) for all quantitative parameters for detecting segments with active inflammation of any type are shown in Fig. 1. The optimal cut-off values were 4.5 mm for wall thickness (sensitivity 81%, specificity 93%), 0.19 for mural T2w ratio (sensitivity 74%, specificity 90%), 1.32 for SUVmax ratio (sensitivity 71%, specificity 90%), 2.1 for RPVCE (sensitivity 71%, specificity 86%), 3.94 for SUVmax of the segment (sensitivity 74%, specificity 85%), 1.36 for RACE (sensitivity 76%, specificity 64%) and 1.541 × 10−3 mm2/s for mean ADC value (sensitivity 48%, specificity 46%).
As shown in Table 3 and Fig. 2, the median values of five of the seven quantitative parameters increased with increasing severity of inflammation. The exceptions were mean ADC value and RPVCE. There were statistically significant differences in six of the seven parameters between segments without active inflammation and segments with mild to moderate inflammation (p < 0.01). The exception was mean ADC value. There were statistically significant differences in mean ADC value, SUVmax ratio and wall thickness between segments with mild to moderate inflammation and segments with severe inflammation (p = 0.009, p = 0.024 and p = 0.042, respectively).
In dectecting severely inflamed bowel segments, the ROCs and their calculated AUCs for all quantitative parameters are shown in Fig. 3. The optimal cut-off values were 4.5 mm for wall thickness (sensitivity 100%, specificity 86%), 1.48 for SUVmax ratio (sensitivity 86%, specificity 90%), 0.19 for mural T2w ratio (sensitivity 100%, specificity 81%), 4.76 for SUVmax of the segment (sensitivity 93%, specificity 86%), 1.74 for RPVCE (sensitivity 93%, specificity 59%), 1.47 for RACE (sensitivity 71%, specificity 73%).
The importance of diverse PET/MR variables for predicting inflamed segments according to the random forest regression test
Wall thickness was the most important parameter for detecting bowel segments with active inflammation of any type, followed by the comb sign (Fig. 4). SUVmax ratio was the most important parameter for predicting severely inflamed segments, followed by the comb sign and wall thickness.
Correlations between PET/MR quantitative parameters and the severity of inflammation on endoscopy as well as SES-CD
There was a very strong correlation between the SES-CD and the severity of inflammation on endoscopy with a Spearman’s correlation coefficient of 0.932 (p < 0.001). Wall thickness was the only parameter that correlated moderately with severity of inflammation on endoscopy and SES-CD (ρ = 0.56 and 0.589; Table 4). For the other parameters, the correlations were weak or very weak.
Discussion
Three findings of this study can be considered important. First, in detecting ileocolonic segments with active inflammation of any type, MR parameters were generally superior to PET parameters. SUVmax ratio was the most important parameter for detecting severely inflamed segments. Second, SUVmax ratio performed better than SUVmax of the bowel segment in detecting inflamed segments. Third, wall thickness correlated moderately with the severity of inflammation on endoscopy as well as with SES-CD, while for other quantitative parameters the correlations were weak or very weak.
The comb sign that reflects acute exacerbation of inflammation is a well-accepted diagnostic parameter in IBD [19]. With an accuracy of 91%, the comb sign was the second most important parameter for detecting inflamed segments of any type and occurred significantly more frequently in severely inflamed segments than in segments with only mild or moderate inflammation (93% vs. 40%). A stratified pattern of mural contrast enhancement showed much higher diagnostic sensitivity in detecting inflamed segments of any type than did a homogeneous transmural pattern (50% vs. 23%). A thickened bowel wall with a stratified enhancement pattern has been reported to be associated with active disease, while a homogeneous pattern suggests quiescence [6, 20]. In this study, 14 severely inflamed segments were found in seven patients, of whom two had a short history of disease (2 and 3 years) and showed a stratified pattern. The other five patients had a longstanding history of disease (mean 24 years, range 18–44 years) and surgical operations such as ileocoecal resection and subtotal colectomy had been performed in four of these patients. Interestingly half of the severely inflamed segments in these five patients showed a stratified pattern and the other half showed a homogeneous transmural pattern. The increased transmural fibrosis in the acute-on-chronic inflamed bowel segments through the whole course of the disease history might have caused the change from a stratified to a homogeneous transmural enhancement pattern. As shown in this study and also in other studies [21], a stratified appearance of the bowel wall was not associated with the severity of inflammation, and we believe that the complex pathogenesis of chronic inflammation in the bowel wall may not be simply associated with or reflected by the appearance of the enhancement pattern. Except for DWI, all the MR qualitative parameters were found to have an excellent specificity over 96% in detecting inflamed segments of any type, but only a low or moderate sensitivity (between 21% and 57%).
In actively inflamed segments, wall thickening and mural oedema are common findings that not only reflect the pathogenesis of CD but are also associated with disease severity [1, 2, 4, 22]. In this study, wall thickness was the most important parameter for detecting inflamed segments of any type. For wall thickness the correlations with the severity of inflammation on endoscopy and SES-CD were moderate (ρ = 0.56 and 0.589; p < 0.001) and for mural T2w ratio the correlations were weak (ρ = 0.43 and 0.46; p < 0.001). Similar correlations with histopathological scores from surgical specimens have been reported by other groups [3, 23]. No significant differences in RACE and RPVCE were seen between segments with mild to moderate inflammation and segments with severe inflammation, but this finding has been discussed as a matter of controversy by others [2, 4]. Furthermore, although both parameters were significantly correlated with the severity of inflammation on endoscopy and with SES-CD (p < 0.001), the correlations was very weak for RACE (ρ = 0.2–0.3) and weak for RPVCE (ρ = 0.4–0.5). Therefore, we agree with Steward et al. who found that the enhanced quantitative MR parameters are less helpful [4] and both of them were of little importance as compared with other parameters in the regression analysis.
There were no significant differences in mean ADC values between segments with and without active inflammation. This finding confirms the findings of Tielbeek et al., who studied 20 patients using a 3.0-T MR scanner with histopathology as the reference standard [23]. However, most published data show a significantly lower ADC values in segments with active inflammation [7, 24,25,26], whereas we found the same only in ulcerative segments with severe inflammation. An explanation might be the small proportion (32%) of severely inflamed segments among all the inflamed segments in this study. Another reason could be the known variability in measurement, since measurement of mean ADC values in normal bowel segments without thickened walls is challenging and hardly reproducible. The adjacent air may also cause artefacts that further influence the mean ADC value. On the contrary, the hyperintensity in the b1,000 DWI images appeared to be more suitable for detecting inflamed segments (sensitivity 71%, specificity 82%) and similar results have been reported by others [25, 26].
As demonstrated in our study and confirmed by other groups [10, 12, 27], the SUVmax ratio was superior to the SUVmax of the segment in terms of diagnostic accuracy and importance in detecting inflamed segments. Compared with a study by Louis et al. [10], who examined 95 ileocolonic segments with [18F]FDG PET/CT and ileocolonoscopy, this study showed nearly the same sensitivity (71% for SUVmax ratio with 1.32 as the threshold) but markedly higher specificity (90% vs. 55%). Louis et al. chose a SUVmax ratio threshold of 1.47 for detecting severely inflamed segments with a sensitivity of 100% and a specificity of 67%. Almost the same threshold (1.48 in our study) was defined and resulted in a sensitivity of 86%, but much higher specificity of 90%. An explanation could be the different endoscopic definition of the severity of inflammation, where only deep ulcers or strictures were defined as severe lesions in the previous study [10]. In other studies with comparable designs, the reported sensitivities in detecting inflamed segments ranged from 82% to 90% and specificities from 89% to 97% [9, 28, 29]. Although the intensity of [18F]FDG uptake was significantly associated with the severity of inflammation on endoscopy, the correlations with SUVmax ratio and SUVmax of the segment were weak (ρ = 0.4–0.5, p < 0.001).
Only a few prospective studies have compared the performance of PET or PET/CT and MR enterography in the assessment of disease activity. Neurath et al. [28] in 2002 found that the sensitivity of hydro-MRI in detecting inflamed areas in ileocolonic segments was strikingly lower than that of [18F]FDG/PET (67% and 85%, respectively) and similar results (66% vs. 90%) were found by Holtmann et al. in 2012 [29]. The inferior sensitivity of hydro-MRI might be explained by the low magnetic field strength of the MR scanners (1 and 1.5 T), T2w imaging without fat saturation and also the thickness of the slices. As shown in the random forest regression test and according to the ROC analysis in our study, SUVmax ratio was of raised importance in predicting inflamed segments of any type, but its predictive value and diagnostic performance were inferior to those of the other four MR parameters. SUVmax ratio was the most important parameter only for predicting severely inflamed segments. Wall thickness was measured at intervals of 0.5 mm and was therefore more like an ordinal variable, while SUVmax ratio is definitely a numerical variable. This might explain the better ability of SUVmax ratio to predict severely inflamed segments, although the ROC AUC for wall thickness was even slightly larger than that for SUVmax ratio (0.96 vs. 0.95).
The MR parameters and PET findings are complementary and the combined morphological and metabolic information increased the overall subjective diagnostic confidence (Fig. 5). In noncompliant patients without optimal bowel distension, the evaluation of bowel segments depends more on the PET findings than on MR parameters (Fig. 6), since proper bowel distension is a prerequisite for optimal evaluation with MR parameters. Only a few double false-negative and double false-positive results were observed in our study. In the terminal ileum of a 47-year-old woman none of the 14 PET/MR parameters revealed any sign of inflammation (e.g. wall thickness 1 mm, SUVmax ratio 0.7). Nevertheless, minimal inflammatory activity could still be detected by ileocolonoscopy on the basis of two small aphthae, but the clinical relevance of such a minimal grade of inflammation was doubtful. In another case, the signs of active inflammation in the sigmoid and descending colon in a 44-year-old man were present according to both PET and MR parameters (e.g. wall thickness 5 mm, SUVmax ratio 2), but the endoscopy with mucosal biopsy revealed no active inflammation. This type of false-positive case has often been described in the literature [10, 30], since transmural inflammation in CD without mucosal involvement might be missed by endoscopy with superficial biopsy.
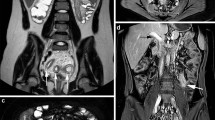
Images in a 39-year-old woman with Crohn’s disease and active inflammation confirmed by endoscopy from the terminal ileum to the rectum. The severely inflamed terminal ileum was clearly demonstrated on both MR and PET images. a Coronal fat-saturated T1w 3D VIBE image in the portal venous phase obtained after intravenous injection of contrast medium shows increased contrast enhancement in the inner wall of the terminal ileum and irregular mucosal surface as an indication of ulcerations. b, c Coronal reconstructed PET image (b) and coronal maximum intensity projection image (c) show intense tracer uptake from the terminal ileum to the rectum. d Fused PET/MR image. e Coronal fat-saturated T2w HASTE image. f Endoscopic image of the terminal ileum shows deep ulceration, marked erythema, granularity and an absent vascular pattern
Images in a 20-year-old woman with Crohn’s disease and active inflammation confirmed by endoscopy at the splenic flexure of the colon. Because of insufficient bowel cleansing and digestion of biphasic solution, the colon was filled with faeces and gas that made evaluation of the bowel wall on MR images difficult. a Coronal fat-saturated T1w 3D VIBE image obtained in the portal venous phase after intravenous injection of contrast medium shows only subtle wall thickening at the splenic flexure of the colon (white arrow) without any other signs of inflammation. b, c Coronal reconstructed PET image (b) and coronal maximum intensity projection image (c) show focal and intense tracer uptake. d Fused PET/MR image. e b1,000 DWI image does not show a hyperintense signal at the splenic flexure of the colon (white arrow). f Endoscopic image shows marked erythema, an absent vascular pattern and granularity, but no ulceration
In four patients, acute inflammatory activity was detected on PET/MR proximal to the terminal ileum but not detectable on ileocolonoscopy, and in one patient therapy with antibiotics was additionally initiated due to an acute mesenteric abscess, because PET/MR enterography visualized the entire bowel and detected extraluminal complications. PET/MR enterography was used in this study for regular patient care to directly influence the medication plan and if necessary arrange other interventions.
One of the limitations of this study was the small sample of inflamed bowel segments (15% of the studied segments). Furthermore severely inflamed segments made up only 32% of all the inflamed segments. Another limitation was the lack of evaluation of interobserver agreement, since the PET/MR parameters were measured in consensus by two experienced radiologists and nuclear medicine physicians. Because over 90% of the PET/MR scans were performed after ileocolonoscopy with an average delay of 3 days, the transmural inflammatory condition might have changed at the time of the PET/MR scan, which would have influenced its diagnostic accuracy.
Conclusion
In providing simultaneous morphological, functional and metabolic information, PET/MR is a promising and noninvasive imaging tool for assessing inflamed segments in CD, and has the potential to serve as a high-quality noninvasive alternative for assessment and therapy monitoring of CD. Both PET and MR parameters provided high diagnostic accuracy. Wall thickness and the comb sign were the most important parameters for detecting segments with active inflammation of any type. SUVmax ratio from PET was the most important parameter for detecting severely inflamed segments with ulceration.
References
Rimola J, Ordas I, Rodriguez S, Garcia-Bosch O, Aceituno M, Llach J, et al. Magnetic resonance imaging for evaluation of Crohn's disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011;17(8):1759–68. https://doi.org/10.1002/ibd.21551.
Rimola J, Rodriguez S, Garcia-Bosch O, Ordas I, Ayala E, Aceituno M, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn's disease. Gut. 2009;58(8):1113–20. https://doi.org/10.1136/gut.2008.167957.
Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E, Bloom S, et al. Mural inflammation in Crohn disease: location-matched histologic validation of MR imaging features. Radiology. 2009;252(3):712–20. https://doi.org/10.1148/radiol.2523082167.
Steward MJ, Punwani S, Proctor I, Adjei-Gyamfi Y, Chatterjee F, Bloom S, et al. Non-perforating small bowel Crohn's disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol. 2012;81(9):2080–8. https://doi.org/10.1016/j.ejrad.2011.07.013.
Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor SA, Baumgart DC, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7(7):556–85. https://doi.org/10.1016/j.crohns.2013.02.020.
Del Vescovo R, Sansoni I, Caviglia R, Ribolsi M, Perrone G, Leoncini E, et al. Dynamic contrast enhanced magnetic resonance imaging of the terminal ileum: differentiation of activity of Crohn’s disease. Abdom Imaging. 2008;33(4):417–24. https://doi.org/10.1007/s00261-007-9267-4.
Oto A, Kayhan A, Williams JTB, Fan X, Yun L, Arkani S, et al. Active Crohn's disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging. 2011;33(3):615–24. https://doi.org/10.1002/jmri.22435.
Hahnemann ML, Nensa F, Kinner S, Maderwald S, Umutlu L, Gerken G, et al. Improved detection of inflammatory bowel disease by additional automated motility analysis in magnetic resonance imaging. Invest Radiol. 2015;50(2):67–72. https://doi.org/10.1097/rli.0000000000000097.
Dabritz J, Jasper N, Loeffler M, Weckesser M, Foell D. Noninvasive assessment of pediatric inflammatory bowel disease with 18F-fluorodeoxyglucose-positron emission tomography and computed tomography. Eur J Gastroenterol Hepatol. 2011;23(1):81–9. https://doi.org/10.1097/MEG.0b013e3283410222.
Louis E, Ancion G, Colard A, Spote V, Belaiche J, Hustinx R. Noninvasive assessment of Crohn's disease intestinal lesions with 18F-FDG PET/CT. J Nucl Med. 2007;48(7):1053–9.
Saboury B, Salavati A, Brothers A, Basu S, Kwee TC, Lam MG, et al. FDG PET/CT in Crohn’s disease: correlation of quantitative FDG PET/CT parameters with clinical and endoscopic surrogate markers of disease activity. Eur J Nucl Med Mol Imaging. 2014;41(4):605–14. https://doi.org/10.1007/s00259-013-2625-2.
Shyn PB, Mortele KJ, Britz-Cunningham SH, Friedman S, Odze RD, Burakoff R, et al. Low-dose 18F-FDG PET/CT enterography: improving on CT enterography assessment of patients with Crohn disease. J Nucl Med. 2010;51(12):1841–8. https://doi.org/10.2967/jnumed.110.080796.
Torigian DA, Zaidi H, Kwee TC, Saboury B, Udupa JK, Cho ZH, et al. PET/MR imaging: technical aspects and potential clinical applications. Radiology. 2013;267(1):26–44. https://doi.org/10.1148/radiol.13121038.
Beiderwellen K, Kinner S, Gomez B, Lenga L, Bellendorf A, Heusch P, et al. Hybrid imaging of the bowel using PET/MR enterography: feasibility and first results. Eur J Radiol. 2016;85(2):414–21. https://doi.org/10.1016/j.ejrad.2015.12.008.
Catalano OA, Gee MS, Nicolai E, Selvaggi F, Pellino G, Cuocolo A, et al. Evaluation of quantitative PET/MR Enterography biomarkers for discrimination of inflammatory strictures from fibrotic strictures in Crohn disease. Radiology. 2016;278(3):792–800. https://doi.org/10.1148/radiol.2015150566.
Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc. 2004;60(4):505–12.
Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: a conditional inference framework. J Comput Graph Stat. 2006;15(3):651–74. https://doi.org/10.1198/106186006X133933.
Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70.
Meyers MA, McGuire PV. Spiral CT demonstration of hypervascularity in Crohn disease: "vascular jejunization of the ileum" or the "comb sign". Abdom Imaging. 1995;20(4):327–32.
Choi D, Lee SJ, Cho YA, Lim HK, Kim SH, Lee WJ, et al. Bowel wall thickening in patients with Crohn's disease: CT patterns and correlation with inflammatory activity. Clin Radiol. 2003;58(1):68–74. https://doi.org/10.1053/crad.2002.1068.
Ziech ML, Bipat S, Roelofs JJ, Nio CY, Mearadji B, van Doorn S, et al. Retrospective comparison of magnetic resonance imaging features and histopathology in Crohn's disease patients. Eur J Radiol. 2011;80(3):e299–305. https://doi.org/10.1016/j.ejrad.2010.12.075.
Rimola J, Rodriguez S, Garcia-Bosch O, Ricart E, Pages M, Pellise M, et al. Role of 3.0-T MR colonography in the evaluation of inflammatory bowel disease. Radiographics. 2009;29(3):701–19. https://doi.org/10.1148/rg.293085115.
Tielbeek JA, Ziech ML, Li Z, Lavini C, Bipat S, Bemelman WA, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol. 2014;24(3):619–29. https://doi.org/10.1007/s00330-013-3015-7.
Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn's disease. Acad Radiol. 2009;16(5):597–603. https://doi.org/10.1016/j.acra.2008.11.009.
Shenoy-Bhangle AS, Nimkin K, Aranson T, Gee MS. Value of diffusion-weighted imaging when added to magnetic resonance enterographic evaluation of Crohn disease in children. Pediatr Radiol. 2016;46(1):34–42. https://doi.org/10.1007/s00247-015-3438-1.
Kiryu S, Dodanuki K, Takao H, Watanabe M, Inoue Y, Takazoe M, et al. Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn's disease. J Magn Reson Imaging. 2009;29(4):880–6. https://doi.org/10.1002/jmri.21725.
Das CJ, Makharia GK, Kumar R, Kumar R, Tiwari RP, Sharma R, et al. PET/CT colonography: a novel non-invasive technique for assessment of extent and activity of ulcerative colitis. Eur J Nucl Med Mol Imaging. 2010;37(4):714–21. https://doi.org/10.1007/s00259-009-1335-2.
Neurath MF, Vehling D, Schunk K, Holtmann M, Brockmann H, Helisch A, et al. Noninvasive assessment of Crohn's disease activity: a comparison of 18F-fluorodeoxyglucose positron emission tomography, hydromagnetic resonance imaging, and granulocyte scintigraphy with labeled antibodies. Am J Gastroenterol. 2002;97(8):1978–85.
Holtmann MH, Uenzen M, Helisch A, Dahmen A, Mudter J, Goetz M, et al. 18F-Fluorodeoxyglucose positron-emission tomography (PET) can be used to assess inflammation non-invasively in Crohn’s disease. Dig Dis Sci. 2012;57(10):2658–68. https://doi.org/10.1007/s10620-012-2190-8.
Shyn PB. 18F-FDG positron emission tomography: potential utility in the assessment of Crohn’s disease. Abdom Imaging. 2012;37(3):377–86. https://doi.org/10.1007/s00261-011-9793-y.
Acknowledgments
We acknowledge the support of the German Research Foundation (DFG, Deutsche Forschungsgemeinschaft).
Funding
This study was funded by the German Research Foundation (DFG, Deutsche Forschungsgemeinschaft; grant number LA 1439/1-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Beiderwellen, K., Nensa, F. et al. [18F]FDG PET/MR enterography for the assessment of inflammatory activity in Crohn’s disease: comparison of different MRI and PET parameters. Eur J Nucl Med Mol Imaging 45, 1382–1393 (2018). https://doi.org/10.1007/s00259-018-3962-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-3962-y