Abstract
Purpose
Early side effects including oesophagitis are potential prognostic factors in patients undergoing radiochemotherapy (RCT) for locally advanced oesophageal cancer (LAEC). We assessed the prognostic value of 18F-fluorodeoxyglucose (FDG) uptake within irradiated non-tumour-affected oesophagus (NTO) during restaging positron emission tomography (PET) as a surrogate for inflammation/oesophagitis.
Methods
This retrospective evaluation included 64 patients with LAEC who had completed neoadjuvant RCT and had successful oncological resection. All patients underwent FDG PET/CT before and after RCT. In the restaging PET scan maximum and mean standardized uptake values (SUVmax, SUVmean) were determined in the tumour and NTO. Univariate Cox regression with respect to overall survival, local control, distant metastases and treatment failure was performed. Independence of clinically relevant parameters was tested in a multivariate Cox regression analysis.
Results
Increased FDG uptake, measured in terms of SUVmean in NTO during restaging was significantly associated with complete pathological remission (p = 0.002) and did not show a high correlation with FDG response of the tumour (rho < 0.3). In the univariate analysis, increased SUVmax and SUVmean in NTO was associated with improved overall survival (p = 0.011, p = 0.004), better local control (p = 0.051, p = 0.044), a lower rate of treatment failure (p < 0.001 for both) and development of distant metastases (p = 0.012, p = 0.001). In the multivariate analysis, SUVmax and SUVmean in NTO remained a significant prognostic factor for treatment failure (p < 0.001, p = 0.004) and distant metastases (p = 0.040, p = 0.011).
Conclusions
FDG uptake in irradiated normal tissues measured on restaging PET has significant prognostic value in patients undergoing neoadjuvant RCT for LAEC. This effect may potentially be of use in treatment personalization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trimodality treatment is a frequently chosen therapeutic approach for locally advanced oesophageal cancer. The combination of neoadjuvant radiochemotherapy (RCT) and surgery has been shown to lead to higher rates of complete (R0) resections and improved overall survival (OS) compared with surgery alone [1]. As an alternative to this procedure, patients can also receive definitive RCT to higher radiation doses than prescribed in the neoadjuvant situation. As this nonsurgical approach was shown to have a slightly worse OS in a meta-analysis [2], it is usually reserved for medically unfit patients in whom the perioperative risks are too high. Though postoperative mortality and morbidity have decreased during recent decades and may be further reduced by laparoscopic resection methods [3] and other novel surgical approaches, RCT remains a potentially risky and costly procedure. Given that 20–30% of patients show complete pathological remission even after neoadjuvant RCT, identification of these patients is a pivotal issue in avoiding surgery of highly RCT-sensitive tumours. A promising method for prediction of pathological complete response is the evaluation of 18F-FDG PET parameters, as FDG PET is often performed for restaging prior to surgery [4].
Several studies have assessed the utility of an FDG PET scan during or at the end of RCT to identify patients with a pathological complete response. Some studies have shown associations between pathological complete remission/OS and tumour maximum standardized uptake value (SUVmax) and metabolic tumour volume [5, 6]. Others have not been able to confirm these findings [7, 8] and have raised doubt as to the usefulness of FDG PET during RCT, especially in patients with squamous cell carcinoma [9]. Even positive studies have had insufficient sensitivity and specificity to identify complete responders on an individual basis. Hence recommendations are that FDG PET response of the tumour should not be used as the only marker to decide whether surgery can be omitted. Nevertheless, it has been shown that treatment adaptation based on the FDG PET response is feasible in patients receiving neoadjuvant chemotherapy for carcinomas of the oesophagogastric junction [10] and the FDG PET response seems to be a promising parameter for stratifying patients to trimodality treatment or definitive RCT [11].
Regarding radiotherapy/RCT several studies of tumours at different sites have demonstrated a correlation between acute side effects and patient outcome [12, 13]. In patients with oesophageal cancer, oesophagitis, defined as worsening of odynophagia during treatment, was associated with an almost fourfold increased probability of complete pathological remission and better OS than in patients without worsening of odynophagia during therapy [14]. However, clinical assessment of oesophagitis is difficult as it needs careful consideration of the temporal course and is often observer-biased, especially in retrospective analyses. We recently identified and validated oral non-tumour FDG uptake measured on midtreatment PET, that can be assumed to ba a surrogate for inflammation, i.e. radiation-induced mucositis, as a prognostic marker for long-term local control (LC) and OS in head and neck squamous cell carcinomas [15]. The aim of the present study was to determine if FDG uptake by non-tumour-affected oesophagus (NTO) within the irradiated volume has prognostic value in oesophageal cancer.
Materials and methods
Patient characteristics
In the present study 64 consecutive patients with FDG PET/CT-staged oesophageal carcinoma were analysed retrospectively. Evaluation of the data was approved by the Institutional Ethics Committee and all patients signed written informed consent. All cases were discussed in an interdisciplinary tumour board and all patients received curatively intended neoadjuvant RCT between October 2005 and December 2014. Inclusion criteria for the analysis were: age >18 years, histologically confirmed potentially R0 resectable oesophageal carcinoma, FDG PET/CT before and at the end (last week of treatment) of RCT, no evidence of distant metastases (DM) on both PET/CT and surgery, curative treatment intention and a minimum follow up of 12 months. Only patients with successful surgical resection were included in the final analysis, which was defined as oncological resection without postoperative mortality. However, analysis of all patients undergoing surgery was also performed and yielded similar results.
Treatment and follow up
Patients were treated with normofractionated RCT with single doses of 2 Gy per fraction up to 40 Gy, and concomitant cisplatin (70 mg/m2 of body surface area) and 5-fluorouracil (5-FU, 3,000 mg/m2of body surface area) during weeks 1 and 4 of radiotherapy, or alternatively weekly carboplatin (targeted area under the curve 2) and paclitaxel (50 mg/m2 of body surface area) [1]. Radiation was prescribed to the whole elective nodal drainage and was administered as three-dimensional conformal or intensity-modulated radiotherapy. Treatment planning was performed with OTP software using the collapsed cone algorithm. The dose output of the linear accelerators was validated at least once per week with an acceptable deviation of less than ±1%. Patient position was verified by two-dimensional imaging and ExacTrac if indicated at the discretion of the treating physician. Surgery was scheduled for 6 weeks after the end of RCT.
Postoperative follow-up usually consisted of clinical examination and CT scans of the thorax and abdomen every 3 months for the first 2 years after treatment and every 6 months thereafter until completion of 5 years of follow up. Endoscopic examination was performed at least at every second follow-up visit. Additional diagnostic procedures were performed as indicated at the discretion of the treating physician. Pathological tumour regression was retrospectively evaluated from patients’ files and scored according to the method of either Mandard et al. [16] or of Becker et al. [17]. For analysis and ease of comparison Becker scores were transformed to Mandard scores (Becker 1a to Mandard 1 and Becker 1b to Mandard 2 etc.) as suggested by Thies and Langer [18]. Side effects were scored clinically according to the Common Terminology Criteria For Adverse Events (CTCAE). Usually side effects were scored during weekly visits. Table 1 provides an overview of tumour and treatment characteristics.
FDG PET/CT protocol
All patients underwent a hybrid 18F-FDG PET/CT scan prior to treatment. Scans (3D PET acquisition, 3 min emission per bed position) were performed using a Biograph 16 (Siemens Medical Solutions Inc., Knoxville, TN). A second scan was performed during the last week of RCT (restaging PET). Data acquisition started 85 ± 16 min (range 59–153 min) after injection of 248–401 MBq 18F-FDG. All patients had fasted for at least 6 h prior to FDG injection. The average serum glucose concentration measured prior to injection was 6.1 mmol/ml. Tomographic images were reconstructed using attenuation-weighted OSEM reconstruction (four iterations, eight subsets, 5 mm FWHM Gaussian filter). The resulting image data had a voxel size of 4.1 × 4.1 × 5 mm.
Data analysis
To determine the tracer uptake in the NTO, a roughly cylindrical region of interest (ROI) was manually delineated. The minimum longitudinal distance to the tumour or affected lymph nodes was four slices of the PET/CT scan (20 mm). The ROI had to be within the high-dose elective treatment volume, which was verified by inspecting the treatment plan in patient files, and the minimum longitudinal length was 20 mm. The minimum volume of NTO was 5 cm3. Figure 1 shows an example NTO ROI with the corresponding PET images and treatment plan. As radiotherapy and chemotherapy can each alone cause inflammation, another ROI was placed outside the irradiated volume in the oral cavity and pharynx, comprising the lymphatic tonsils and oral mucosa and submucosa tissue (SMT). For the delineated ROIs, SUVmax and SUVmean were computed. In this retrospective study the tracer uptake times were not standardized. Therefore all SUVs were corrected for scan time to T 0 = 75 min after injection using the expression:
where T is the time at which the SUV was actually measured and b = 0.31 describes the shape and decrease in the arterial input function over time [19]. Since only the time-corrected values were investigated the index “tc” is omitted in the following discussion.
The fraction difference between SUVmax or SUVmean between the first and second scans was computed as:
where the indices 1 and 2 refer to the pretherapy and restaging PET scans, respectively. For comparison, we also determined the SUVmax of the primary tumour on the pretherapy and restaging PET scans and computed the fractional difference as described above. In the following discussion we refer to these quantities as SUVlesion/1, SUVlesion/2 and ΔSUVlesion. The ROIs were defined and analysed using the ROVER software, version 2.1.26 (ABX, Radeberg, Germany).
Statistical analysis
Survival was analysed with respect to the endpoints OS, LC, occurrence of DM and treatment failure (TF, defined as any locoregional recurrence or occurrence of DM) for the period from the start of radiotherapy to death and/or event. Patients who did not keep follow-up appointments and for whom information on survival or tumour status was thus unavailable were censored with the date of the last follow-up. The associations between the endpoints and clinically relevant parameters (age, grade, histology) as well as quantitative PET parameters were analysed using univariate Cox proportional hazards regression in which the PET parameters were included as binarized parameters. The clinical parameters gender, T stage and N stage were not included because the values were asymmetrically distributed (Table 1). The cut-off values used for binarization were calculated by performing univariate Cox regression for each measured value. The value leading to the hazard ratio (HR) with the highest significance was used as the cut-off value. To avoid groups being too small, only values within the interquartile range were considered as potential cut-off values. The cut-off values were separately computed for all endpoints. The probabilities of survival were computed and rendered as Kaplan-Meier curves.
The independence of PET parameters and clinically relevant parameters was analysed by multivariate Cox regression. Those parameters with at least a trend for significance in a univariate Cox regression (p < 0.1) were included. Each PET parameter was analysed separately together with the clinical parameters. The HRs and the p values of the clinical parameters were averaged over all analyses. The independence of the prognostic value of NTO SUV and tumour SUV was tested in a multivariate analysis with the clinical parameters significant in the univariate analysis as confounding factors. Goodness of fit of nested Cox regression models was compared using analysis of variance based on log likelihoods.
Correlations were tested using Spearman’s rank correlation method. Statistical significance was assumed for p values less than 0.05. Statistical analysis was performed with the R language and environment for statistical computing, version 3.1.2 [20].
Results
The 2-year, 3-year, and 5-year OS rates were 62%, 47% and 40%, respectively. These values are in line with data from current literature [1, 21]. Overall, 50% of patients died during the observation period. The rates for local tumour control, absence of TF and freedom from DM at 5 years were 75%, 42% and 53%, respectively. As expected, there were strong correlations between NTO SUVmax and SUVmean and between NTO ∆SUVmax and ∆SUVmean (Spearman’s rho 0.81 and 0.9, respectively). Spearman’s rho for the correlations between absolute NTO SUV and NTO ∆SUV ranged from 0.55 to 0.68. There were rather weak correlations between SMT SUV and NTO parameters (Spearman’s rho from 0.25 to 0.28), which indicates that both regions have the potential to provide independent prognostic information. All correlations were significant. Supplementary Fig. 1 shows the correlations between SMT and NTO SUVmean values in individual patients. SUVlesion and NTO SUV were essentially uncorrelated (p > 0.2) in most cases. The only exceptions were the correlations between ΔSUVlesion and NTO ΔSUVmax (p = 0.07) and between ΔSUVlesion and NTO ΔSUVmean (p = 0.02). However, in both cases the correlation was rather weak (rho <0.3).
Of the 64 patients, 18 (28%) showed pathological complete remission after RCT. Patients with pathological complete remission were compared to patients without pathological complete remission. Complete responders had a significantly higher NTO SUVmean, irrespective of the tumour histology (SUVmean 1.9 vs. 1.5, p = 0.002; Fig. 2b–d). Furthermore, NTO SUVmean was inversely correlated with tumour regression scores (rho −0.35, p = 0.005; Fig. 2a). No significant correlation between NTO SUV and clinical oesophagitis score was observed (data not shown).
Correlations between SUVmean of non-tumour-affected oesophagus (NTO) and pathological response parameters in patients with squamous cell carcinomas (SCC) and adenocarcinomas (ADC). a Correlation with postsurgical pathological tumour regression (1 complete regression, 5 no response). b–d NTO SUVmean in patients with complete remission (CR, group 1 in a) and without complete remission (groups 2-5 in a) in all patients (b), in patients with SCC (c) and in patients with ADC (d)
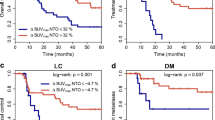
Figures 3 and 4 show the Kaplan Meier plots for the clinical outcome parameters evaluated (OS, TF, LC, DM) in patients stratified by NTO SUVmax and SUVmean. In the univariate Cox regression all normal tissue PET parameters investigated (SUVmax, SUVmean, ∆SUVmax and NTO ∆SUVmean and SMT SUVmean) were prognostic for OS, TF and DM (Tables 2 and 3; Supplementary Table 1). Univariate analysis showed significant associations between LC and NTO SUVmean (p = 0.044) and NTO ∆SUVmax (p = 0.042; Supplementary Table 2. ΔSUVlesion showed a trend for significant associations with OS, TNM and DM. SUVlesion on restaging was prognostic for TNM (P = 0.032) and showed a trend for significance for DM.
In multivariate Cox regression only histology was an independent significant prognostic factor for OS (p = 0.03). A trend for significance was found for the NTO parameters (SUVmax p = 0.064, ∆SUVmean p = 0.064; Table 3). In the multivariate analysis with respect to TF and DM, all NTO parameters were significant prognostic factors (Table 2; Supplementary Table 1). Histology, SUVlesion/2 and ΔSUVlesion showed a trend for significance with respect to DM. SUVlesion/2 was an independent prognostic factor for TNM (P = 0.028). In the multivariate analysis with respect to LC only NTO ∆SUVmax showed a significant association (p = 0.036). However, due to the low number of events (9 of 64), the results for LC are of limited value. For SMT SUVmean no significant associations with any of the clinical endpoints were found in the multivariate analysis.
To determine whether NTO SUV provided information in addition to tumour SUV, multivariate Cox regressions including histology, NTO ΔSUVlesion and NTO SUVmax were performed. Inclusion of NTO SUVmax in the Cox regression model resulted in a significantly improved fit for OS (p = 0.028), TF (p = 0.00012) and DM (p = 0.035), whereas a nonsignificant increase in log likelihood was observed for LC (p = 0.12).
Discussion
We report here the first use of nontumour 18F-FDG PET parameters in irradiated normal tissues as prognostic markers in oesophageal carcinoma. FDG PET is known to reflect tissue inflammation and is therefore in used clinically not only in oncology but also for the staging and localization of inflammatory diseases [22, 23]. An interim FDG PET scan was recently proposed as a tool for predicting oesophageal injury in patients undergoing radiotherapy for non-small-cell lung cancer [24]. In our study inflammation in the NTO was identified as a strong prognostic factor for important oncological outcome parameters (pathological regression, TF, local recurrence). In addition it provides information in addition to clinical parameters as well as to tumour SUV.
Acute side effects of oncological treatment and their association with outcome in patients with gastrointestinal malignancies are receiving increasing attention. Pronounced side effects have been shown to be a favourable prognostic factor in patients with colorectal carcinoma undergoing chemotherapy [25, 26]. This association has also been seen in patients with rectal carcinoma treated with radiotherapy or RCT. in patients treated with RCT, it was a positive prognostic factor for pathological complete tumour remission [27, 28]. In patients with oesophageal carcinoma, one study found an association between worsening of odynophagia during treatment and higher rates of tumour regression and improved OS [14].
The underlying reason for the observed association between side effects and tumour response is not well understood. One obvious explanation is that responses of normal tissue and tumour are determined to a significant extent by genetically defined intrinsic or cellular radiation sensitivity. Thus, higher normal tissue toxicity would be associated with a better tumour response. This explanation seems reasonable as an early in vivo study suggested similar radiosensitivities in tumour and normal tissue of different strains of mice [29]. Furthermore several ex vivo studies indicated an association between ex vivo radiosensitivity of fibroblasts and lymphocytes and pronounced side effects suggesting similar individual radiosensitivities of different tissues [30, 31]. However, other large studies did not show such an association [32, 33]. Another explanation is that a proinflammatory immunological response leads to increased inflammation and tumour clearance at the same time. This is reasonable as polarization of immune cells by radiotherapy is possible [34] and CD8-positive lymphocytes have been shown to have prognostic value in head and neck cancers [35]. Besides these explanations, it is well known that side effects increase with radiation dose. However, in the present study all patients received equal fractionation schedules and total radiation doses, and the high-dose treatment volumes were evaluated.
Another possible confounding factor was the use of concomitant chemotherapy, which is known to increase side effects [36]. This is even more important, as several studies have also shown associations between chemotherapy side effects and tumour response and patient outcome [25, 37], including oral mucositis after administration of 5-fluorouracil [26]. To gain further insight into the contribution of chemotherapeutics, we additionally contoured the oral submucosa and mucosa. Increased uptake in this structure should not represent a radiation response, as it is completely outside the irradiated volume. Although increased oral FDG uptake was associated with several outcome parameters in the univariate analysis, it did not remain a significant factor nor did it show a trend in the multivariate analysis. Therefore, it is unlikely that the observed differences for NTO were only due to increased activity of concomitant chemotherapy. Furthermore, the observed correlations between NTO FDG parameters and outcome were much stronger than any correlation between tumour FDG parameters and outcome. Normal tissue FDG PET parameters seem to be of astonishing robustness, as several investigated parameters (SUVmax, SUVmax, ∆SUV) showed significant differences between prognostic groups.
Although this study was limited by its retrospective nature and lacked an independent cohort for validation, it indicates that non-tumour parameters may be relevant when analysing the prognostic impact of functional imaging. A combination of tumour and normal tissue parameters may be a very promising way to further personalize cancer treatment and identify patients with highly RCT-sensitive tumours, in whom trials on omission of surgery may be performed. This is even more important as in recent analyses the use of the full spatial and temporal FDG PET information instead of only classical PET parameters has been shown to be very promising for the identification of these patients [38,38,39,41]. However, even these features may not be sufficient to predict pathological complete responses [42]. Inclusion of tumour and nontumour parameters in current models could further increase sensitivity and specificity so that sufficient information is available to personalize treatment in clinical trials on omission of surgery and to individualize radiotherapy dose prescription [43].
Overall, our study revealed a high prognostic impact of NTO FDG PET parameters obtained during the last week of neoadjuvant RCT. This finding corroborates the prognostic importance of non-tumour parameters in PET imaging and, after validation, has the potential to be included in novel strategies for personalizing therapy.
References
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Kranzfelder M, Schuster T, Geinitz H, Friess H, Büchler P. Meta-analysis of neoadjuvant treatment modalities and definitive non-surgical therapy for oesophageal squamous cell cancer. Br J Surg. 2011;98:768–83.
Gurusamy KS, Pallari E, Midya S, Mughal M. Laparoscopic versus open transhiatal oesophagectomy for oesophageal cancer. Cochrane Database Syst Rev. 2016;3:CD011390.
Rebollo Aguirre AC, Ramos-Font C, Villegas Portero R, Cook GJR, Llamas Elvira JM, Tabares AR. 18F-fluorodeoxiglucose positron emission tomography for the evaluation of neoadjuvant therapy response in esophageal cancer: systematic review of the literature. Ann Surg. 2009;250:247–54.
Swisher SG, Erasmus J, Maish M, Correa AM, Macapinlac H, Ajani JA, et al. 2-Fluoro-2-deoxy-D-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101:1776–85.
Jayachandran P, Pai RK, Quon A, Graves E, Krakow TE, La T, et al. Postchemoradiotherapy positron emission tomography predicts pathologic response and survival in patients with esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:471–7.
Elimova E, Wang X, Etchebehere E, Shiozaki H, Shimodaira Y, Wadhwa R, et al. 18-fluorodeoxy-glucose positron emission computed tomography as predictive of response after chemoradiation in oesophageal cancer patients. Eur J Cancer 1990. 2015;51:2545–52.
Malik V, Lucey JA, Duffy GJ, Wilson L, McNamara L, Keogan M, et al. Early repeated 18F-FDG PET scans during neoadjuvant chemoradiation fail to predict histopathologic response or survival benefit in adenocarcinoma of the esophagus. J Nucl Med. 2010;51:1863–9.
Palie O, Michel P, Ménard J-F, Rousseau C, Rio E, Bridji B, et al. The predictive value of treatment response using FDG PET performed on day 21 of chemoradiotherapy in patients with oesophageal squamous cell carcinoma. A prospective, multicentre study (RTEP3). Eur J Nucl Med Mol Imaging. 2013;40:1345–55.
Lordick F, Ott K, Krause B-J, Weber WA, Becker K, Stein HJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805.
Lloyd S, Chang BW. Current strategies in chemoradiation for esophageal cancer. J Gastrointest Oncol. 2014;5:156–65.
Wolff HA, Daldrup B, Jung K, Overbeck T, Hennies S, Matthias C, et al. High-grade acute organ toxicity as positive prognostic factor in adjuvant radiation and chemotherapy for locally advanced head and neck cancer. Radiology. 2011;258:864–71.
Wolff HA, Raus I, Jung K, Schüler P, Herrmann MK, Hennies S, et al. High-grade acute organ toxicity as a positive prognostic factor in primary radiochemotherapy for anal carcinoma. Int J Radiat Oncol Biol Phys. 2011;79:1467–78.
Hennies S, Hermann RM, Gaedcke J, Grade M, Hess CF, Christiansen H, et al. Increasing toxicity during neoadjuvant radiochemotherapy as positive prognostic factor for patients with esophageal carcinoma. Dis Esophagus. 2014;27:146–51.
Zschaeck S, Loeck S, Leger S, Haase R, Bandurska-Luque A, et al. FDG uptake in normal tissues assessed by PET during treatment has prognostic value for treatment results in head and neck squamous cell carcinomas undergoing radiochemotherapy. Radiother Oncol. 2017;122(3):437–44.
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–6.
Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521–30.
Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment. Front Oncol. 2013;3:262.
van den Hoff J, Lougovski A, Schramm G, Maus J, Oehme L, Petr J, et al. Correction of scan time dependence of standard uptake values in oncological PET. EJNMMI Res. 2014;4:18.
R Core Team. R: A Language and Environment for Statistical Computing. The R Foundation.
Duan X-F, Tang P, Yu Z-T. Neoadjuvant chemoradiotherapy for resectable esophageal cancer: an in-depth study of randomized controlled trials and literature review. Cancer Biol Med. 2014;11:191–201.
Buscombe J. PET imaging of inflammation. Q J Nucl Med Mol Imaging. 2014;58:284–9.
Hess S, Hansson SH, Pedersen KT, Basu S, Høilund-Carlsen PF. FDG-PET/CT in infectious and inflammatory diseases. PET Clin. 2014;9:497–519.
Niedzielski JS, Yang J, Liao Z, Gomez DR, Stingo F, Mohan R, et al. (18)F-Fluorodeoxyglucose positron emission tomography can quantify and predict esophageal injury during radiation therapy. Int J Radiat Oncol Biol Phys. 2016;96:670–8.
Schuell B, Gruenberger T, Kornek GV, Dworan N, Depisch D, Lang F, et al. Side effects during chemotherapy predict tumour response in advanced colorectal cancer. Br J Cancer. 2005;93:744–8.
Soveri LM, Hermunen K, de Gramont A, Poussa T, Quinaux E, Bono P, et al. Association of adverse events and survival in colorectal cancer patients treated with adjuvant 5-fluorouracil and leucovorin: is efficacy an impact of toxicity? Eur J Cancer. 2014;50:2966–74.
Dahl O, Horn A, Mella O. Do acute side-effects during radiotherapy predict tumour response in rectal carcinoma? Acta Oncol. 1994;33:409–13.
Wolff HA, Gaedcke J, Jung K, Hermann RM, Rothe H, Schirmer M, et al. High-grade acute organ toxicity during preoperative radiochemotherapy as positive predictor for complete histopathologic tumor regression in multimodal treatment of locally advanced rectal cancer. Strahlenther Onkol. 2010;186:30–5.
Budach W, Hartford A, Gioioso D, Freeman J, Taghian A, Suit HD. Tumors arising in SCID mice share enhanced radiation sensitivity of SCID normal tissues. Cancer Res. 1992;52:6292–6.
Barber JB, Burrill W, Spreadborough AR, Levine E, Warren C, Kiltie AE, et al. Relationship between in vitro chromosomal radiosensitivity of peripheral blood lymphocytes and the expression of normal tissue damage following radiotherapy for breast cancer. Radiother Oncol. 2000;55:179–86.
Borgmann K, Hoeller U, Nowack S, Bernhard M, Röper B, Brackrock S, et al. Individual radiosensitivity measured with lymphocytes may predict the risk of acute reaction after radiotherapy. Int J Radiat Oncol Biol Phys. 2008;71:256–64.
Peacock J, Ashton A, Bliss J, Bush C, Eady J, Jackson C, et al. Cellular radiosensitivity and complication risk after curative radiotherapy. Radiother Oncol. 2000;55:173–8.
Russell NS, Grummels A, Hart AA, Smolders IJ, Borger J, Bartelink H, et al. Low predictive value of intrinsic fibroblast radiosensitivity for fibrosis development following radiotherapy for breast cancer. Int J Radiat Biol. 1998;73:661–70.
Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602.
Balermpas P, Rödel F, Rödel C, Krause M, Linge A, Lohaus F, et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: a multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int J Cancer. 2016;138:171–81.
Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–8.
Cameron DA, Massie C, Kerr G, Leonard RCF. Moderate neutropenia with adjuvant CMF confers improved survival in early breast cancer. Br J Cancer. 2003;89:1837–42.
Zhang H, Tan S, Chen W, Kligerman S, Kim G, D'Souza WD, et al. Modeling pathologic response of esophageal cancer to chemoradiation therapy using spatial-temporal 18F-FDG PET features, clinical parameters, and demographics. Int J Radiat Oncol Biol Phys. 2014;88:195–203.
Yip SS, Coroller TP, Sanford NN, Mamon H, Aerts HJ, Berbeco RI. Relationship between the temporal changes in positron-emission-tomography-imaging-based textural features and pathologic response and survival in esophageal cancer patients. Front Oncol. 2016;6:72.
Beukinga RJ, Hulshoff JB, van Dijk LV, Muijs CT, Burgerhof JG, Kats-Ugurlu G, et al. Predicting response to neoadjuvant chemoradiotherapy in esophageal cancer with textural features derived from pre-treatment 18F-FDG PET/CT imaging. J Nucl Med. 2017;58:723–9.
Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges JP, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52:369–78.
van Rossum PSN, Fried DV, Zhang L, Hofstetter WL, van Vulpen M, Meijer GJ, et al. The incremental value of subjective and quantitative assessment of 18F-FDG PET for the prediction of pathologic complete response to preoperative chemoradiotherapy in esophageal cancer. J Nucl Med. 2016;57:691–700.
Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16:234–49.
Acknowledgment
Falk Tillner is acknowledged for providing screenshots of treatment planning.
Author information
Authors and Affiliations
Contributions
S.Z. provided ideas for the study; S.Z. and F.H. performed the analysis, and drafted the manuscript, designed the figures and calculated the underlying statistics, and together with S.L.. C.J., K.Z., J.K., G.B. and J.W. were responsible for treatment, histology and imaging of patients. R.B., J.S. and S.Z. collected patient data and performed the follow-up examinations. M.K., K.Z., F.H. and M.B. provided ideas, supervised the analysis and interpretation of the data, and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
The study was partly supported by the German Federal Ministry of Education and Research (BMBF contract 03ZIK42/OncoRay). The funding source had no influence on data acquisition, evaluation or writing of the manuscript.
Conflicts of interest
None.
Ethical approval
The study was approved by the Institutional Ethics Committee and all patients signed written informed consent.
Rights and permissions
About this article
Cite this article
Zschaeck, S., Hofheinz, F., Zöphel, K. et al. Increased FDG uptake on late-treatment PET in non-tumour-affected oesophagus is prognostic for pathological complete response and disease recurrence in patients undergoing neoadjuvant radiochemotherapy. Eur J Nucl Med Mol Imaging 44, 1813–1822 (2017). https://doi.org/10.1007/s00259-017-3742-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3742-0