Abstract
Purpose
The standard evaluation of multisystem Langerhans cell histiocytosis (LCH) includes a clinical evaluation, laboratory tests and a skeleton/skull X-ray survey, with chest high-resolution computed tomography (HRCT) in the case of pulmonary involvement. Preliminary reports suggest that 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET-CT) may be useful for evaluating patients with LCH.
Methods
Fourteen consecutive adult patients with multisystem LCH were included in this retrospective study, and were evaluated using standard procedures and 18F-FDG PET-CT. The two sets of findings were compared both at baseline and during follow-up. Serial HRCT and pulmonary function tests were used to evaluate outcome in patients with lung involvement.
Results
At the baseline evaluation, PET-CT identified every LCH localization found with the standard evaluation (except a mild cecum infiltration). PET-CT showed additional lesions in seven patients, mostly involving bones, and differentiated inactive from active lesions. Thyroid 18F-FDG uptake was identified in three cases. No pituitary stalk 18F-FDG uptake was observed in patients with pituitary LCH. Only 3/12 (25 %) patients with pulmonary LCH displayed moderate pulmonary 18F-FDG uptake. During follow-up, variations (≥50 % of maximum standardized uptake) in bone 18F-FDG uptake intensity were correlated with disease state and response to treatment. The absence of lung 18F-FDG uptake did not preclude lung function improvement after treatment.
Conclusions
Except for cases with pulmonary and pituitary involvement, 18F-FDG PET-CT could replace the standard evaluation for staging of adult patients with multisystem LCH. Serial PET-CT scans are useful for evaluating treatment responses, particularly in cases with bone LCH involvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Langerhans cell histiocytosis (LCH) is a rare disease of unknown etiology that is characterized by the accumulation of CD1a-positive Langerhans-like cells in involved tissues, which are often organized in granulomas [1–4]. LCH may occur in patients of all ages, from neonates to the elderly, with peak incidence occurring in adults between 20 and 40 years of age [3, 4]. While LCH may affect virtually any organ, it most frequently affects the bones, pituitary stalk and skin. Lung involvement is also frequent in adults; it can be present as part of a multisystem disease or as a single localization of LCH, and it is strongly associated with smoking [5, 6].
The clinical presentation and prognosis of LCH are highly variable. The Histiocyte Society classifies the clinical forms of LCH according to the number and type of organs involved [7]. Single-system LCH affects only one organ/system, particularly bone (unifocal or multifocal) and the lungs in adults [3–6]. Multisystem LCH involves two or more organs/systems, with some localizations, known as “risk organs,” i.e., the hematopoietic system, spleen, liver and central nervous system, bearing less favorable prognoses [3, 4]. Thus, the accurate staging of LCH is an important step in managing these patients. Therefore, at diagnosis, a comprehensive clinical evaluation, several laboratory tests and bone imaging are recommended for all adult LCH patients, regardless of the primary manifestation of the disease [8]. A skeletal X-ray survey (including a dental panoramic radiograph) and standard chest X-rays are systematically performed, followed by chest high-resolution computed tomography (HRCT) in case of lung involvement [8]. Bone CT is recommended for specific LCH localizations, such as lesions in the mastoid or vertebrae [8]. Bone magnetic resonance imaging (MRI) may be useful for better assessing contiguous extensions into soft tissues [8]. Additional investigations are performed as needed according to clinical symptoms and signs suggesting specific LCH localizations, such as head MRI in the presence of diabetes insipidus [8].
The investigations that are serially performed during spontaneous or post-treatment patient follow-up examinations vary according to the initial localizations and the eventual occurrence of new symptoms or signs of LCH. Assessing the outcome of LCH in bone using standard imaging techniques remains difficult, because osteolytic lesions require months to heal [9].18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET-CT), which combines an anatomical evaluation (CT) with an assessment of the metabolic activity (18F-FDG uptake) of bone lesions, should be particularly useful in this context [10]. Phillips et al. evaluated the usefulness of 18F-FDG PET-CT in children with LCH and bone involvement, and found that PET-CT could identify more bone lesions than standard imaging techniques. This approach also allowed the detection of early responses to treatment [11].
The use of PET-CT in adult LCH patients has been reported in individual or small numbers of cases, and the PET-CT results were not systematically compared with the standard imaging findings [12–14]. The usefulness of PET-CT for managing pulmonary LCH, which affects a substantial proportion of adult patients, remains questionable. Krajiceck et al. reported on PET-CT findings at a given time in a series of adult patients with pulmonary LCH [15]. Nodular lesions displayed significant 18F-FDG uptake, whereas this was rarely the case for cystic lung lesions. This is an important drawback, because the impairment of lung function has been shown to be correlated with the extent of cystic lung lesions found by HRCT [16, 17].
To address these issues, we retrospectively analyzed a series of consecutive patients with systemic LCH (most of them with lung involvement) who were assessed by both standard and PET-CT evaluations at the time the disease was initially staged and during follow-up.
Methods
Study design and subject selection
This retrospective study was conducted by the French National Reference Center for LCH. In this center, 18F-FDG PET-CT has been systematically included as part of the evaluation of adult patients with multisystem LCH since April 2009. All consecutive patients 18 years of age or older with multisystem LCH referred from April 2009 to May 2014 were eligible for this study, provided that they had a PET-CT scan within 8 weeks of their standard evaluation. The diagnosis of LCH was either histologically confirmed by a biopsy of an involved site showing the accumulation of CD1a-positive cells, or based on a typical lung HRCT pattern associated with lytic bone lesions and/or diabetes insipidus and the exclusion of alternative diagnoses [6].
The study was performed in accordance with the tenets of the Declaration of Helsinki and was approved by the institutional review board (CPP Ile de France IV, IRB number 00003835). All patients provided written informed consent for the use of their medical reports for research.
Standard evaluation
The standard patient assessment included a comprehensive clinical evaluation with a thorough history of LCH manifestations, blood laboratory tests, a skeletal X-ray survey, chest X-rays and bone CT and/or MRI scans for specific areas, as recommended [8]. In the case of lung involvement, chest HRCT and lung function tests were performed [6]. For patients with hypothalamic/pituitary manifestations, a head MRI scan and a comprehensive endocrinological evaluation were performed.
The skeletal X-ray survey consisted of anteroposterior and lateral views of the skull and spine, as well as anteroposterior views of the ribs, pelvis, and upper and lower limbs. A dental panoramic radiograph was also obtained. Bone CT examinations comprised multiplanar reconstructions with a bone filter and a millimetric section thickness. MRI studies included short tau inversion recovery (STIR), T2-weighted and T1-weighted (with and without gadolinium injection) images in two orthogonal planes. Lung CT scans were performed with patients in the supine position during end-inspiratory breath-hold, and were obtained with a reconstructed section thickness of 1 mm or less and a high-spatial frequency algorithm. The images were displayed with a lung window setting (window level: −600 HU; window width 1600 HU). Semi-quantitative nodular and cystic scores were used to assess the extent of lung lesions, as previously described [17]. Chest CT images were also systematically analyzed with a bone window setting (width: 2000; level: 300) to search for eventual LCH involvement in the thoracic bones.
Lung volumes were evaluated by plethysmography, and forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were determined by the flow curve volume. The diffusing capacity of the lung for carbon monoxide (DLCO) was measured using the single-breath method. Predictive values were determined according to the European Respiratory Society criteria [18]. Obstruction was defined as a ratio of FEV1 to FVC <70 % [19].
Additional investigations were performed as needed for specific LCH localizations.
18F-FDG PET-CT
Patients fasted for at least 6 h before undergoing whole-body PET-CT, and blood sugar levels were checked to ensure that glycemia was in the normal range. Images were acquired 60 min after an intravenous injection of 5 MBq/kg of 18F-FDG (not exceeding 500 MBq) using a Gemini GXL instrument (germanium oxyorthosilicate-based PET + 16-slice CT; Philips). CT data were acquired without contrast enhancement using the following parameters: 120 kV; 100 mAs; pitch 0.94; slice thickness 2.5 mm. PET data were collected in a 3-dimensional mode, with 2 min per-table-position, and were reconstructed with a line-of-response row-action maximum-likelihood algorithm (LOR-RAMLA).
Visual analysis was performed, and maximum standardized uptake value (SUVmax) was measured for each pathological uptake shown on the manufacturer’s review workstation (PETView, Philips Medical Systems). For patients with serial PET-CT scans, a variation of 50 % or greater in the SUVmax of the lesion was considered significant [11].
Data collection
Data on patient demographics, clinical symptoms and signs, and treatments of interest at diagnosis, at the time of each PET-CT scan, and at the last time of follow-up were retrieved from the medical records. Both the standard bone imaging (i.e., skeletal X-ray survey, bone CT and/or MRI) and lung CT scans were interpreted by an expert radiologist (C de MM). All PET-CT scans were analyzed by two experienced nuclear medicine physicians (LV and AV), who had no knowledge of the related clinical history or standard imaging findings.
Evaluation of disease state and response to treatment
The disease state was assessed based on standard evaluations as defined by Histiocyte Society criteria [20]. In brief, if all signs and symptoms were resolved, patients were considered as having non-active disease (NAD). Otherwise, they were classified as having active disease (AD). AD was further subdivided into regressive (improvement of symptoms or signs, no new lesions), stable (persistence of symptoms or signs, no new lesions) or progressive (progression of symptoms or signs and/or appearance of new lesions) disease. LCH response to treatment was classified as better (complete resolution or regression), intermediate (stable or mixed, i.e., new lesions in one site, regression in another site) or worse (progression) [20]. Because specific LCH treatments have virtually no influence on pituitary involvement, this localization was not considered in LCH staging unless new endocrine dysfunction occurred (progressive disease) [8].
The outcome of pulmonary LCH involvement was based on the results of serial lung function tests [17]. The overall lung function outcome was defined by whether there was an increase or decrease of ≥10 % in the FEV1 and/or FVC, and/or of ≥15 % in the DLCO. In cases of discrepancies, the impaired parameter was used as the overall lung function outcome. If the changes were <10 % for FEV1 and FVC and <15 % for the DLCO, lung function was considered to be stable. Additionally, the occurrence of a new pneumothorax during follow-up was considered a sign of pulmonary LCH progression [17].
In all cases, disease state and disease response to treatment were assessed without knowledge of the PET-CT results.
Statistical analysis
Descriptive statistics are shown as the mean ± standard deviation or the median (interquartile range [IQR]). The mean SUV variation over time was compared across treatment groups using the nonparametric Mann–Whitney test. The treatment response (defined by a variation of at least 50 % in mean SUVmax) was compared across the treatment groups using Fisher’s exact test. All tests were two-sided, and p values of 0.05 or less denoted statistical significance.
Analysis was performed using R 3.2.2 (https://www.R-project.org/) software.
Results
Study population
The medical records of 15 patients with multisystem LCH were reviewed. One patient was excluded because the interval between the standard bone imaging and 18F-FDG PET-CT scans was greater than 8 weeks. Fourteen patients (37 ± 13 years, 7 men) were included in the study. The diagnosis of LCH was histologically confirmed in 12 patients (bone n = 6, lung n = 4, skin n = 1, peripheral lymph node n = 1, cecum n = 1; one patient had two-site biopsies). The remaining two patients had a typical lung HRCT pattern with multifocal lytic bone lesions; one of these two patients also had diabetes insipidus.
The initial PET-CT scan was performed at the time of diagnosis of LCH for five patients and at the time of disease reactivation for the remaining nine patients. At this time, all patients had AD, and none were receiving specific treatments for LCH, except substitutive hormonal therapy in the case of pituitary stalk involvement. The clinical manifestations and staging of LCH based on the standard evaluation for each patient are shown in Table 1. In summary, the involved organs were as follows: bone (n = 13; unifocal n = 3, multifocal n = 10), lung (n = 12), pituitary stalk (n = 9), skin (n = 3), mucosa (n = 4; genital n = 2, oral n = 2), lymph node (n = 1), soft tissue (n = 1), and cecum (n = 1). Among the 12 patients with pulmonary LCH involvement, six were current smokers, five were ex-smokers and one was a non-smoker.
18F-FDG PET-CT findings
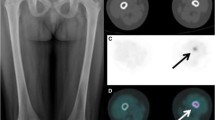
The initial PET-CT results for each patient are detailed in Table 1. In all but one patient, PET-CT showed hypermetabolic lesions, with a median SUVmax of 5.9 (IQR [4.1–8.7]). PET-CT identified every LCH bone lesion visualized by standard imaging. Bone lesions were hypermetabolic in 11/13 patients. In five patients, PET-CT showed additional hypermetabolic osteolytic lesions. Thyroid 18F-FDG uptake was observed in three patients; this uptake was missed by the standard evaluation in two patients (Table 1 and Figs. 1 and 2). Thyroid 18F-FDG uptake was homogeneous for two patients and was localized to the right thyroid lobe for the third patient. Further evaluation demonstrated specific thyroid LCH localization in two patients, one of whom had associated papillary carcinoma; the other patient was diagnosed with Hashimoto thyroiditis.
None of the nine patients with diabetes insipidus and/or anterior pituitary dysfunction had significant pituitary stalk 18F-FDG uptake, despite abnormal MRI scans in all but one patient (pituitary stalk thickening and/or absence of posterior pituitary bright spot). Because of the initial suspicion of metastatic cancer, patient #4 had a systematic colonoscopy that showed a mild LCH infiltration of the cecum, which was not visualized by PET-CT.
Serial 18F-FDG PET-CT findings
Ten patients had one (n = 6) or two (n = 4) serial PET-CT scans during their follow-up (Tables 2 and 3). Serial PET-CT scans were performed in six patients after the systemic treatment of LCH (i.e., prednisone and vinblastine and/or cladribine; Table 2). In five of these patients, the serial PET-CT findings after treatment were consistent with the category of disease response (i.e., better, intermediate or worse), based on the standard evaluation and as assessed blinded to the PET-CT results (Table 2, Fig. 3). The remaining patient (#9) was classified as having stable AD after first-line treatment, whereas PET-CT evaluation identified new hypermetabolic osteolytic lesions of both clavicles, denoting disease progression (Table 2).
Patient #1. A PET: maximum-intensity projection before treatment with cladribine, showing multiple hypermetabolic lesions. PET-CT fused images showing hypermetabolism of a bilateral cervical lymph nodes, b lytic lesions of the left sixth rib, c lytic lesion of the right sacrum. B PET: maximum-intensity projection after treatment with cladribine, showing the resolution of all metabolic lesions, d regression of the cervical lymph nodes, e regression of the hypermetabolism of the left sixth rib with the persistence of a small lytic lesion on CT, f regression of the hypermetabolism of the right sacrum with the persistence of a lytic lesion on CT
The serial PET-CT scan results of four untreated patients during the follow-up period were also available. Patients #5 and #14 had refused chemotherapy, patient #8 had inactive multifocal bone disease with new-onset diabetes insipidus, and patient #11 had pulmonary involvement with recurrent pneumothoraces that precluded the use of cladribine because of a disproportionate risk for pleuropulmonary infection complications. As shown in Table 3, the serial PET-CT scan results were in agreement with those of the disease state assessment that was blinded to the PET-CT findings. Patient #11 was classified as AD progressive because of recurrent pneumothoraces during the follow-up period, for which PET-CT assessment does not provide additional information.
Overall, the median (IQR) value of SUV variation over time was −136 % (−484; −95) after cladribine, versus −11 % (−18; +9) after prednisone and vinblastine, and −42 % (−63; −21) in untreated patients (p = 0.01, by the Kruskal-Wallis test). When considering the variations in SUVmax ≥50 %, all six patients treated with cladribine improved, none of the three patients who received prednisone and vinblastine improved, and two of the four untreated patients also improved (p = 0.016, by Fisher’s exact test).
18F-FDG PET-CT assessment of bone LCH
In the 13 patients with bone LCH involvement visualized by standard imaging, PET-CT identified osteolytic lesions in all cases. LCH lesions involved the ribs (n = 5), vertebrae (n = 6), skull (including the mastoid and maxillae/mandibulae; n = 19), pelvis (n = 20), long bones (n = 5), sternum (n = 1) and scapula (n = 1). Among these 57 bone lesions, which varied in size from 7 to 47 mm, 41 displayed 18F-FDG uptake (median SUVmax = 5.4, IQR [4.5–7.5]), even for lesions <10 mm in size. At the time of the initial LCH evaluation, PET-CT identified 12 additional lytic bone lesions of the pelvis (patients #1, #11 and #14), the ribs (patient #14), the vertebrae (patient #11) and the jaw (patients #3 and #5), which were missed by the standard evaluation (Table 1).
Serial PET-CT scans were most informative for the evaluation of bone lesions during patient follow-up. The 18F-FDG uptake of bone lesions (SUVmax values) generally evolved concordantly with disease state, as assessed by the standard evaluation (Tables 2 and 3). Notably, for patients whose bone disease improved, the SUVmax of the bone lesions resolved or decreased by ≥50 % despite the persistence of osteolytic lesions on the concomitant CT scans (Fig. 3). Conversely, for patients whose bone disease was stable or progressive, the SUVmax of the bone lesions remained stable or increased, or new hypermetabolic osteolytic lesions were identified (Tables 2 and 3).
18F-FDG PET-CT findings in pulmonary LCH involvement
Twelve patients of the present series had pulmonary LCH involvement. Lung HRCT scans showed a nodulocystic pattern in six cases (4 with a low nodular and 2 with high nodular score) and an isolated cystic pattern in the remaining six patients. The lung HRCT cystic scores of the 12 patients were as follows: low (n = 3), moderate (n = 1), high (n = 4) and very high (n = 4).
As shown in Table 1, only three patients had 18F-FDG uptake in the lungs with low SUVmax values. Patients #4 and #14 (both with a high nodular score) had moderate 18F-FDG uptake in the lung nodules. Among patients with a cystic pattern on the lung HRCT scan, only one patient (#12) had moderate 18F-FDG uptake in thick-walled lung cysts (Fig. 4).
The presence of 18F-FDG uptake in the lungs was not predictive of the response to treatment for pulmonary LCH (Table 4). Notably, patients #6 and #7 had no lung 18F-FDG uptake, and nevertheless experienced significant improvement in lung function after treatment with cladribine (Table 4 and Fig. 5).
Patient #6. a Lung HRCT before treatment showing diffuse, thick- and thin-walled lung cysts (arrows). b Fused PET-CT image before treatment with no significant lung uptake. c Lung HRCT after treatment with cladribine showing the partial regression of cysts (arrows). d Fused PET-CT image after treatment showing no significant changes
Finally, it should be stressed that the 18F-FDG uptake in the pleura observed in patients #7 and #10 did not reflect specific LCH involvement, but instead reflected the inflammatory pleural reaction induced by previous talc pleurodesis performed 42 and 3 months before the PET-CT evaluation, respectively.
Discussion
In this study evaluating the role of 18F-FDG PET-CT in the management of adult multisystem LCH, we found the following main results: (1) PET-CT identified every LCH localization found by the standard evaluation, with the exception of a mild cecum infiltration, which was incidentally discovered by colonoscopy; (2) in half of the patients, PET-CT showed additional localized 18F-FDG uptake, mostly by osteolytic lesions but also by thyroid abnormalities; (3) during follow-up, PET-CT was very useful for the assessment of the spontaneous outcome and response to treatment of the disease; (4) PET-CT was only slightly helpful for the management of pulmonary involvement.
The assessment of bone LCH remains difficult because of the absence of a gold standard examination. A skeletal X-ray survey is the first investigation recommended, but has poor sensitivity regarding specific localizations such as those in the vertebrae and pelvic bones [8]. Bone CT and MRI scans can provide additional information, but are not systematically performed and are not among the first investigations to be carried out in a given patient [8]. Whole-body MRI scans have been proposed as an alternative for assessing bone LCH involvement in children, but this method does not accurately assess the activity of bone disease [21].
An important advantage of 18F-FDG PET-CT is that it provides both a precise anatomical evaluation (CT) and an assessment of the metabolic activity (18F-FDG uptake) of bone lesions. In the present study, 18F-FDG PET-CT scans were more accurate than the standard evaluation in identifying bone LCH localizations. Importantly, in all cases, these additional bone LCH sites had an osteolytic pattern on the CT scans, which was consistent with LCH involvement. PET-CT scans showed additional lesions in 5/13 (40 %) patients with bone LCH. More precisely, this approach identified 12 additional lesions that mainly concerned not only the pelvis and the jaw but also the ribs and vertebrae. These findings resulted in a change in disease status from unifocal to multifocal bone disease, which has consequences for the management of these patients, who warrant closer follow-up and eventually the initiation of systemic treatment [8]. Our results are consistent with those of a study conducted by Phillips et al. with a cohort of pediatric LCH patients, in which 18F-FDG PET identified 35 % more lesions than did the standard evaluation [11]. In this study, PET was found to be less sensitive than MRI for spine LCH localizations, but importantly, PET images were not coupled with CT scans at the same time. The absence of concomitant bone CT scans also explains the results of another pediatric study which found 18F-FDG PET to be less sensitive than bone MRI [22]. Furthermore, PET missed very small bone lesions, whose clinical impact is questionable.
18F-FDG uptake provided valuable information by differentiating old, inactive lesions from active lesions. Indeed, bone lesion sclerosis, which is indicative of bone lesion healing, may take months to occur [9]. Furthermore, significant variations (≥50 % of SUVmax) in the intensity of bone 18F-FDG uptake were correlated with disease state or response to treatment during follow-up, based on the standard evaluation. Thus, a decrease in SUVmax was associated with a favorable LCH outcome, despite the persistence of bone lysis on bone CT scans. Conversely, an increase in or the appearance of bone 18F-FDG uptake during follow-up was indicative of LCH progression. This finding is in agreement with Phillips’s study of pediatric LCH, as well as with preliminary reports of adult LCH [11, 13, 14, 22, 23]. The strength of our study is that the PET-CT findings were uniformly and blindly compared with those of the recommended, standard patient evaluation.
Another salient finding of our study was the identification by PET-CT of thyroid 18F-FDG uptake in three of 14 patients, which is more frequent than previously reported in LCH [24–26]. In two of these three patients, this 18F-FDG uptake was related to thyroid LCH involvement, whereas it was secondary to Hashimoto thyroiditis in the third patient. Most importantly, in one patient, thyroid LCH involvement was associated with papillary carcinoma. Thus, histological confirmation should be obtained in the case of thyroid 18F-FDG uptake in patients with LCH [27].
Other LCH localizations were also identified by PET-CT in this study, including lesions in the lymph nodes, soft tissues and genitals. In the case of lymph node involvement, a tissue biopsy should be promptly considered to rule out alternative diagnoses, particularly lymphoma [28, 29].
A minority of our patients (25 %) with pulmonary LCH involvement shown by lung CT scans displayed moderate 18F-FDG uptake on the PET-CT scans. Two of these three patients had a high nodular lung CT score, and the remaining patient had thick-walled cysts; these findings are consistent with those of a previous report, although the intensity of lung 18F-FDG uptake was lower in our series [15]. Importantly, the presence or absence of lung 18F-FDG uptake was not correlated with disease severity, outcome or response to treatment. Therefore, PET-CT appears to be of little use in the management of LCH with pulmonary involvement. Finally, 18F-FDG uptake in the pleura may be observed in cases with previous talc pleurodesis for pneumothorax.
Interestingly, efficient new PET-CT systems allow the acquisition of additional dedicated (full inspiratory/breath-hold) lung CT scans with quality equivalent to that of standalone CT. Thus, when a PET-CT is performed in a patient with LCH lung involvement, a “one-stop shop” approach, providing the valuable information of both PET-CT and lung HRCT in a single visit, could improve patient convenience.
We did not observe any pituitary stalk 18F-FDG uptake in our patients with diabetes insipidus and/or anterior pituitary dysfunction, despite abnormalities on the MRI scans (pituitary stalk thickening and/or absence of posterior pituitary bright spot). Thus, although PET-CT scans may show 18F-FDG uptake in cases with a hypothalamic and/or pituitary mass [14], pituitary MRI appears to be a more valuable mode of investigation for this LCH localization.
This study has several limitations, which are mainly due to the small sample size and its retrospective design. In particular, serial PET-CT evaluations were not performed at similar time points during patient follow-up. For children treated with vinblastine, disease response to treatment is usually evaluated 6 weeks after the end of the induction phase of weekly injection treatments [11]. In the case of treatment with cladribine, which is administered in monthly cycles, evaluating the response at 3 months of treatment is reasonable. In untreated patients, serial PET-CT scans should be performed on a case-by-case basis, guided by clinical outcome.
In summary, with the exception of pulmonary and pituitary involvement, our study strongly suggests that whole-body 18F-FDG PET-CT is a valuable technique for evaluating adult patients with multisystem LCH, and could replace the standard evaluation. Serial PET-CT scans are also very useful for evaluating response to treatment, particularly for cases with bone LCH involvement.
References
Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672–81.
Picarsic J, Jaffe R. Nosology and pathology of Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 2015;29:799–823.
Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76:363–8.
Weitzman S, Egeler RM. Langerhans cell histiocytosis: update for the pediatrician. Curr Opin Pediatr. 2008;20:23–9.
Suri H, Yi ES, Nowakowski GS, Vassallo R. Pulmonary langerhans cell histiocytosis. Orphanet J Rare Dis. 2012;7:16.
Tazi A, de Margerie C, Naccache JM, Fry S, Dominique S, Jouneau S, et al. The natural history of adult pulmonary Langerhans cell histiocytosis: a prospective multicentre study. Orphanet J Rare Dis. 2015;10:30.
Writing Group of the Histiocyte Society. Histiocytosis syndromes in children. Lancet. 1987;1:208–9.
Girschikofsky M, Arico M, Castillo D, Chu A, Doberauer C, Fichter J, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis. 2013;8:72.
Meyer JS, Harty MP, Mahboubi S, Heyman S, Zimmerman RA, Womer RB, et al. Langerhans cell histiocytosis: presentation and evolution of radiologic findings with clinical correlation. Radiographics. 1995;15:1135–46.
Kaste SC, Rodriguez-Galindo C, McCarville ME, Shulkin BL. PET-CT in pediatric Langerhans cell histiocytosis. Pediatr Radiol. 2007;37:615–22.
Phillips M, Allen C, Gerson P, McClain K. Comparison of FDG-PET scans to conventional radiography and bone scans in management of Langerhans cell histiocytosis. Pediatr Blood Cancer. 2009;52:97–101.
Binkovitz LA, Olshefski RS, Adler BH. Coincidence FDG-PET in the evaluation of Langerhans’ cell histiocytosis: preliminary findings. Pediatr Radiol. 2003;33:598–602.
Blum R, Seymour JF, Hicks RJ. Role of 18FDG-positron emission tomography scanning in the management of histiocytosis. Leuk Lymphoma. 2002;43:2155–7.
Lee HJ, Ahn BC, Lee SW, Lee J. The usefulness of F-18 fluorodeoxyglucose positron emission tomography/computed tomography in patients with Langerhans cell histiocytosis. Ann Nucl Med. 2012;26:730–7.
Krajicek BJ, Ryu JH, Hartman TE, Lowe VJ, Vassallo R. Abnormal fluorodeoxyglucose PET in pulmonary Langerhans cell histiocytosis. Chest. 2009;135:1542–9.
Canuet M, Kessler R, Jeung MY, Metivier AC, Chaouat A, Weitzenblum E. Correlation between high-resolution computed tomography findings and lung function in pulmonary Langerhans cell histiocytosis. Respiration. 2007;74:640–6.
Tazi A, Marc K, Dominique S, de Bazelaire C, Crestani B, Chinet T, et al. Serial computed tomography and lung function testing in pulmonary Langerhans’ cell histiocytosis. Eur Respir J. 2012;40:905–12.
European Respiratory Society. Standardized lung function testing. Eur Respir J Suppl. 1993;16:1–100.
Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76.
Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12:327–38.
Goo HW, Yang DH, Ra YS, Song JS, Im HJ, Seo JJ, et al. Whole-body MRI of Langerhans cell histiocytosis: comparison with radiography and bone scintigraphy. Pediatr Radiol. 2006;36:1019–31.
Mueller WP, Melzer HI, Schmid I, Coppenrath E, Bartenstein P, Pfluger T. The diagnostic value of 18F-FDG PET and MRI in paediatric histiocytosis. Eur J Nucl Med Mol Imaging. 2013;40:356–63.
Adam Z, Szturz P, Vanicek J, Moulis M, Pour L, Krejci M, et al. Cladribine (2-chlorodeoxyadenosine) in frontline chemotherapy for adult Langerhans cell histiocytosis: a single-center study of seven cases. Acta Oncol. 2013;52:994–1001.
Cai YF, Wang QX, Ni CJ, Dong SY, Lv L, Li Q, et al. A case report: the diagnosis and therapeutic evaluation for a rare disease of langerhans cell histiocytosis involving thyroid. Medicine (Baltimore). 2015;94:e1891.
Giovanella L, Ceriani L, Crippa S, Mazzucchelli L. Imaging in endocrinology: Langherans cell histiocytosis of the thyroid gland detected by 18FDG-PET/CT. J Clin Endocrinol Metab. 2007;92:2866–7.
Uchiyama M, Watanabe R, Ito I, Ikeda T. Thyroid involvement in pulmonary Langerhans cell histiocytosis. Intern Med. 2009;48:2047–8.
Vergez S, Rouquette I, Ancey M, Serrano E, Caron P. Langerhans cell histiocytosis of the thyroid is a rare entity, but an association with a papillary thyroid carcinoma is often described. Endocr Pathol. 2010;21:274–6.
Das DK, Sheikh ZA, Alansary TA, Amir T, Al-Rabiy FN, Junaid TA. A case of Langerhans’ cell histiocytosis associated with Hodgkin’s lymphoma: fine-needle aspiration cytologic and histopathological features. Diagn Cytopathol. 2016;44:128–32.
Naumann R, Beuthien-Baumann B, Fischer R, Kittner T, Bredow J, Kropp J, et al. Simultaneous occurrence of Hodgkin’s lymphoma and eosinophilic granuloma: a potential pitfall in positron emission tomography imaging. Clin Lymphoma. 2002;3:121–4.
Acknowledgments
The authors thank M. Mao (Assistance Publique-Hôpitaux de Paris; Service de Pneumologie, Hôpital Saint-Louis, Paris, France) and E. Savariau (Institut Universitaire d’Hématologie, Service d’Infographie, Hôpital Saint-Louis, Paris, France) for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study received no funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Obert, J., Vercellino, L., Van Der Gucht, A. et al. 18F-fluorodeoxyglucose positron emission tomography-computed tomography in the management of adult multisystem Langerhans cell histiocytosis. Eur J Nucl Med Mol Imaging 44, 598–610 (2017). https://doi.org/10.1007/s00259-016-3521-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3521-3