Abstract
We report the case of a 34-year-old female who was evaluated for a right lower extremity soft-tissue mass, found to be a large cystic lesion bound by fibrous tissue containing innumerable, freely mobile nodules of fat. Her presentation suggested the diagnosis of nodular cystic fat necrosis (NCFN), a rare entity that likely represents a morphological subset of fat necrosis potentially caused by vascular insufficiency secondary to local trauma. Her lesion was best visualized using MRI, which revealed characteristic imaging features of NCFN including nodular lipid-signal foci that suppress on fat-saturated sequences, intralesional fluid with high signal intensity on T2-weighted imaging, and a contrast-enhancing outer capsule with low signal intensity on T1-weighted imaging. Ultrasound imaging offered the advantage of showing mobile hyperechogenic foci within the anechoic cystic structure, and the lesion was otherwise visualized on radiography as a nonspecific soft-tissue radiopacity. She was managed with complete surgical excision with pathologic evaluation demonstrating, similar to the radiologic features, innumerable free-floating, 1–5 mm, smooth, nearly uniform spherical nodules of mature fat with widespread necrosis contained within a thick fibrous pseudocapsule. Follow-up imaging revealed no evidence of remaining or recurrent disease on postoperative follow-up MRI. The differential diagnosis includes lipoma with fat necrosis, lipoma variant, atypical lipomatous tumor, and a Morel-Lavallée lesion. There is overlap in the imaging features between fat necrosis and both benign and malignant adipocytic tumors, occasionally making this distinction based solely on imaging findings challenging. To our knowledge, this is the largest example of NCFN ever reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nodular cystic fat necrosis (NCFN) is a non-neoplastic soft-tissue lesion that presents as distinct subcutaneous nodules of necrotic adipocytes bound by fibrous tissue [1]. The term “nodular-cystic fat necrosis” was first used in 1977 by Przyjemski and Schuster to describe this entity in which one or more discrete fat nodules were found within a fluid-filled simple cystic cavity, either free-floating or attached to the cyst wall by a thin stalk [2]. NCFN has since also been reported in the literature as “mobile encapsulated lipoma” and “encapsulated fat necrosis” [3, 4]. NCFN has been observed occurring predominantly in adolescent boys or middle-aged women and often presents in the lower extremity as one or multiple palpable subcutaneous masses [1,2,3,4,5]. Vascular insufficiency secondary to local trauma has been implicated in the pathogenesis of NCFN [1, 2], although less than half of reported cases have a known history of trauma to the affected area; however, the true incidence may be higher, as these lesions have been observed to evolve over a time course ranging from days to years, with antecedent trauma having occurred up to 10 years prior to the onset of disease [4,5,6].
Here, we report a 34-year-old female who presented with unilateral soft-tissue masses in the right lower extremity consistent with NCFN. Her lesions were detectable on radiography and visualized by ultrasound, but MRI provided the most adequate characterization of her disease. When interpreting imaging studies of heterogeneous lipid-containing soft-tissue masses, NCFN has a distinctive appearance in the differential diagnosis alongside entities such as lipoma with fat necrosis, lipoma variants, atypical lipomatous tumor, and Morel-Lavallée lesion. Pathologic evaluation confirmed the diagnosis of NCFN which, to our knowledge, is the largest known manifestation of NCFN in the literature.
Case report
A 34-year-old woman presented with the chief complaint of right knee swelling associated with pain caused by long periods of standing. On physical exam, a soft, nontender, non-erythematous swelling on the posterior lateral aspect of the right knee was noted. The swelling persisted and grew for several months, and she additionally developed a separate small, firm mass above the knee, at which point she was referred to orthopedic oncology and imaging. She noted ecchymosis on the lateral knee early in her course of symptoms, but she did not endorse any history of trauma to the knee. She had no other significant past medical or surgical history.
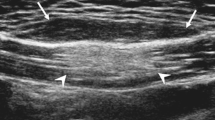
Ultrasound imaging of the knee revealed a frond-like intramuscular lesion in the posterior distal right thigh measuring 9.7 cm, containing several mobile echogenic nodules with surrounding hypoechoic fluid (Fig. 1). A separate 0.4-cm cystic lesion was also noted superficial to the primary lesion. On MRI, the lesion was visualized as a heterogeneous, thick-walled fluid collection filled with innumerable, nearly uniform, ovoid, lipid-signal foci demonstrating high signal intensity on T1-weighted imaging (Fig. 2a and b) and low intensity on T1- and T2-weighted fat suppressed sequences (Fig. 2c and d). There was enhancement of the wall of the lesion, but not of the internal nodules, on T1-weighted postcontrast imaging (Fig. 2e). The lesion was measured to be 5.6 cm × 2.6 cm in axial dimension and at least 19.9 cm craniocaudally, with full measurement limited by the field of view. No suspicious features were present by diffusion-weighted imaging, and dynamic contrast-enhanced imaging showed homogeneous delayed enhancement of the capsule, without early arterial enhancement. There was mild mass effect upon the lateral aspect of the biceps femoris, but no other changes associated with the lesion were noted in the muscles or other structures of the lower extremity. The lesion was also visualized on radiography as an ovoid nonspecific soft-tissue radiopacity (Fig. 3).
MRI of the right knee. A–B T1-weighted MRI in coronal (A) and axial (B) planes showing a large cystic lesion (white arrows) containing innumerable, round, lipid-signal foci located lateral to the right knee and extending superiorly into the right thigh (black arrow). C Fat-suppressed T1-weighted MRI showing suppression of intralesional fat signal (arrow). D Fat-suppressed T2-weighted MRI showing suppression of intralesional fat signal and high signal intensity of cystic fluid (arrow), with mild perilesional edema. E Fat-suppressed T1-weighted MRI with intravenous contrast medium showing enhancement of the cyst wall (arrow). No enhancement of intralesional structures is seen
The patient was managed operatively with surgical excision. Two irregular cystic structures were removed from the right thigh, measuring 11.2 cm and 12.4 cm with externally attached yellow-tan, lobulated adipose tissue which were removed in separate masses but were connected anatomically. Upon opening the specimen, the lumen of each cystic mass was filled with innumerable yellow-tan, solid nodules measuring between 1–5 mm in greatest dimension and lined by a thick fibrous pseudocapsule (Fig. 4a). Histologic evaluation revealed innumerable, nearly uniform, individual nodules composed entirely of necrotic fat with no evidence of viable adipocytes. These nodules were seen freely floating within but bound by this thick fibrous pseudocapsular cyst wall (Fig. 4b–d). RNA in situ hybridization for MDM2 product amplification was negative, excluding an unusual manifestation of atypical lipomatous tumor [7]. The final diagnosis was nodular cystic fat necrosis.
Gross and microscopic pathology of the right lower extremity lesions. A Two masses surgically excised from the right thigh measuring 11.2 cm and 12.4 cm have been opened, each containing innumerable, freely mobile yellow-tan nodules measuring 1–5 mm. B–D High-power (200X, (B)) microscopy of the cystic structures showing nodules composed of necrotic fat which, at low power (10X, (C–D)), are seen bound by a thick fibrous pseudocapsule cyst wall containing freely floating nodules of mature fat necrosis
Clinical follow-up at three months, there were no signs of recurrence. Follow-up MRI four months postoperatively showed no evidence of remaining disease (Fig. 5).
Discussion
The mechanism underlying the formation of NCFN is not completely understood, but it has been proposed that the lesions begin as fat lobules which undergo inflammation and subsequent vascular insufficiency, perhaps secondary to local trauma, which causes necrosis and fibrous encapsulation [1, 8]. As a result, the fat is unable to be resorbed and is retained as discrete nodules which often become freely mobile after they detach from surrounding tissues [1, 2]. The presence of lipomembranous changes alongside necrotic adipocytes has been proposed as the primary role of ischemia in the pathogenesis of NCFN [6]. However, NCFN without evidence of adipocytes has been reported, suggesting that the cellular components of the lesion are eventually resorbed in end-stage disease [9]. Calcifications have also been reported in end-stage disease [5, 8, 10]. Importantly, the absence of viable adipocytes and the discrete innumerable nodules of NCFN is quite distinct and helps separate it from other lipomatous tumors [1].
Przyjemski and Schuster described the gross and microscopic appearance of NCFN as similar to detached epiploic appendages in the abdomen [2]. In terms of imaging findings, NCFN has a heterogeneous appearance corresponding to its nodular and cystic components. On MRI, fat nodules appear as round hyperintense areas on T1-weighted imaging which are hypointense on fat-suppressed sequences, while the surrounding cystic fluid is hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging [9, 11, 12]. The fibrous cyst wall is hypointense on T1-weighted imaging, and gadolinium enhancement of the cyst wall, but not the fatty nodules, is seen on T1-weighted postcontrast imaging [11, 12]. Ultrasound imaging of these lesions shows anechoic cystic structures that contain mobile echogenic foci [11], although no hyperechogenicities were visualized in one case report [8]. Calcifications, if present, may be detected on CT scan [10], or potentially, radiography. On radiography, our patient’s lesion was visualized as a soft-tissue radiopacity with no clear evidence of internal heterogeneity.
Based on her imaging findings, the differential diagnosis for our patient’s presentation included a lipoma with fat necrosis, a lipoma variant, an atypical lipomatous tumor, and a Morel-Lavallée lesion. In the evaluation and diagnosis of soft-tissue masses, MRI is widely regarded as the preferred imaging modality owing to its excellent soft-tissue contrast [13], particularly useful in the characterization of masses for lipid content.
Among adipocytic tumors, lipoma is the most common entity and appears as a lesion composed almost exclusively of fat [14]. There is some overlap in MRI features among lipoma, normal subcutaneous fat, and fat necrosis, such as high signal intensity on T1-weighted imaging and signal loss on fat-suppressed or short tau inversion recovery (STIR) sequences [15,16,17,18]. It may be possible to distinguish fat necrosis based on morphological characteristics such as a spiculated or “bunch-of-grapes” appearance, attributed to encapsulating fibrous tissue [17, 19]. Furthermore, the lack of a discrete mass has been proposed as a criterion for identifying fat necrosis [20], although histologically confirmed mass-like fat necrosis has also been reported [21]. In this regard, NCFN may be considered a morphological subset of fat necrosis, with key features including a fibrous cystic wall that contains the freely mobile nodules suspended in fluid. Ultrasound, therefore, offers the advantage of showing mobility of the nodules in real time.
Fat necrosis occurring within a lipoma may appear as amorphous stranding or curvilinear heterogeneity within regions of lipid signal, with gadolinium rim enhancement that is attributed to vascularized fibrous tissue [21]. In addition, several rare lipoma variants, such as angiolipoma, myolipoma, chondroid lipoma, spindle cell lipoma, and pleomorphic lipoma, may be considered in the differential diagnosis of fat necrosis [16, 22, 23]. These lesions contain varying degrees of fatty and non-fatty tissue, such as blood vessels, smooth muscle cells, or chondromyxoid matrix, depending on the specific variant [16, 23]. It is often difficult to diagnose lipoma variants based solely on imaging findings because they may share imaging characteristics with both normal tissue and various malignancies [16, 23].
In contrast to a lipoma, fatty lesions with a significant volume of non-fatty components may raise suspicion for malignancy [14]. Atypical lipomatous tumor (ALT), also called well-differentiated liposarcoma (WDLPS) when located in deep anatomic locations (such as the retroperitoneum, groin/paratestis, or deep visceral sites), is a relatively common, locally aggressive lipomatous tumor with the capacity to dedifferentiate into a mitotically active sarcoma [14, 23]. MRI findings that are associated with increased risk of malignancy include sparsity of fat within a complex mass, intrinsic T1- and T2-signal heterogeneity, cystic or necrotic areas, ill-defined margin, perilesional signal, intraosseous or neurovascular extension, and size greater than 5 cm [24]. Additional features associated with ALT/WDLPS include septa measuring greater than 2 mm in thickness, septal enhancement, and nodular or patchy non-fatty components [25], easily identified on non-contrast MR sequences [26]. Therefore, the heterogeneous appearance of fat necrosis, which may occasionally be mass-like and feature thick internal septa, may raise concern for an ALT/WDLPS or dedifferentiated liposarcoma [12, 27]. Ultimately, while the protean imaging findings of fat necrosis have significant overlap with ALT/WDLPS or other liposarcomas, a low index of suspicion should lead to adequate tissue sampling to secure the diagnosis [12].
A Morel-Lavallée lesion (MLL) is a fusiform collection of fluid between the subcutaneous fat and underlying fascia caused by a shearing traumatic injury [28]. Similar to NCFN, MLL characteristically occurs in the lower extremities, with the thigh, pelvis, and knee being the most frequently affected regions [29]. The pathophysiologic mechanism of MLL involves shear forces between soft-tissue layers causing the leakage of blood and lymph from ruptured vessels [30]. As MLL evolves, continued inflammation results in the formation of a fibrous pseudocapsule surrounding the fluid collection [30]. As with most soft-tissue masses, MRI is regarded as the imaging modality of choice for MLL [28, 30]. MLL can be categorized into six types based on lesion characteristics and imaging findings: type I (seroma), type II (subacute hematoma), type III (chronic organizing hematoma), type IV (closed laceration), type V (pseudonodular), and type VI (infected) [31]. Lesion contents may appear homogeneous or heterogeneous depending on type, and internal hyperintensities on T1-weighted imaging, suggestive of entrapped fatty tissue, or on T2-weighted imaging, suggestive of intralesional blood products, may be seen [31]. The fibrous pseudocapsule of MLL, if present, is hypointense on all MRI sequences and may show mild contrast enhancement [32]. However, unlike in NCFN, patchy contrast enhancement of internal structures may be identified in MLL [32].
Of note, similar imaging appearances have been reported in lesions that may arise as a complication of autologous fat transfer procedures, often performed in the breast and buttocks [33,34,35]. These lesions also appear as heterogeneous soft-tissue masses with cystic and solid components, encapsulated by a contrast-enhancing cyst wall [33,34,35]. However, our patient had no prior history of fat transfer procedures or other surgeries in the lower extremity.
Simple surgical resection is the recommended approach for benign soft-tissue masses, while wide resection with or without adjuvant radiation therapy is generally indicated for malignant tumors [25]. NCFN may therefore be managed with observation or simple excision, and there is no evidence to suggest any risk of malignant behavior [2, 5, 8, 11].
In conclusion, we report a case of a 34-year-old female who presented with lower extremity NCFN based on imaging and histopathological findings. The characteristic imaging appearance of NCFN includes the presence of discrete nodules of lipid signal surrounded by fluid within a cystic structure, best characterized using MRI, although the dynamic nature of ultrasound offers the advantage of showing the mobility of the internal fatty nodules. A history of physical trauma, although implicated in the pathophysiology, is frequently absent or unknown. Therefore, regardless of known inciting trauma to the affected region, a diagnosis of NCFN should be considered in the workup of soft-tissue masses when imaging shows a heterogeneous fatty lesion.
References
Hurt MA, Santa Cruz DJ. Nodular-cystic fat necrosis: A reevaluation of the so-called mobile encapsulated lipoma. J Am Acad Dermatol. 1989;21:493–8.
Przyjemski CJ, Schuster SR. Nodular-cystic fat necrosis. J Pediatr. 1977;91:605–7.
Ban M. Nodular-cystic fat necrosis-A review of 147 Japanese patients. J Dermatol Res. 2016;1:65–8.
Kiryu H, Rikihisa W, Furue M. Encapsulated fat necrosis – A clinicopathological study of 8 cases and a literature review. J Cutan Pathol. 2000;27:19–23.
Oh C-W, Kim K-H. A case of nodular cystic fat necrosis: The end stage lesion showing calcification and lipomembranous changes. J Dermatol. 1998;25:616–21.
Pujol RM, Wang C-Y, Gibson LE, Daniel Su WP. Lipomembranous changes in nodular-cystic fat necrosis. J Cutan Pathol. 1995;22:551–5.
Weaver J, Downs-Kelly E, Goldblum JR, Turner S, Kulkarni S, Tubbs RR, et al. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Mod Pathol. 2008;21:943–9.
De Wilde R, Hautekiet A, Geers S, Bossche LV, De Muynck M. Ultrasonographic presentation of nodular cystic fat necrosis after a low-velocity trauma: A case report. J Rehabil Med-Clin Commun. 2022;5:jrmcc00083.
Nakamura Y, Ishizuki S, Ishitsuka Y, Watanabe R, Saito A, Furuta J, et al. Multiple, lipid-containing, subcutaneous cysts suspected of end-stage nodular cystic fat necrosis. J Dermatol. 2020;47:e85–7.
Yi-Hsin H, Ya-Wen H, Yi-Chin S. Multiple floating fat balls on the right lower leg. Indian J Dermatol Venereol Leprol. 2011;77:731.
Gocmen R, Kerimoglu U. An unusual appearance of traumatic fat necrosis: Floating fat balls. European Journal of Radiology Extra. 2008;66:e43–5.
Burkholz KJ, Roberts CC, Lidner TK. Posttraumatic pseudolipoma (fat necrosis) mimicking atypical lipoma or liposarcoma on MRI. Radiol Case Rep. 2007;2:56–60.
Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses. AJR Am J Roentgenol. 2000;175:575–87.
Einarsdottir H, Söderlund V, Larson O, Jenner G, Bauer HCF. MR imaging of lipoma and liposarcoma. Acta Radiol SAGE Publications. 1999;40:64–8.
Sundaram M, McLeod RA. MR imaging of tumor and tumorlike lesions of bone and soft tissue. AJR Am J Roentgenol Ray Soc. 1990;155:817–24.
Bancroft LW, Kransdorf MJ, Peterson JJ, O’Connor MI. Benign fatty tumors: Classification, clinical course, imaging appearance, and treatment. Skelet Radiol. 2006;35:719–33.
López JA, Saez F, Alejandro Larena J, Capelastegui A, Martín JI, Canteli B. MRI diagnosis and follow-up of subcutaneous fat necrosis. J Magn Reson Imaging. 1997;7:929–32.
Galant J, Martí-Bonmatí L, Sáez F, Soler R, Alcalá-Santaella R, Navarro M. The value of fat-suppressed T2 or STIR sequences in distinguishing lipoma from well-differentiated liposarcoma. Eur Radiol. 2003;13:337–43.
Canteli B, Saez F, de los Ríos A, Alvarez C. Fat necrosis. Skeletal Radiol. 1996;25:305–7.
Tsai TS, Evans HA, Donnelly LF, Bisset GS, Emery KH. Fat necrosis after trauma: A benign cause of palpable lumps in children. AJR Am J Roentgenol Ray Soc. 1997;169:1623–6.
Chan LP, Gee R, Keogh C, Munk PL. Imaging features of fat necrosis. AJR Am J Roentgenol Ray Soc. 2003;181:955–9.
Drevelegas A, Pilavaki M, Chourmouzi D. Lipomatous tumors of soft tissue: MR appearance with histological correlation. Eur J Radiol. 2004;50:257–67.
Gupta P, Potti TA, Wuertzer SD, Lenchik L, Pacholke DA. Spectrum of fat-containing soft-tissue masses at MR imaging: The common, the uncommon, the characteristic, and the sometimes confusing. Radio Graphics. 2016;36:753–66.
Ahlawat S, Fritz J, Morris CD, Fayad LM. Magnetic resonance imaging biomarkers in musculoskeletal soft tissue tumors: Review of conventional features and focus on nonmorphologic imaging. J Magn Reson Imaging. 2019;50:11–27.
Yee EJ, Stewart CL, Clay MR, McCarter MM. Lipoma and its doppelganger: The atypical lipomatous tumor/well-differentiated liposarcoma. Surg Clin N Am. 2022;102:637–56.
Shannon BA, Ahlawat S, Morris CD, Levin AS, Fayad LM. Do contrast-enhanced and advanced MRI sequences improve diagnostic accuracy for indeterminate lipomatous tumors? Radiol Med. 2022;127:90–9.
Andaç N, Baltacıoǧlu F, Çimşit NÇ, Tüney D, Aktan Ö. Fat necrosis mimicking liposarcoma in a patient with pelvic lipomatosis: CT findings. Clin Imaging. 2003;27:109–11.
Diviti S, Gupta N, Hooda K, Sharma K, Lo L. Morel-Lavallee lesions-Review of pathophysiology, clinical findings, imaging findings and management. J Clin Diagn Res. 2017;11:TE01–4.
Vanhegan IS, Dala-Ali B, Verhelst L, Mallucci P, Haddad FS. The Morel-Lavallée lesion as a rare differential diagnosis for recalcitrant bursitis of the knee: Case report and literature review. Case Rep Orthop. 2012;2012:593193.
Bonilla-Yoon I, Masih S, Patel DB, White EA, Levine BD, Chow K, et al. The Morel-Lavallée lesion: Pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol. 2014;21:35–43.
Mellado JM, Bencardino JT. Morel-Lavallée lesion: Review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13:775–82.
Mellado JM, del Palomar LP, Díaz L, Ramos A, Saurí A. Long-standing Morel-Lavallée lesions of the trochanteric region and proximal thigh: MRI features in five patients. AJR Am J Roentgenol Ray Soc. 2004;182:1289–94.
Masarapu V, Wang PS, Gorbachova T. Slow-growing buttock mass after failure of incorporation of autologous fat transfer for gluteal augmentation: Ultrasound and MRI features. Skelet Radiol. 2020;49:1669–75.
Kontoes P, Gounnaris G. Complications of fat transfer for breast augmentation. Aesthet Plast Surg. 2017;41:1078–82.
Lin DJ, Wong TT, Ciavarra GA, Kazam JK. Adventures and misadventures in plastic surgery and soft-tissue implants. RadioGraphics Radiol Soc North Am. 2017;37:2145–63.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Shivani Ahlawat: Neurofibromatosis Therapeutic Acceleration Program. Laura M. Fayad: NIH R61, Neurofibromatosis Therapeutic Acceleration Program, Johns Hopkins University. Minsoo Kim, John M. Gross, and Adam S. Levin declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, M., Gross, J.M., Ahlawat, S. et al. Nodular cystic fat necrosis: a distinctive rare soft-tissue mass. Skeletal Radiol 53, 583–588 (2024). https://doi.org/10.1007/s00256-023-04426-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-023-04426-0