Abstract
Intra-articular masses are not a rare finding in routine imaging. This is particularly true in patients with underlying joint diseases such as degenerative arthritis. Nevertheless, concomitant presentation is rather uncommon in imaging studies. The authors report an unusual concomitant lipoma arborescens and synovial osteochondromatosis (which has not previously been reported in the literature to the best of the authors’ knowledge) in a man in his 60 s with a long-standing history of knee osteoarthritis. In this case presentation, we review the differential diagnosis for noninfectious synovial proliferative disorders presenting as intra-articular masses, their potential association with underlying joint pathology, and discuss the key imaging features and appropriate treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intra-articular masses are not uncommon radiologic findings whether in asymptomatic patients or discovered in the course of joint pain, swelling, locking, or grinding symptoms. Benign intra-articular masses can be classified as noninfectious synovial proliferative processes, infectious granulomatous diseases, deposition diseases, vascular malformations, and miscellaneous conditions [1]. An example of such pathology is lipoma arborescens, which is an uncommon cause of intra-articular masses that are slowly progressive, typically involving the suprapatellar bursa of the knee [2]. Another example is synovial osteochondromatosis, which is the result of proliferative and metaplastic changes in the synovium leading to hyaline cartilage nodules in the joint [3]. Even though lipoma arborescens and synovial osteochondromatosis are both commonly described in association with degenerative arthropathy, to the best of our knowledge, this is the first case of concomitant lipoma arborescens and secondary synovial osteochondromatosis of the knee reported in the literature. Treating physicians, radiologists, and pathologists should be aware that such co-occurrence of these two pathologies is possible, although extremely rare, to prevent potential confusion in achieving the correct diagnosis and management in a seemingly complex case.
Case report
A man in his 60 s with a long-standing history of knee osteoarthritis presented with worsening right knee pain and progressive swelling of the joint. At physical examination, mild tenderness and restriction of movements were present but no other inflammatory signs were observed. The patient was a runner for approximately 40 years. There was no history of prior surgery, systemic disease, or previous infection. The patient was eventually eligible for total knee arthroplasty, and a preoperative workup was performed.
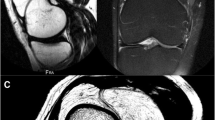
Anteroposterior and lateral radiographs of the knee showed patellofemoral and tibiofemoral osteoarthritis (Fig. 1). They also show multiple intra-articular ossified bodies of varied sizes and shapes in the popliteal region of the knee as well as in the supra- and infrapatellar regions. There was a small effusion and some radiolucent areas of the suprapatellar, prefemoral area, suggestive of fat. CT of the right knee demonstrated the fullness of the suprapatellar pouch with multiple, nodular fat density foci and joint effusion (Fig. 2). CT confirmed that the loose calcified bodies were likely intra-articular. Magnetic resonance imaging (MRI) of the knee was requested to further evaluate intra-articular pathology. MRI showed a multi-lobulated mass in the suprapatellar bursa with a signal equivalent to that of the fat, advanced tricomparimental degenerative arthropathy, and multiple intra-articular ossified bodies (Fig. 3).
a, b Sagittal proton density-weighted and T2-weighted fat-suppressed MR images reveal multiple high signal intensity suprapatellar nodules (arrow) with a signal decrease on fat-suppressed MR images, confirming their fat content. The large loose body in the popliteal area shows signal intensities similar to that of bone, consistent with a fully ossified mass
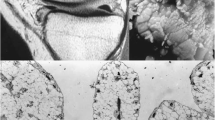
A voluminous ossified mass in the popliteal region was also depicted with predominantly high proton density signal intensity and some areas of intermediate to low signal intensity areas on T2-weighted images (Fig. 3). These findings were highly suggestive of lipoma arborescens in the suprapatellar bursa and concomitant multiples intra-articular synovial osteochondromatosis bodies as illustrated in a schematic representation of the knee joint (Fig. 4). The patient eventually underwent knee arthroplasty. The suprapatellar mass was resected for pathologic evaluation. At the time of surgery, a large loose body was identified within Hoffa’s fat pad and was excised together with the fat pad itself. Significant synovitis with synovial hypertrophy was also noted, and a partial synovectomy of the knee was also performed. Within the posterolateral knee joint recess, a large cartilaginous loose body was noted and excised and sent for pathology. As representatively depicted in Fig. 5, the lesion consisted of multiple proliferative cartilaginous nodules in variable sizes with osseous differentiation on the surface and focal mineralization within the lesion. The cut surface of the specimen showed a vaguely multinodular appearance embedded in the surrounding synovial tissue. The cartilaginous matrix was intensely basophilic. Proliferative chondrocytes showed mild atypia, but no mitosis/necrosis was noted. The histopathologic features are compatible with synovial osteochrondromatosis, also known as synovial chondrometaplasia (Fig. 6). The differential diagnosis includes, but is not limited to, tenosynovial chondromatosis that arises from tenosynovial membranes and intra-articular bodies, which were incompatible with the imaging and pathologic findings in this case.
Representative histology image of knee joint nodules displayed hyalinized fibrous surface (arrow) and underneath proliferative benign chondrocytes (asterisks) at high power view (hematoxylin–eosin stain, × 400 original). Proliferative chondrocytes showed mild atypia, but no mitosis/necrosis was noted
Discussion
The differential diagnosis for benign intra-articular masses in noninfectious and nonsystemic conditions includes lipoma arborescens, synovial osteochondromatosis, and pigmented villonodular synovitis [1].
Lipoma arborescens is a benign, usually monoarticular disorder characterized by the replacement of the subsynovial tissue by mature fat cells and occurs most commonly in the knee [2]. This is particularly well seen on MRI as villous lipomatous proliferation with masses typically following the signal intensity of fat on all sequences, located most commonly in the supra-patellar pouch in the knee. MRI including T1-weighted and fat-suppressed sequences is the modality of choice for the diagnosis. In our case, the diagnosis of lipoma arborescence was made based solely on imaging findings because the histopathological diagnosis was not possible due to a lack of specimens. However, MRI findings were indeed characteristic of lipoma arborescence. Affected patients are usually in the 5th to 7th decades of life. Clinical presentation is nonspecific and consists of joint pain and swelling. In the secondary lipoma arborescens, background degenerative changes and meniscal tears are commonly present, while this is not observed in the primary lipoma arborescens [4]. Although lipoma arborescens is usually monoarticular, bilateral, and polyarticular presentation is not uncommon, particularly in the secondary forms. Lipoma arborescens can also occur in the extra-articular tissues of synovial origin, such as in the bursae and tendon sheaths [5]. These are usually related to idiopathic primary forms and have been previously reported (e.g., in the subacromial bursa of the shoulder, in the bicipitoradial bursa of the elbow, and along the periarticular tendon sheaths in the ankle and wrist.
In the largest series of cases, degenerative changes were observed in 87% and 97% of lipoma arborescence cases [6, 7]. The strong association of lipoma arborescence with osteoarthritic joint changes suggests that secondary lipoma arborescence reflects a nonspecific synovial response to chronic irritation [6]. Lipoma arborescens does not require an aggressive surgical treatment unless symptomatic despite conservative management. However, in advanced primary cases and difficult cases of secondary lipoma arborescens, surgery can be considered. The surgical treatment of choice for lipoma arborescens is either open or arthroscopic synovectomy [4].
Synovial osteochondromatosis most frequently involves the knee and the hip joints [3]. The clinical presentation consists of joint pain, swelling, and limitation of motion. Men are more frequently affected than women, usually in the 5th decade of life. Primary synovial osteochondromatosis is diagnosed when there is no underlying joint abnormality. It is an uncommon benign neoplastic process with hyaline cartilage nodules in the subsynovial tissue of a joint, tendon sheath, or bursa. Imaging findings are variable and depend on the three phases of the disease [8]. The initial phase corresponds to synovial proliferation and formation of intrasynovial cartilaginous nodules that eventually detached from the synovium forming free intra-articular bodies in the transitional phase. The final phase is characterized by inactive synovial disease with persistent loose bodies in the joint that are calcified or ossified over time. Radiographs reveal multiple intraarticular calcifications of similar size and shape, distributed throughout the joint, with typical “ring-and-arc” chondroid mineralization. CT optimally depicts the calcified intraarticular fragments and extrinsic bone erosion. MRI findings are more variable, although the typical pattern reveals low-to-intermediate signal intensity with T1 weighting and very high signal intensity with T2 weighting (reflecting the high water content) with hypointense calcifications. The gross pathologic appearance of primary synovial osteochondromatosis consists of hyperplastic synovium covering bluish-white, multilobulated, nodular projections of hyaline cartilage diffusely involving the entire joint surface [3, 9,10,11]. These nodules may be numerous and give a “cobblestone” appearance to the synovium. The chondral bodies in the joint space, bursa, or tendon sheath are usually similar in size and shape, suggesting their origin within a similar time frame. Histologically, primary synovial osteochondromatosis is composed of lobules of hyaline cartilage, surrounded by synovial lining that is usually attenuated. The hyaline cartilage in primary synovial osteochondromatosis is often hypercellular with atypical histologic features, including multinucleation, nuclear crowding, nuclear enlargement and hyperchromasia, and mild myxoid changes. These atypical features, which would otherwise suggest a grade 1 to 2 chondrosarcoma, are typically present in primary synovial osteochondromatosis [3, 9,10,11]. Thus, histologic correlation with the radiologic appearance (particularly the location of the process centered in a joint, bursa, or tendon sheath) is essential for correct diagnosis and treatment strategy [3, 9,10,11]. Particularly with long-standing diseases, the lobules of hyaline cartilage frequently undergo peripheral enchondral ossification that may progress to contain central yellow marrow. Treatment of primary synovial osteochondromatosis is surgical synovectomy with the removal of chondral fragments. Local recurrence is not uncommon and ranges from 3 to 23% [7]. Malignant transformation to chondrosarcoma is uncommon. Because local recurrence of primary synovial osteochondromatosis is not infrequent, distinguishing recurrent disease from malignant transformation can be difficult [3]. However, a rapid increase in the size of the lesion in a patient with a known primary disease or a rapidly deteriorating clinical course should raise suspicion of malignant transformation.
In contrast, secondary synovial osteochondromatosis is associated with joint abnormalities, such as mechanical or arthritic conditions that cause intraarticular chondral bodies. Indeed, our case was a secondary synovial osteochondromatosis associated with osteoarthritis and loose bodies of different shapes and sizes. Secondary synovial osteochondromatosis can be distinguished histologically from primary synovial osteochondromatosis by the reactive, proliferative chondral bodies that often have a central nidus of nonneoplastic hypocellular cartilage that grows in concentric rings, lack of atypia, and presence of fragments of articular hyaline cartilage [3, 12]. In addition, there are a smaller number of chondral fragments with variable sizes. The underlying arthropathy such as osteoarthritis is usually appreciable clinically and radiologically. Secondary synovial osteochondromatosis is managed by anti-inflammatory medication with the additional management of the inflammatory joint symptoms until or unless mechanical symptoms prohibit adequate function. At this point, surgical management is indicated to improve long-term function and prognoses, and removal of joint bodies, as well as arthroplasty or joint reconstruction, can be performed [13].
The differential diagnosis includes pigmented villonodular synovitis and is easily made on imaging. MRI typically shows lobulated mass-like synovial proliferation. Because of the tendency of the lesions to bleed, pigmented villonodular synovitis is characterized by a low signal intensity due to hemosiderin deposition. Some areas of high signal intensity on T2-weighted images may be present, due to inflamed synovium or effusion. Intense gadolinium enhancement is common, reflecting the high vascularity of synovial proliferation. A well-defined single nodule in the less common localized form has been also described [14]. The knee is the most frequently involved joint, but unlike lipoma arborescens and synovial osteochondromatosis, pigmented villonodular synovitis most often occurs in young to middle-aged adults [15]. Local recurrence following surgical synovectomy is frequent [14].
These two noninfectious synovial proliferative processes (LA and synovial osteochondromatosis) occur most frequently in the knee, particularly when an underlying articular disease such as osteoarthritis is present. This case highlights the uncommon but possible combination of various types of intra-articular masses. With the increasing use of MRI, more such cases are likely to be seen. Moreover, understanding and recognizing the spectrum of radiologic appearances and their pathologic basis for the primary versus secondary lipoma arborescens and synovial osteochondromatosis allow improved patient assessment by avoiding misinterpretation of these entities as other aggressive articular masses and are important to optimize clinical management.
Change history
25 April 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00256-022-04063-z
References
Sheldon JP, Forrester DM, Learch TJ. Imaging of intra-articular masses. Radiographics. 2005;25:105–19. https://doi.org/10.1148/rg.251045050.
Narváez J, Narváez JA, Ortega R, Juan-Mas A, Roig-Escofet D. Lipoma arborescens of the knee. Rev Rhum Engl Ed. 1999;66:351–3.
Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27:1465–88. https://doi.org/10.1148/rg.275075116.
Sanamandra SK, Ong KO. Lipoma arborescens. Singapore Med J. 2014;55:5–11. https://doi.org/10.11622/smedj.2014003.
Minami S, Miyake Y, Kinoshita H. SICOT J. 2016;2:28. https://doi.org/10.1051/sicotj/2016019.
Vilanova JC, Barceló J, Villalón M, Aldomà J, Delgado E, Zapater I. MR imaging of lipoma arborescens and the associated lesions. Skeletal Radiol. 2003;32:504–9. https://doi.org/10.1007/s00256-003-0654-9/.
Howe BM, Wenger DE. Lipoma arborescens: comparison of typical and atypical disease presentations. Clin Radiol. 2013;68:1220–6. https://doi.org/10.1016/j.crad.2013.07.002.
McKenzie G, Raby N, Ritchie D. A pictorial review of primary synovial osteochondromatosis. Eur Radiol. 2008;18:2662–9. https://doi.org/10.1007/s00330-008-1024-8.
Resnick D. Tumors and tumor-like lesions of soft tissues. In: Diagnosis of bone and joint disorders. 4th ed. Philadelphia, Pa: Saunders, 2002;4204–4273.
Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Synovial tumors. In: Tumors of the bone and joints. 4th ed. Silver Spring, Md: ARP Press, 2005;386–432.
Weiss SW, Goldblum JR. Cartilaginous soft tissue tumors. In: Enzinger and Weiss’s soft tissue tumors. 4th ed. Philadelphia, Pa: Mosby, 2001;1368–1388.
Villacin AB, Brigham LN, Bullough PG. Primary and secondary synovial chondrometaplasia: histopathologic and clinicoradiologic differences. Hum Pathol. 1979;10:439–51.
Habusta SF Tuck JA. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing;2022 Jan. 2021 Dec 28. PMID:29262110. Bookshelf ID: NBK470463.
Murphey MD, Rhee JH, Lewis RB, Fanburg-Smith JC, Flemming DJ, Walker EA. Pigmented villonodular synovitis: radiologic-pathologic correlation. Radiographics. 2008;28:1493–518. https://doi.org/10.1148/rg.285085134.
Garner HW, Ortiguera CJ, Nakhleh RE. Pigmented villonodular synovitis. Radiographics. 2008;28:1519–23.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to all four categories established by the International Committee of Medical Journal Editors (http://www.icmje.org) including (1) conception and design, acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be published, and (4) agree to be accountable for all aspects of the work if questions arise related to its accuracy or integrity. More specifically, ST, LD, DM, and AG prepared all radiological images and legends. QH prepared macroscopic and histological specimen images and legends, and description of histopathological findings in the case report manuscript. Ali Guermazi, as the corresponding author, hereby verifies each author’s contribution.
Corresponding author
Ethics declarations
Informed Consent
The privacy officer at our institution was presented with the de-identified images and the manuscript and subsequently delivered a written approval for the publication of the materials. HIPAA requirements were followed. Informed consent was not required.
Conflict of interest
One author (initials blinded for review) reports a conflict of interest that is unrelated to the submitted work: he is a shareholder of BICL, LLC, and a consultant to Pfizer, Novartis, Regeneron, AstraZeneca, Merck Serono, and TissueGene. All other authors have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised. The correct name of the first author should be: Sofia Takkal.
Rights and permissions
About this article
Cite this article
Takkal, S., Diaz, L., Manuel, D. et al. Concomitant lipoma arborescens and synovial osteochondromatosis of the knee. Skeletal Radiol 51, 2211–2216 (2022). https://doi.org/10.1007/s00256-022-04053-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-022-04053-1