Abstract
Objective
Running is among the most popular recreational activities; nonetheless, the acute post-race changes of cartilage or meniscus have rarely been determined. The current study aimed to review the acute changes in knee cartilage and meniscus among habituate runners following long-distance running detected by using quantitative magnetic resonance imaging (MRI).
Materials and methods
Systematic literature search was performed on those dominate clinical databases which including MEDLINE, Cochrane, Embase, ScienceDirect, and Web of Science. Included studies should be conducted on healthy marathon runners, and the participants should be examined before and after running by using MRI. Intervention studies were excluded.
Results
A total number of 14 studies were finally included in this review which all examined the cartilage or meniscus by using MRI functional sequences. Among them, six studies quantitatively measured the changes regarding volume of the knee cartilage or/and meniscus. Five studies found that the volume would decrease initially after running. Ten studies reported T2 (T2*) would decrease after running and returned to the baseline in a short term, while T1ρ may remain increased in months. Five studies measured subareas for T2 (T2*) value, and found that the superficial and medial subarea changed more vastly than other regions after running.
Conclusion
Runners experience transient changes in the volume and signals of knee cartilage and meniscus after long-distance running. A liquid exchange and material interaction in cartilage and meniscus was observed after running. Superficial and medial areas of knee cartilage and meniscus might be more susceptible to mechanical loading.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Running is one of the most popular recreational activities globally, and cartilage and meniscus of the knee joint play essential roles in transferring the mechanical loading to subchondral bone and reducing friction in the joint during the activity. Literatures have suggested that long-distance running will overload the knee joint, thus leading to chronic damage [1]. Previous studies have shown that exercises exert significant impact on the biochemical components and microscopic morphology of the cartilage, which may result in deformation [2,3,4]. In addition, both animal and human studies have found that long-term repetitive mechanical loading could cause health hazards, such as cartilage degeneration in the knee joint and additional chondrocyte apoptosis [5,6,7]. More importantly, such change could lead to imbalance in the cartilage composition, thus accelerate the development of osteoarthritis (OA) [8].
There is a close spatial relationship between meniscus and cartilage in knee joint; the function of meniscus is to distribute loads in the knee and to lubricate cartilage surfaces [9,10,11]. Both articular cartilage and meniscus possess type II collagen [2]. Furthermore, meniscus and articular cartilage originate from the interzone [12]. Therefore, they are similar in physiological composition.
Magnetic resonance imaging (MRI) is a valid tool for the quantitative evaluation of microstructural and compositional changes as well as the detection of early-stage knee injuries: specialized MRI quantification techniques can detect changes in water content, proteoglycan, and collagen arrays in cartilage and meniscus to visible imaging changes of OA [13]. T2 value, measured by quantitative MRI, has been widely used to assess changes in water interactions and anisotropy of collagen arrays within the extracellular cartilage or meniscus matrix [14].T2 value of cartilage and meniscus also provides information of water content interaction with each other and with surrounding macromolecules [15, 16]. Compared to T2 value, T2* value takes shorter scanning time and shows higher resolution [17]. The significant positive association between T2 value and T2* value has been reported previously in knee cartilage [18]. T1ρMRI is a noninvasive method to study biophysical condition of in vivo cartilage. T1ρ is used to reflect interactions between motion-restricted water content, especially water and proteoglycan interactions [19, 20]. The slow motion between water and proteoglycan has been reported to be efficiently detected by the T1ρ mechanism [21]. It has been suggested that the increase of T1ρ value represents a decrease in proteoglycan content and tissue hydration in articular cartilage [22]. Therefore, the local macromolecular environment reflected by MRI parameters could potentially indicate cartilage damage and OA risks.
Up to date, the acute post-race changes of cartilage and meniscus are still largely unknown. It is of great clinical significance to elucidate the acute effect of long-distance running on knee cartilage and meniscus. Therefore, the main aim of this systematic review is to consolidate the related publications and to provide robust evidence of the changes in cartilage and meniscus by using T2 (T2*), T1ρ, thickness, and volume parameters from MRI. We also hope this study could give implications on the training distances for recreational runners and athletes to lower the risk of knee damage.
Materials and methods
Systematic review scales
We performed the current systematic review to examine the acute changes of knee cartilage and meniscus by quantitative MRI following long-distance running by two independent reviewers (DBS, SYD). Methodology of the current review was aligned with the Cochrane Handbook for Systematic Review as well as the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [23, 24].
Clinical question of this review was: what are the acute knee and meniscus changes in the post-race phase (within 48 h) when characterized by T2 (T2*) value, T1ρ value, volume and thickness among healthy runners?
Literature search
We searched six major electronic databases systematically, which were PubMed, Cochrane, Embase, ScienceDirect and Web of Science, with the strategy of using keywords “(marathon OR half-marathon OR run OR runner) AND (mri OR magnetic resonance imaging) AND (knee cartilage OR meniscus).” Related articles were identified up to July 1, 2021.
Study selection
Discussions were carried out to make the definitive decision where unanimous agreement could not be reached. After eliminating duplicates, articles were screened according to abstract and title to find all relevant literature for this review according to the inclusion and exclusion criteria as follows: Inclusion criteria: (1) healthy runners without known risk factors of knee injuries; (2) the runners were examined pre and post-race by using quantitative MRI examination; (3) quantitative examination of knee cartilage and/or meniscus were done; (4)the running distance ≥ 5 km and/or the running time ≥ 30 min; (5) English literature; (6) the post-race examination was done within 48 h. Exclusion criteria: (1) review studies, editorials, letters or abstracts only; (2) ultramarathons; (3) intervention studies.
Data extraction
Data extracted of the current review including: the authors of the publication, subject demographics (age, BMI, gender and training stages), time points of MRI examination, grading system, and MRI parameters. All data were extracted by independent investigators (DBS, SYD).
Narrative synthesis
Because of the heterogeneity, it was impossible to perform a formal meta-analysis. Thus, a comprehensive qualitative review of the existing literature was performed.
Quality assessment/risk of bias assessment
Newcastle–Ottawa scale (NOS) was used for the evaluation of the study quality and risk of bias [25]. NOS is one of the most commonly adopted tools for the quality assessment of non-randomized studies included in a systematic review. The scaling results of our included articles are shown in Table 1.
Results
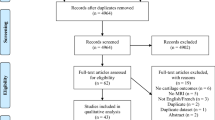
After removing duplicate articles, 652 articles were screened. After reading titles and abstracts, 565 articles were excluded as they were irrelevant. Among the remaining 87 full-text articles, 51 were further excluded for the following reasons: no quantitative testing; neither cartilage nor meniscus was studied; the study participants were not recreational runners.
Finally, a total of 14 studies were included in this systematic review. The detailed screening process is shown in Fig. 1. Demographic characteristics of the involved subjects are shown in Table 2; Table 3 describes the research methods and research instruments; main results of each article are summarized in Table 4.
Qualitative synthesis results
Standard knee MRI examinations pre and post running were performed in all 14 studies. Six studies quantitatively measured the changes in morphology of the knee cartilage or meniscus [26,27,28,29,30,31]. Ten studies quantitatively measured MRI functional sequences of knee cartilage or meniscus [26, 28, 32,33,34,35,36,37,38,39]. Among them, seven studies measured T2 value [28, 32,33,34,35,36, 38], two studies measured T2* value [26, 39] and four studies measured T1ρ value [32, 33, 36, 37].
MRI parameter changes with time
A number of included studies found that cartilage and meniscus volume or thickness would transiently decrease in a short period of time after running [27,28,29,30,31]. Willwacher et al. examined the volume in cartilage immediately after running and reported a significant decrease [30]; Mosher et al. examined the thickness of cartilage in 15 min after running in both runners and sedentary controls, and the researchers reported a significant decrease [28]; Kersting et al. examined the volume of cartilage and meniscus after running and reported the volume decreased significantly [29]; Kessler et al. reported the volume of cartilage and meniscus decreased immediately after running and then increased to a slightly lower level compared with pre-run (p > 0.05) in 60 min [27, 31]..
In terms of quantitative measurement of knee cartilage and meniscus by MRI functional sequence, most studies showed that T2 (T2*) value decreased immediately after running and increased after a convalescence period. The immediate post-run time difference among studies, ranging from one minute to around 20 min, has been clearly addressed in most of the studies. Mosher et al. examined the subjects in 15 min after running and found cartilage T2 value significantly decreased [28]; Crowder et al. reported a significant cartilage T2 value decrease immediately after running, T2 of superficial femur and superficial patella remained decreased until the 25 min and 15 min, respectively [34]; Esculier et al. observed that T2 values of tibia and femur decrease insignificantly at 19 min after running, but then started to increase at 55 min and 91 min [36]. Cha et al. also reported cartilage T2 value decreased first and increased in 10 min and 2 h after running [35]. Other studies designed a longer convalescence period for another examination and reported T2 (T2*) value increased in 10 h [38], 12 h [26], and 48 h [32, 33, 39].
We encounter a controversial result of T1ρ value changes in the 4 studies examined T1ρ value [32, 33, 36, 37]. Esculier et al. found that in subjects with OA, T1ρ value insignificantly decreased at 31 min after running and increased at 67 min when comparing to pre-run levels. However, the trend is different in healthy controls that T1ρ presented a significant decrease at 31 min and remained at similar level at 67 min [36]. Heckelman et al. reported T1ρ value decreased after running and recovered to pre-run level in 24 h [37]. Luke et al. and Stehling et al. reported T1ρ value in runners significantly increased in 48 h than baseline after running [32, 33]. The recovery periods in each study were different. It is rational to speculate that T1ρ would decrease in a certain period after running, and then increase to a level even greater than baseline.
MRI parameter changes with subarea
Most studies found that the T2 (T2*) value of the superficial and medial subarea of cartilage and meniscus changed vastly in a short period time after running [32, 34, 35, 39]. As for volume and thickness, there were some controversial results: Kersting reported a volume decrease in lateral and medial tibia after running [29]. Zhang et al. has divided the regions into 21 sub-regions by using automatic segmentation and reported that at the time point of 12 h after running, the thickness of lateral central femoral cartilage and the volume of lateral posterior tibial cartilage increased, and at the time point of 2 mon, the thickness and volume of medial anterior tibia significantly decreased [26]. It is worth mentioning that the majority of the included studies segment the cartilage or meniscus manually, two studies used semi-automatic segmentation methods [28, 38], and another two studies used automatic segmentation [26, 32].
Quality assessment/risk of bias assessment
Blinded assessments were carried out to ensure the reliability of MRI reading in some studies. Three studies reported blinded assessments performed by two independent investigators, and the disagreement was resolved by negotiation [26, 32, 33]. In another two studies, the blinded assessments were performed by a panelist [36, 37]. Two studies reported that blinded assessments were performed, however the exact numbers of their evaluators were unclear [27, 31]. In seven studies, assessors and methods were not specified [28,29,30, 34, 35, 38, 39].
Discussion
The current systematic review study is trying to provide solid evidence for the transient structural changes of knee cartilage and meniscus after long-distance running. Healthy runners without known risk factors of knee injuries can experience transient changes in T2 (T2*), T1ρ, volume, and thickness of knee cartilage and meniscus after running. There would be a liquid exchange period in cartilage and meniscus after running, which indicates an interaction between water and cartilage matrix.
Volume of knee cartilage and meniscus
Five studies assessed volume of cartilage and meniscus reported controversial results with various convalescence period. However, it is not hard to find a certain trend when comparing to T2 and T1ρ values that volume would decrease immediately after running and recover to baseline after a certain period of rest. Due to the limited number of the included studies in this systematic review, we were unable to conclude the exact time points for the changes. The cartilage deformation might be caused by high impact load mainly [40], and the recovery of cartilage volume in a short period of time after running may be due to its adaptability to loading.
Moreover, the volume or thickness cartilage subarea changed differently. Willwacher et al. reported the volume decreased in medial tibia [30]; Zhang et al. reported the thickness of lateral central femoral cartilage and the volume of lateral posterior tibial cartilage increased at the time point of 12 h after running, the thickness and volume of medial anterior tibia significantly decreased after two months [26]; Kersting et al. reported that the volume of lateral tibia cartilage decreased the most after running [29]; Kessler et al. reported lateral and medial meniscus significantly decreased after running in the studies done in 2006 and 2008; in 2008 they also observed an significant increase after 60 min recovery[27, 31]. The decreased thickness and volume of medial cartilage could be explained by the large contact area of the medial cartilage during exercise. It has been suggested that the medial cartilage transmitted most of the knee loading [26]. The increased thickness and volume of cartilage might be due to the vacuum effect caused by the uneven stress. In this phenomenon, the pressure in the cartilage compression area increases and accumulates, while the pressure in the adjacent cartilage structure decreases. At this time, the cartilage structure in the compression area deformed, water was extruded, and the content of proteoglycan and collagen in the cartilage increased accordingly [41]. This different result of decreased volume of lateral tibial cartilage after running might be due to the uneven loading caused by the runner’s different postures. Willwacher et al. found the reduction was more significant in the group with larger deviation of habitual motion path (HMP) [30]. They suggested that uneven local stress could be an important factor in cartilage volume reduction. Future studies are strongly suggested to clarify the relationship between longer recovery time and the changes of cartilage subarea after running.
T2 value and T2* value of knee cartilage and meniscus
A certain trend was observed in the T2 value and T2* value of cartilage and meniscus during running: T2 value or T2* value would decrease initially after running and started to increase to baseline in months. Nag et al. suggested that water was squeezed out from the cartilage matrix during running and would exchange back after the race [42]. Thus, the possible mechanism could be the cartilage still in a compressed condition after running, and the increased T2 value indicates the water absorbing after running, which could be the repairing process. The alternative mechanism for the increase of T2 value can be the enhanced post-race inflammatory response in cartilage matrix. The rapid increase of T2 value may be resulted from the articular cartilage of the elders that was more intolerable to long-term running load, leading to the enhanced inflammatory response in cartilage matrix. Future trials with larger sample sizes and longer follow-up period would be needed to understand the exact mechanisms.
Most studies found that the T2 (T2*) value of the superficial and medial subarea of cartilage and meniscus changed most in a short period time after running [28, 32, 34, 35, 39]. This might be that superficial and medial cartilage was more vulnerable to mechanical loading. Previous study has suggested that the degeneration of type II collagen associated with aging starts from the shallow layer, which might be led by repeated mechanical loading. As mentioned above, the medial cartilage might transmit most of the knee loading [26]. Luke et al. observed similar trend as T2 value and T1ρ value increased in the medial part of knee cartilage after running [32]. Hesper et al. found that T2* value of superficial cartilage layers changed more significant after running [39], and Zhang et al. found that the T2 value of medial knee cartilage increased 12 h after running [26]. The detection time after running was longer than 12 h in the abovementioned three studies, which is 10–12 weeks, 4 weeks, and 8 weeks, respectively. It is reasonable to consider the long recovery period as the rising period of liquid exchange.
T1ρ value of knee cartilage and meniscus
There was a similar post-race change trend of T1ρ value, volume and T2 value, except for T1ρ value, which changed slower than volume and T2 value. A similar interactive physiological change is speculated between the change of T1ρ value and T2 value after running. T2 value of cartilage and meniscus decrease after running and could start to rise in about 24 h after running, whereas T1ρ value increased in about 48 h after running. However, very limited studies reported T1ρ values. More studies are suggested to provide thorough information at more frequent examination time points.
The shorter running time and distance may also have an essential influence on the rapid recovery of T1ρ value. Heckelman et al. reported that T1ρ value of knee cartilage decreased of 4% and 11% with 3 miles and 10 miles running, respectively. After running, the cartilage of knee joint recovered up to 2% of its baseline value within 24 h after running (no significant difference) [37]. They regarded the recovery as a normal physiological change, thus suggested running within this distance range would be safe. It was worth mentioning that Luke et al. and Stehling et al. found T1ρ value stayed higher after marathon than pre-run level after 3 months [32, 33]. The difference in test distance would be a possible reason for the T1ρ value changes. Esculier et al. found that runners showed better tolerance to the load with the running time of 30 min than those without running habits [36]. Therefore, running and training distance may play an important role in the change of T1ρ value. And in the studies of Luke et al. and Stehling et al. on T1ρ [32, 33], it might still be in the rising period of liquid exchange period 3 months after running. Future trial with longer follow-ups and consistent design are recommended to study the clinical significance of T1ρ value and the recovery time of T1ρ value in the knee cartilage and meniscus of runners.
Conclusions
A fluid exchange period in knee cartilage and meniscus has been observed by using MRI parameters after long-distance running among runners. The exchange has been speculated as the interaction between water molecules and cartilage matrix. In general, the values of those MRI parameters would decrease initially after running, and then experience an increase to the baseline level. The volume of cartilage and meniscus and the collagen arrays represented by T2 (T2*) changes more rapidly than the proteoglycan represented by T1ρ changes after running. Superficial and medial areas of knee cartilage and meniscus are more susceptible to mechanical loading. More studies are needed in superficial, medial and deep sub-areas using MRI parameters more than T2 values, especially volume and T1ρ values in at more frequent time intervals in order to draw a picture on a clear picture of the trend.
Data availability
Not applicable.
References
Schueller-Weidekamm C, Schueller G, Uffmann M, Bader T. Incidence of chronic knee lesions in long-distance runners based on training level: findings at MRI. Eur J Radiol. 2006;58(2):286–93.
Subburaj K, Kumar D, Souza RB, Alizai H, Li X, Link TM, Majumdar S. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med. 2012;40(9):2134–41.
Souza RB, Stehling C, Wyman BT, Hellio Le Graverand MP, Li X, Link TM, Majumdar S. The effects of acute loading on T1rho and T2 relaxation times of tibiofemoral articular cartilage. Osteoarthritis Cartilage. 2010;18(12):1557–63.
Chen M, Qiu L, Shen S, Wang F, Zhang J, Zhang C, Liu S. The influences of walking, running and stair activity on knee articular cartilage: quantitative MRI using T1 rho and T2 mapping. PLoS One. 2017;12(11): e0187008.
Horisberger M, Fortuna R, Valderrabano V, Herzog W. Long-term repetitive mechanical loading of the knee joint by in vivo muscle stimulation accelerates cartilage degeneration and increases chondrocyte death in a rabbit model. Clin Biomech. 2013;28(5):536–43.
Smith RL, Carter DR, Schurman DJ. Pressure and shear differentially alter human articular chondrocyte metabolism: a review. Clin Orthop Relat Res. 2004;(427 Suppl):S89–95.
Fang T, Zhou X, Jin M, Nie J, Li X. Molecular mechanisms of mechanical load-induced osteoarthritis. Int Orthop. 2021;45(5):1125–36.
Alentorn-Geli E, Samuelsson K, Musahl V, Green CL, Bhandari M, Karlsson J. The association of recreational and competitive running with hip and knee osteoarthritis: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2017;47(6):373–90.
Subburaj K, Kumar D, Souza RB, et al. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med. 2012;40(9):2134–41.
Liu B, Lad NK, Collins AT, et al. In vivo tibial cartilage strains in regions of cartilage-to-cartilage contact and cartilage-to-meniscus contact in response to walking. Am J Sports Med. 2017;45(12):2817–23.
McCann L, Ingham E, Jin Z, Fisher J. Influence of the meniscus on friction and degradation of cartilage in the natural knee joint. Osteoarthritis Cartilage. 2009;17(8):995–1000.
Chen S, Fu P, Wu H, Pei M. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res. 2017;370(1):53–70.
Kleemann RU, Krocker D, Cedraro A, Tuischer J, Duda GN. Altered cartilage mechanics and histology in knee osteoarthritis: relation to clinical assessment (ICRS Grade). Osteoarthritis Cartilage. 2005;13(11):958–63.
Liess C, Lüsse S, Karger N, Heller M, Glüer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10(12):907–13.
Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–8.
Van Breuseghem I. Ultrastructural MR imaging techniques of the knee articular cartilage: problems for routine clinical application. Eur Radiol. 2004;14(2):184–92.
Tao H, Qiao Y, Hu Y, et al. Quantitative T2-mapping and T2⁎-mapping evaluation of changes in cartilage matrix after acute anterior cruciate ligament rupture and the correlation between the results of both methods. Biomed Res Int. 2018;2018:7985672.
Behzadi C, Welsch GH, Laqmani A, et al. The immediate effect of long-distance running on T2 and T2* relaxation times of articular cartilage of the knee in young healthy adults at 3.0 T MR imaging. Br J Radiol. 2016;89(1064):20151075.
Bolbos RI, Link TM, Ma CB, Majumdar S, Li X. T1rho relaxation time of the meniscus and its relationship with T1rho of adjacent cartilage in knees with acute ACL injuries at 3 T. Osteoarthritis Cartilage. 2009;17(1):12–8.
Rauscher I, Stahl R, Cheng J, et al. Meniscal measurements of T1rho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology. 2008;249(2):591–600.
Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med. 2005;54(5):1087–93.
Li X, Pai A, Blumenkrantz G, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61(6):1310–8.
Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142.
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Zhang P, Yu B, Zhang R, et al. Longitudinal study of the morphological and T2* changes of knee cartilages of marathon runners using prototype software for automatic cartilage segmentation. Br J Radiol. 2021;94(1119):20200833.
Kessler MA, Glaser C, Tittel S, Reiser M, Imhoff AB. Recovery of the menisci and articular cartilage of runners after cessation of exercise: additional aspects of in vivo investigation based on 3-dimensional magnetic resonance imaging. Am J Sports Med. 2008;36(5):966–70.
Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage. 2010;18(3):358–64.
Kersting UG, Stubendorff JJ, Schmidt MC, Brüggemann GP. Changes in knee cartilage volume and serum COMP concentration after running exercise. Osteoarthritis Cartilage. 2005;13(10):925–34.
Willwacher S, Mählich D, Trudeau MB, et al. The habitual motion path theory: evidence from cartilage volume reductions in the knee joint after 75 minutes of running. Sci Rep. 2020;10(1):1363.
Kessler MA, Glaser C, Tittel S, Reiser M, Imhoff AB. Volume changes in the menisci and articular cartilage of runners: an in vivo investigation based on 3-D magnetic resonance imaging. Am J Sports Med. 2006;34(5):832–6.
Luke AC, Stehling C, Stahl R, et al. High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running: does long-distance running lead to cartilage damage? Am J Sports Med. 2010;38(11):2273–80.
Stehling C, Luke A, Stahl R, et al. Meniscal T1rho and T2 measured with 3.0T MRI increases directly after running a marathon. Skeletal Radiol. 2011;40(6):725–35.
Crowder HA, Mazzoli V, Black MS, et al. Characterizing the transient response of knee cartilage to running: decreases in cartilage T2 of female recreational runners. J Orthop Res. 2021.
Cha JG, Lee JC, Kim HJ, et al. Comparison of MRI T2 relaxation changes of knee articular cartilage before and after running between young and old amateur athletes. Korean J Radiol. 2012;13(5):594–601.
Esculier JF, Jarrett M, Krowchuk NM, et al. Cartilage recovery in runners with and without knee osteoarthritis: a pilot study. Knee. 2019;26(5):1049–57.
Heckelman LN, Smith WAR, Riofrio AD, et al. Quantifying the biochemical state of knee cartilage in response to running using T1rho magnetic resonance imaging. Sci Rep. 2020;10(1):1870.
Wang Z, Ai S, Tian F, et al. Higher body mass index is associated with biochemical changes in knee articular cartilage after marathon running: a quantitative T2-relaxation MRI study. Orthop J Sports Med. 2020;8(8):2325967120943874.
Hesper T, Miese FR, Hosalkar HS, et al. Quantitative T2(*) assessment of knee joint cartilage after running a marathon. Eur J Radiol. 2015;84(2):284–9.
Boocock M, McNair P, Cicuttini F, Stuart A, Sinclair T. The short-term effects of running on the deformation of knee articular cartilage and its relationship to biomechanical loads at the knee. Osteoarthritis Cartilage. 2009;17(7):883–90.
Qiu L, Perez J, Emerson C, et al. Biochemical changes in knee articular cartilage of novice half-marathon runners. J Int Med Res. 2019;47(11):5671–9.
Nag D, Liney GP, Gillespie P, Sherman KP. Quantification of T(2) relaxation changes in articular cartilage with in situ mechanical loading of the knee. J Magn Reson Imaging. 2004;19(3):317–22.
Otterness IG, Eckstein F. Women have thinner cartilage and smaller joint surfaces than men after adjustment for body height and weight. Osteoarthritis Cartilage. 2007;15(6):666–72.
Funding
This study was funded by the Medical and Health Science & Technology Project of Zhejiang Province (grant number 2020KY711) and the Key Medical Disciplines of Hangzhou (grant number YDYX).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Institutional review board statement
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shu, D., Chen, F., Guo, W. et al. Acute changes in knee cartilage and meniscus following long-distance running in habituate runners: a systematic review on studies using quantitative magnetic resonance imaging. Skeletal Radiol 51, 1333–1345 (2022). https://doi.org/10.1007/s00256-021-03943-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03943-0