Abstract
Objectives
To investigate the associations of medial and lateral patellofemoral osteoarthritis (PF-OA) at baseline with symptomatic and radiographic OA outcomes in the medial tibiofemoral compartment (MTFC) over 4 years, according to baseline overweight status.
Methods
Data and MRI images of 600 subjects in the FNIH-OA biomarkers consortium were used. Symptomatic worsening and radiographic progression of MTFC-OA were defined using Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain scores and MTFC joint space narrowing (JSN) from baseline to 4-year follow-up. Baseline MRIs were read to establish PF-OA diagnosis. The association between baseline regional PF-OA pattern and odds for MTFC-OA progression was evaluated using regression models (adjusted for relevant confounding covariates including body mass index (BMI), age, sex, PF alignment measurements, KL grade, and knee alignment). To evaluate the effect modifying role for overweight status, stratification analysis was performed (BMI ≥ 25 vs. < 25 kg/m2).
Results
At baseline, 340 (56.7%), 255 (42.5%), and 199 (33.2%) subjects had OA in the medial, lateral, and both PF compartments. Baseline medial PF-OA was associated with WOMAC pain score and MTFC JSN progression at 4 years (Adjusted OR:1.56[95%CI:1.09–2.23] and 1.59[1.11–2.28], respectively) but not lateral PF-OA. In stratification analysis, overweight status was found to be an effect modifier for medial PF-OA and WOMAC pain (OR in overweight vs. non-overweight subjects:1.65[1.13–2.42] vs. 0.50[0.12–1.82]) as well as MTFC-JSN progression (1.63[1.12–2.4] vs. 0.75[0.19–2.81]).
Conclusions
In addition to the known confounding effect of BMI for PF-OA and MTFC-OA, the overweight status may also play an effect modifier role in the association between baseline medial PF-OA and MTFC-OA progression, which is amenable to secondary prevention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The knee is commonly subdivided into three compartments, the medial and lateral tibiofemoral (TF) and the patellofemoral (PF) compartments. Compared with the lateral TF compartment, symptomatic osteoarthritis (OA) of the medial TF compartment (MTFC) is more prevalent and the most common reason for knee replacement [1, 2]. The high prevalence and burden of knee OA and lack of effective disease-modifying medical treatments [3] emphasize the importance of primary and secondary preventive measures in managing this condition. Identifying risk factors for incidence and progression of MTFC-OA is warranted to implement risk modifications and relevant preventative measures optimally.
Recent reports suggested that the OA pathogenesis in the knee compartments is interrelated [4, 5]. PF-OA is also very common, and compared with TF-OA, it tends to occur in the younger population and is primarily associated with underlying abnormal PF morphology measurements [5,6,7]. It has been shown that up to 60% of subjects with PF-OA develop OA in the TF joint in the future [8], suggesting that PF-OA may precede TF-OA development [9]. PF-OA and TF-OA share several common risk factors, ranging from demographic characteristics [10, 11] (e.g., age, gender, BMI, family history, and previous knee injuries) to specific biomechanical derangements (e.g., knee alignment and PF morphology measurements) [4, 5, 7, 12]. It is yet to be determined whether the sequential development and high prevalence of concurrent PF-OA and TF-OA are merely due to these confounding risk factors.
The Osteoarthritis Initiative (OAI) study represents an extensive public database containing longitudinal imaging and clinical data of knee OA based on annual visits from thousands of subjects from four centers. Within the OAI, the Foundation for the National Institutes of Health (FNIH) OA biomarkers consortium study was conducted to investigate several clinical, imaging, and biochemical biomarkers as indicators for symptomatic or radiographic MTFC-OA progression [13]. The FNIH OA biomarkers consortium study design provides an opportunity to evaluate MTFC-OA progression risk factors with the possibility of controlling for confounding effects [14, 15] of relevant covariates (e.g., knee alignment and PF morphology measurements). We hypothesized that baseline PF-OA is associated with OA progression in the MTFC according to baseline overweight status. Hence, we used the FNIH OA biomarkers consortium study database to assess the possible role of the regional pattern of PF-OA (medial or lateral) as predictors for symptomatic, radiographic, and MRI-based TF-OA progression over the 4-year follow-up and to evaluate roles of overweight status as an effect modifier.
Materials and methods
In this analysis, several TF-OA-related outcomes were defined using baseline and follow-up clinical, radiographic, and magnetic resonance imaging (MRI) data. The primary exposures of this study (medial and lateral PF-OA features) were defined using findings of the baseline knee MRI images. The institutional review boards of the University of California, San Francisco (OAI Coordinating Center; Approval Number: 10–00532), and all other OAI clinical centers approved the OAI study, and informed consent was taken from subjects before the study.
Study population
In the current analysis, all 600 subjects of the FNIH OA biomarkers consortium, a nested study with a case-control design within the OAI, were included. The FNIH OA biomarkers consortium is primarily designed and aimed at the comprehensive investigation of various biomarkers for predicting symptomatic and radiographic MTFC-OA progression. Demographic, clinical, radiographic, and MRI data and images were obtained from the OAI open-access database for this analysis. The detailed description of the FNIH OA biomarkers consortium design can be found in a previous report [13] and the study database at https://data-archive.nimh.nih.gov/oai.
Subjects were included in the FNIH OA biomarkers consortium if they had available demographic and clinical data as well as knee radiographs and 3-T MRI images without artifacts at baseline and 24 months. Subjects with at least one knee with a baseline Kellgren Lawrence grade (KLG) of at least one or higher (from the central readings of the radiographs) were eligible. Subjects with histories of surgeries with metal implants in bones or total knee/hip replacement and subjects with advanced knee OA at baseline (based on standardized Western Ontario and McMaster universities osteoarthritis [WOMAC] pain score > 91 or minimum medial joint space width [JSW] < 1.0 mm) were excluded. WOMAC is a 24-item, condition-specific questionnaire, which consists of three subscales (pain, stiffness, and physical function) used for assessing hip and knee OA pain [14].
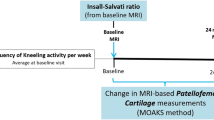
Subjects were evaluated for symptomatic worsening or radiographic MTFC-OA progression from baseline to 24–48-month follow-up visits, and using a random selection method, 194 knees with both symptomatic worsening and radiographic progression, 103 with only symptomatic worsening, 103 with only radiographic progression, and 200 without symptomatic worsening nor radiographic progression were included in the FNIH OA biomarkers consortium (Fig. 1).
Study Timeline. BMI: Body Mass Index; ISR, Insall–Salvati Ratio; JSN: Joint Space Narrowing; KLG, Kellgren Lawrence Grade; LPT, Lateral Patellar Tilt; MRI: Magnetic Resonance Imaging; MTFC: Medial TF Compartment; OA: Osteoarthritis; PF: Patellofemoral; TF: Tibiofemoral; TGD, Trochlear Groove Depth; TT-TG, Tibial Tuberosity to Trochlear Groove; WOMAC: The Western Ontario and McMaster Universities Osteoarthritis Index
MTFC-OA symptomatic worsening, radiographic progression, and MRI-based worsening (outcomes)
Symptomatic MTFC-OA worsening
Annual clinical visits of subjects in the OAI included assessment of knee symptoms with WOMAC pain score. Symptomatic TF-OA worsening was defined as a nine or more points increase in the standardized WOMAC pain scores (scaled to 0–100) between baseline and 24–48 months (this cutoff was based on previous literature on the minimum clinically significant threshold) [13].
Radiographic MTFC-OA progression
Non-fluoroscopic fixed flexion protocol was used to perform radiography of knees in each visit [16]. Images were read centrally to determine (medial) JSW and (medial) joint space narrowing grade (JSN grade, OARSI atlas grades 0-to-3) [17]. Interval decrease in JSW equating 0.7 mm or more from baseline to 24 or 48 months was defined as radiographic MTFC-OA progression (this cutoff was based on the mean and standard deviation [SD] of changes during 1 year in 90 subjects of the OAI control group) [13].
MRI-based TF-OA worsening
In the OAI, 3-T MRI devices (Trio, Siemens Healthcare, Erlangen, Germany) were used to perform image acquisition. Coronal 2D intermediate-weighted (IW) turbo spin-echo (TSE), sagittal 3D dual-echo at steady state (DESS), coronal, and axial multiplanar reformations of the 3D DESS and sagittal IW fat-saturated TSE sequences were included in the standard OAI MRI protocol and used for assessment [18].
The validated semi-quantitative MRI OA knee scoring (MOAKS) method was used to assess OA-related features in the knee compartments at baseline [19]. MOAKS is a semi-quantitative scoring instrument for the assessment of knee OA features in MRI (including cartilage lesions, bone marrow lesions (BMLs), elements of meniscal morphology, etc. [19]). Two musculoskeletal radiologists read the MRIs according to the MOAKS instrument to quantify the extent and severity of cartilage lesions and BMLs in 14 subregions of the knee [19, 20].
The criteria proposed by Runhaar et al. [21] were used to evaluate the clinically meaningful worsening in cartilage lesions and BML MOAKS scores over the 24 months in the anterior, central, and posterior subregions of the medial and lateral femur, tibia, and patella. Cartilage lesions worsening was defined as an increase in the percentage of full-thickness cartilage loss or size of any cartilage loss, and BML worsening was defined as an increase in the size or number of BMLs.
MRI-based regional PF-OA features (exposures)
Using the MOAKS scores, baseline MRI-based PF-OA was defined as the presence of a definite osteophyte (scores >0) with concomitant full- or partial-thickness cartilage lesion (scores >1) in the trochlear or patellar subregions [19].
Demographic and clinical characteristics and MRI-based PF morphology measurements (covariates)
In addition to body mass index (BMI) as a known risk factor for both PF and TF-OA, demographic (age, sex) and biomechanical (knee alignment as varus, valgus, or normal) characteristics, which were extracted from the OAI database, additional MRI-based PF alignment measurements were assessed in MRI images and used for this analysis. Two musculoskeletal radiologists evaluated baseline axial IW TSE knee MRI to measure trochlear groove depth (TGD), tibial tuberosity-to-trochlear groove (TT-TG), and lateral patellar tilt (LPT), and sagittal IW fat-saturated TSE sequences were analyzed to measure the Insall-Salvati ratio (ISR). They had 4 years of experience and were blinded to the outcomes [4, 5, 7]. The reliability of all these MRI-based measurements has been described previously [4, 22,23,24].
Statistical analysis
The associations between baseline regional PF-OA pattern (medial, lateral, and overall) and baseline JSN grade and WOMAC pain scores were assessed using linear regression models. Similar linear regression models were used to study the associations between baseline regional PF-OA pattern and baseline MOAKS-based cartilage lesions and BML scores in subregions of the MTFC and LTFC. Results are presented as the beta for PF-OA presence along with the 95% confidence interval (CI) and p value.
Logistic regression models were used to assess the association between baseline regional PF-OA pattern and symptomatic worsening and radiographic MTFC-OA progressions, as well as the worsening of the MOAKS-based scores of cartilage lesions and BML scores in the MTFC and LTFC. Results are presented as the estimated odds ratio (OR) for the presence of the PF-OA along with the 95% CI and p value.
Baseline BMI was used to stratify the dataset into normal subjects (BMI < 25 kg/m2) and overweight subjects (BMI ≥ 25 kg/m2), and TF-OA features and PF-OA frequency were compared across the two groups. Subsequently, the association between regional PF-OA pattern with baseline WOMAC pain score and JSN grade and symptomatic and radiographic MTFC-OA was studied within each stratum.
All regression models were adjusted for the relevant confounding effects of age, sex, BMI, knee alignment, and PF morphology measurements (i.e., TGD, TT-TG, LPT, and ISR). Logistic regression models were also adjusted for baseline radiographic TF-OA severity (KLG). The reported p values for multiple comparisons were corrected using the Benjamini and Hochberg method, and the corrected p values lower than 0.05 were considered statistically significant. All analyses were performed using the R statistical package version 3.6.1 (foundation for statistical computing, Vienna, Austria).
Results
The mean age and BMI in the FNIH OA biomarkers consortium subjects were 61.55 (± 8.88) years and 30.72 (± 4.78) kg/m2, respectively (539 subjects had BMI ≥ 25 kg/m2, and 60 subjects had BMI < 25 kg/m2). Out of 600 subjects, 353 (58.8%) were female, and 247 (41.2%) were male. Moreover, 340, 255, and 432 subjects had (MRI-based) medial, lateral, and overall PF-OA at baseline, respectively (Table 1).
Associations between regional PF-OA pattern with symptomatic and radiographic MTFC-OA
Medial PF-OA was associated with WOMAC pain score (Beta: 0.69 [0.16–1.21]) and JSN grade (Beta: 0.21 [0.08–0.34]) at baseline, as well as WOMAC pain score worsening (OR: 1.56 [1.09–2.23]), JSN progression (OR: 1.59 [1.11–2.28]), and JSN-and-WOMAC pain score progression (OR: 2.14 [1.45–3.19]) over the follow-ups (Table 2).
In the stratification analysis, BMI did not change significantly between the baseline and the 2-year follow-up visit in overweight and non-overweight subjects. Although baseline JSN grade, medial, and overall PF-OA frequency and longitudinal TF-OA features (JSN progression and WOMAC pain score worsening) were not significantly different between patients with BMI ≥ 25 kg/m2 and < 25 kg/m2 (Table 1), medial PF-OA retained the same associations with baseline WOMAC pain score and JSN grade as well as WOMAC pain score and JSN grade progression in overweight patients (BMI ≥ 25 kg/m2) (Table 2 and Fig. 2). Nonetheless, in patients with normal weight (BMI < 25 kg/m2), no such associations between medial PF-OA and symptomatic or radiographic MTFC-OA were detected (Table 2). Crude models, as well as adjusted models (adjusting for all the mentioned variables before and after inclusion of BMI), are presented in Table 2.
Association Between Medial PF-OA and MTFC-OA Outcomes in Overweight Subjects. Axial DESS sequence obtained from the baseline MRI of a 57-year-old female (BMI: 31.2 kg/m2) which demonstrates features of medial PF-OA (A) with patellar cartilage (arrowhead) and trochlear osteophyte (arrow) MOAKS scores of 2. Between baseline (B) and 48-month (C) visits, there was radiographic progression of MTFC-OA (minimum JSW decrease (≥ 0.7 mm) from 2.0 to 1.2 mm) and symptomatic MTFC-OA (WOMAC pain score increased from 0 to 3) progression. DESS sequence from the baseline MRI of a 56-year-old male (BMI: 32 kg/m2) without medial PF-OA (D). Between baseline (E) and 48-month (F) visits, this subject was not reported with radiographic MTFC-OA (JSW decrease (< 0.7 mm) from 4.9 to 4.7 mm) or symptomatic MTFC-OA (WOMAC pain scores were 0 in both visits) progression. BMI: Body Mass Index; DESS: Dual Echo Steady State; JSW: Joint Space Width; MOAKS: MRI OA Knee Score; MRI: Magnetic Resonance Imaging; MTFC: Medial Tibiofemoral Compartment; OA: Osteoarthritis; PF: Patellofemoral; WOMAC: Western Ontario and McMaster Universities OA; JSW: Joint Space Width
Lateral PF-OA was not associated with symptomatic and radiographic MTFC-OA (Table 3); however, overall PF-OA was associated with WOMAC pain score and JSN grade (at baseline and progression over follow-ups) (Supplementary Table 1).
Associations between regional PF-OA pattern with MRI-based TF-OA features
Existence of medial PF-OA was associated with higher cartilage lesion scores in central (Beta: 0.49 [0.33–0.64]) and posterior (Beta: 0.25 [0.10–0.4]) femoral as well as anterior tibial (Beta: 0.27 [0.15–0.4]) subregions of the MTFC at baseline. The presence of medial PF-OA at baseline was also associated with higher BML scores in central femoral (Beta: 0.17 [0.06–0.27]) and anterior tibial (Beta: 0.19 [0.09–0.29]) subregions of the MTFC at baseline (Table 4).
Lateral PF-OA was associated with higher cartilage lesion scores in central femoral (Beta: 0.17 [0.08–0.26]) and anterior tibial (Beta: 0.22 [0.10–0.34]) subregions in the LTFC as well as higher odds of BML score worsening over follow-ups in the central tibial (OR: 7.42 [2.09–32.61]) subregion of the LTFC (Supplementary Table 2). However, the presence of the lateral PF-OA was not associated with OA-related lesions in subregions of the MTFC. Overall, PF-OA was also associated with MRI-based TF-OA features in certain subregions of the MTFC (Supplementary Table 3).
Discussion
In this analysis, we assessed the associations between regional patterns of PF-OA and the development/worsening of symptomatic and radiographic MTFC-OA after proper adjustment for several relevant demographic (age, sex, and BMI) and biomechanical (knee alignment and PF morphology measurements) confounders. We showed whether medial PF-OA is associated with both higher prevalence of symptomatic and radiographic MTFC-OA in the baseline and their worsening after 48 months, as well as MRI-based features of MTFC-OA and their worsening over this period, suggesting a potential role for medial PF-OA as a predictor for the MTFC-OA. However, Lateral PF-OA was not associated with symptomatic and radiographic MTFC-OA at baseline or during follow-up. We also proposed an effect modification role [14, 15] for the overweight status in the association between baseline medial PF-OA and MTFC-OA outcomes.
It has been shown that elevated BMI is associated with higher incidence and progression of TF-OA [2]. BMI is also considered a risk factor for PF-OA and a combined disease pattern [10]. Moreover, higher BMI is observed in adults with PF pain compared with healthy controls [25]. In this regard, it has been proposed that weightloss in obese subjects with PF-OA and TF-OA might result in reduced PF joint compressive loading during walking [26]. We included the BMI in our regression model as a relevant confounder, as it is a risk factor for both PF-OA and TF-OA. Besides, as high BMI is amenable to secondary prevention measures such as weightloss, we investigated the role of overweight status as a potential effect modifier in association between PF-OA and MTFC-OA outcomes. Herein, we showed that medial PF-OA is associated with the progression of MTFC-OA only in the overweight subjects (≥ 25 kg/m2). This finding points to the potential mechanical derangement as the mechanism behind the association of OA in the PF and medial TF compartments and also highlights the beneficial impact of weightloss as a preventive measure for the MTFC-OA outcomes, specifically in those overweight subjects, who have diagnosed with baseline medial PF-OA. [27]
Previous studies have also suggested that BMI may play a role as an effect modifier for other risk factors of TF-OA outcomes. It has been shown that obese patients have significantly worse scores in Knee Injury and OA Outcome Score (KOOS) subscales [26]. Evaluating OAI patients revealed that higher BMI is correlated with more severe cartilage degeneration in individuals with risk factors for knee OA [28]. Moreover, weight gain increases the chances of worse cartilage degeneration [29], and weight loss modifies the course of knee cartilage degeneration [30]. Here, we showed that although medial PF-OA increases the risk of MTFC-OA progression, the effect may be only seen in overweight patients, which might have implications for more aggressive weight-loss interventions.
Clinical and epidemiologic studies on knee OA are mainly focused on the TF compartments [31], though the PF compartment is an important cause for the OA symptoms. In a meta-analysis, Hart et al. indicated that a relatively high prevalence of radiographic PF-OA is observed in symptomatic subjects [32], and 32% and 52% of these subjects showed BML and cartilage lesions in their PF compartment in MRI assessments [32]. Previous studies suggested that the development of PF-OA usually preceded the development of TF-OA in subjects with concurrent PF- and TF-OA [32]. In this regard, Stefanik et al. investigated developments of MRI-based OA features (BMLs and cartilage lesions) in the PF and TF compartments over 84 months [33]. Their study revealed that although most subjects with isolated PF or TF involvements retain their isolated pattern of OA features over the follow-ups, subjects with isolated MRI-based PF-OA features (vs. subjects with TF-OA features) at baseline have higher odds of developing OA in the other compartment. Moreover, Duncan et al. assessed the association between PF-OA and TF-OA in 414 symptomatic subjects over 36 months and showed that baseline PF-OA might increase TF-OA development and progression risk [9]. Given that PF-OA and TF-OA share several common risk factors (ranging from demographic characteristics to biomechanical derangements), it is not fully understood that the sequential development of PF-OA and TF-OA are due to these shared risk factors or one being an independent risk factor for the other.
In this analysis, we showed that the medial PF-OA is independently associated with symptomatic worsening, radiographic progression, and MRI-based MTFC-OA worsening after adjusting for the effects of these demographic characteristics (age, sex, and BMI) or biomechanical derangements (knee alignment and PF morphology measurements). A similar pattern of associations may exist between lateral PF-OA and OA features in the LTFC. This is in line with the previous reports that OA features tend to medialize or lateralize in the knee compartments during the disease process as Kornaat et al. found that cartilage lesions in either side of the PF compartment are associated with the same side’s cartilage lesions and bone marrow edema in the TF compartments [34].
PF-OA may precede and contribute to TF-OA development [9, 33]. Although these two entities share common risk factors, the presence of the medial PF-OA is associated with a higher prevalence of MTFC-OA features after controlling the effects of these risk factors (showed in this analysis). Considering these two findings, medial PF-OA may be a risk factor for symptomatic worsening and radiographic progression of the MTFC-OA.
Although we used the FNIH OA biomarkers consortium, a good platform for evaluating the association between OA in MTFC and its risk factors, the findings of this analysis may have been tempered by several limitations. First, knee radiography is the accepted imaging modality in the clinical practice for assessing PF-OA. However, the OAI imaging protocol does not include lateral or knee skyline Merchant radiographic views. We used a previously validated PF-OA definition in MRI images for this analysis to address this limitation [19]. Second, OA in knee compartments is a chronic disease, usually developing over several years. Hence, to fully delineate the associations between PF- and TF-OA, longer follow-up assessments are warranted. Third, we only used the baseline BMI measurements for stratification according to the over-weight status as BMI may alter during the follow-up and considered a time-varying covariate. To address this limitation, we have demonstrated that the BMI has not changed between the baseline and 2-year follow-up among overweight and non-overweight subjects. Fourth, the reported associations in the current study should not be interpreted as causal relationships. This research is observational by its nature, and hence, the cause-and-effect relationship between medial PF-OA and MTFC-OA progression cannot be inferred. Fifth, unequaled sample sizes were obtained after grouping the subjects based on their BMI, which may have tempered the power of this analysis to investigate the potential association between PF- and TF-OA in subjects with BMI < 25 kg/m2. Finally, all study subjects in the FNIH OA biomarkers consortium are affected by some degree of the OA (in the TF compartments). Therefore, future studies should confirm these findings before generalizing these results to the general population [4].
In conclusion, our findings showed that medial PF-OA might play the role as a risk factor for symptomatic worsening and radiographic MTFC-OA progression, despite the effects of the common risk factors of PF- and TF-OA. Moreover, this association is probably affected by the overweight status. The findings of this work suggested that PF-OA might be a (stand-alone) TF-OA risk factor (in subjects with high BMI). Future studies are required to confirm the potential cause-and-effect relationship between medial PF-OA and MTFC-OA progression and to study whether weight loss as a secondary preventive measure in overweight subjects with (medial) PF-OA would improve MTFC-OA outcomes.
Abbreviations
- (BMI):
-
Body mass index
- (BML):
-
Bone marrow lesion
- (CI):
-
Confidence interval
- (DESS):
-
Dual-echo at steady state
- (FNIH):
-
Foundation for the National Institutes of Health
- (ISR):
-
Insall-Salvati ratio
- (IW):
-
Intermediate-weighted
- (JSN):
-
Joint space narrowing
- (JSW):
-
Joint space width
- (KLG):
-
Kellgren Lawrence grade
- (LPT):
-
Lateral patellar tilt
- (LTFC):
-
Lateral tibiofemoral compartments
- (MRI):
-
Magnetic resonance imaging
- (MTFC):
-
Medial tibiofemoral compartments
- (MOAKS):
-
MRI osteoarthritis knee scoring
- (MPRs):
-
Multiplane reconstructions
- (OR):
-
Odds ratio
- (OA):
-
Osteoarthritis
- (OAI):
-
Osteoarthritis Initiative
- (PF):
-
Patellofemoral
- (SD):
-
Standard deviation
- (TT-TG):
-
Tibial tuberosity to trochlear groove
- (TF):
-
Tibiofemoral
- (TGD):
-
Trochlear groove depth
- (TSE):
-
Turbo spino-echo
- (WE):
-
Water-excitation
- (WOMAC):
-
Western Ontario and McMaster universities osteoarthritis
References
Huétink K, Nelissen RG, Watt I, van Erkel AR, Bloem JL. Localized development of knee osteoarthritis can be predicted from MR imaging findings a decade earlier. Radiology. 2010;256(2):536–46.
Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252(3):772–80.
Hochberg MC, Guermazi A, Guehring H, Aydemir A, Wax S, Fleuranceau-Morel P, et al. Effect of intra-articular Sprifermin vs placebo on Femorotibial joint cartilage thickness in patients with osteoarthritis: the FORWARD randomized clinical trial. Jama. 2019;322(14):1360–70.
Haj-Mirzaian A, Guermazi A, Pishgar F, Roemer FW, Sereni C, Hakky M, et al. Patellofemoral morphology measurements and their associations with tibiofemoral osteoarthritis-related structural damage: exploratory analysis on the osteoarthritis initiative. Eur Radiol. 2020;30(1):128–40.
Haj-Mirzaian A, Guermazi A, Pishgar F, Pourvaziri A, Roemer F, Sereni C, et al. Association of patella Alta with worsening of patellofemoral osteoarthritis-related structural damage: data from the osteoarthritis initiative. Osteoarthr Cartil. 2019;27(2):278–85.
Lankhorst NE, Damen J, Oei EH, Verhaar JAN, Kloppenburg M, Bierma-Zeinstra SMA, et al. Incidence, prevalence, natural course and prognosis of patellofemoral osteoarthritis: the cohort hip and cohort knee study. Osteoarthr Cartil. 2017;25(5):647–53.
Haj-Mirzaian A, Guermazi A, Hafezi-Nejad N, Sereni C, Hakky M, Hunter DJ, et al. Superolateral Hoffa’s fat pad (SHFP) oedema and patellar cartilage volume loss: quantitative analysis using longitudinal data from the Foundation for the National Institute of health (FNIH) osteoarthritis biomarkers consortium. Eur Radiol. 2018;28(10):4134–45.
Kobayashi S, Pappas E, Fransen M, Refshauge K, Simic M. The prevalence of patellofemoral osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil. 2016;24(10):1697–707.
Duncan R, Peat G, Thomas E, Hay EM, Croft P. Incidence, progression and sequence of development of radiographic knee osteoarthritis in a symptomatic population. Ann Rheum Dis. 2011;70(11):1944–8.
McAlindon T, Zhang Y, Hannan M, Naimark A, Weissman B, Castelli W, et al. Are risk factors for patellofemoral and tibiofemoral knee osteoarthritis different? J Rheumatol. 1996;23(2):332–7.
Zhang W, McWilliams DF, Ingham SL, Doherty SA, Muthuri S, Muir KR, et al. Nottingham knee osteoarthritis risk prediction models. Ann Rheum Dis. 2011;70(9):1599–604.
Elahi S, Cahue S, Felson DT, Engelman L, Sharma L. The association between varus-valgus alignment and patellofemoral osteoarthritis. Arthritis Rheum. 2000;43(8):1874–80.
Roemer FW, Guermazi A, Collins JE, Losina E, Nevitt MC, Lynch JA, et al. Semi-quantitative MRI biomarkers of knee osteoarthritis progression in the FNIH biomarkers consortium cohort - Methodologic aspects and definition of change. BMC Musculoskelet Disord. 2016;17(1):466.
Vander Weele TJ. Confounding and effect modification: distribution and measure. Epidemiol Methods. 2012;1(1):55–82.
Corraini P, Olsen M, Pedersen L, Dekkers OM, Vandenbroucke JP. Effect modification, interaction and mediation: an overview of theoretical insights for clinical investigators. Clin Epidemiol. 2017;9:331–8.
Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skelet Radiol. 2003;32(3):128–32.
Culvenor AG, Engen CN, Øiestad BE, Engebretsen L, Risberg MA. Defining the presence of radiographic knee osteoarthritis: a comparison between the Kellgren and Lawrence system and OARSI atlas criteria. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3532–9.
Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthr Cartil. 2008;16(12):1433–41.
Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI osteoarthritis knee score). Osteoarthr Cartil. 2011;19(8):990–1002.
Guermazi A, Roemer FW, Haugen IK, Crema MD, Hayashi D. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat Rev Rheumatol. 2013;9(4):236–51.
Runhaar J, Schiphof D, van Meer B, Reijman M, Bierma-Zeinstra SM, Oei EH. How to define subregional osteoarthritis progression using semi-quantitative MRI osteoarthritis knee score (MOAKS). Osteoarthr Cartil. 2014;22(10):1533–6.
Thakkar RS, Del Grande F, Wadhwa V, Chalian M, Andreisek G, Carrino JA, et al. Patellar instability: CT and MRI measurements and their correlation with internal derangement findings. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):3021–8.
Ye Q, Yu T, Wu Y, Ding X, Gong X. Patellar instability: the reliability of magnetic resonance imaging measurement parameters. BMC Musculoskelet Disord. 2019;20(1):317.
Pandit S, Frampton C, Stoddart J, Lynskey T. Magnetic resonance imaging assessment of tibial tuberosity-trochlear groove distance: normal values for males and females. Int Orthop. 2011;35(12):1799–803.
Hart HF, Barton CJ, Khan KM, Riel H, Crossley KM. Is body mass index associated with patellofemoral pain and patellofemoral osteoarthritis? A systematic review and meta-regression and analysis. Br J Sports Med. 2017;51(10):781–90.
Larsen P, Engberg AS, Motahar I, Ostgaard SE, Elsoe R. Obesity influences the knee injury and osteoarthritis outcome score. Joints. 2019;7(1):8–12.
Kumar D, Beavers D, DeVita P, Messier S. Effects of weight-loss on patellofemoral loading in overweight and obese adults with patellofemoral osteoarthritis: secondary analysis from the idea randomized trial. Osteoarthr Cartil. 2017;25:S171–2.
Baum T, Joseph GB, Nardo L, Virayavanich W, Arulanandan A, Alizai H, et al. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: thirty-six-month follow-up data from a longitudinal, observational multicenter study. Arthritis Care Res. 2013;65(1):23–33.
Bucknor MD, Nardo L, Joseph GB, Alizai H, Srikhum W, Nevitt MC, et al. Association of cartilage degeneration with four year weight gain--3T MRI data from the osteoarthritis initiative. Osteoarthr Cartil. 2015;23(4):525–31.
Gersing AS, Solka M, Joseph GB, Schwaiger BJ, Heilmeier U, Feuerriegel G, et al. Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the osteoarthritis initiative. Osteoarthr Cartil. 2016;24(7):1126–34.
Felson DT. The epidemiology of knee osteoarthritis: results from the Framingham osteoarthritis study. Semin Arthritis Rheum. 1990;20(3 Suppl 1):42–50.
Hart HF, Stefanik JJ, Wyndow N, Machotka Z, Crossley KM. The prevalence of radiographic and MRI-defined patellofemoral osteoarthritis and structural pathology: a systematic review and meta-analysis. Br J Sports Med. 2017;51(16):1195–208.
Stefanik JJ, Guermazi A, Roemer FW, Peat G, Niu J, Segal NA, et al. Changes in patellofemoral and tibiofemoral joint cartilage damage and bone marrow lesions over 7 years: the multicenter osteoarthritis study. Osteoarthr Cartil. 2016;24(7):1160–6.
Kornaat PR, Watt I, Riyazi N, Kloppenburg M, Bloem JL. The relationship between the MRI features of mild osteoarthritis in the patellofemoral and tibiofemoral compartments of the knee. Eur Radiol. 2005;15(8):1538–43.
Acknowledgments
The OAI was a public-private partnership comprised of several contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health (NIH), a branch of the Department of Health and Human Services, and conducted by the Osteoarthritis Initiative (OAI) Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public-use dataset and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Moreover, several grants and direct or in-kind contributions provide the publicly available data from the FNIH OA Biomarkers Consortium, including AbbVie, Amgen, Arthritis Foundation, Artialis; Bioiberica, BioVendor, DePuy, Flexion Therapeutics, GSK, IBEX, IDS, Merck Serono, Quidel, Rottapharm | Madaus, Sanofi, Stryker, the Pivotal OAI MRI Analyses (POMA) study, NIH HHSN2682010000 21C, and the Osteoarthritis Research Society International.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The institutional review boards of the University of California, San Francisco (OAI Coordinating Center; Approval Number: 10–00532), and all other OAI clinical centers approved the OAI study. All subjects have given informed consent before participating in the OAI project. All procedures performed in studies involving human participants were following the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
None of the authors have any conflicting personal or financial relationships that could have influenced the results of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Pishgar, F., Guermazi, A., Ashraf-ganjouei, A. et al. Association between Patellofemoral and medial Tibiofemoral compartment osteoarthritis progression: exploring the effect of body weight using longitudinal data from osteoarthritis initiative (OAI). Skeletal Radiol 50, 1845–1854 (2021). https://doi.org/10.1007/s00256-021-03749-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03749-0