Abstract
Purpose
The present study aims to analyze the accuracy of injections aimed to hit the proximal and depth part of the patellar tendon “target point” in patellar tendinopathy, comparing ultrasound-guided or non-ultrasound-guided (blind) injections.
Methods
A cadaver randomized study was carried out. Injections were performed under ultrasound control, as well as blinded. There were 26 knees from fresh cadavers and injections were placed by 26 practitioners with experience in the use of musculoskeletal ultrasound and injection treatment. Each participant performed 6 ultrasound-guided and 6 blind punctures in different cadaveric specimens. This provided 312 injections that were analyzed in 2 different anatomical cuts, thus providing a database of 624 measurements for statistical analysis.
Results
Statistically significant differences were observed (p < 0.0001) in the distance from the target point between the ultrasound-guided and the non-guided infiltrations. The “unguided” injections were considered to have been performed on average 10 mm away from the target point compared to the “ultrasound-guided” injections. The ultrasound-guided injections obtained an accuracy of 74.36% while the “non-ultrasound-guided” injections obtained an accuracy of 11.54% (p < 0.0001).
Conclusion
The use of ultrasound to guide the positioning of injections on the dorsal side of the proximal patellar tendon had a significantly higher accuracy compared to blind injections. The finding provides knowledge of importance for injection treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment of proximal patellar tendon disorders includes targeted injection treatments. Injections of platelet-rich plasma (PRP) [1, 2] or ultrasound-guided galvanic electrolysis technique (USGET) [1, 3], polidocanol [4, 5], stem cells [6], and hyaluronic acid [7] are in use.
On one hand, the problem arises when doctors want to know whether the puncture or infiltration has produced the desired biological effect. On the other side, practitioners often do not consider whether it has been performed at the correct point on the target tissue. That is, whether it has been ultrasound-guided to the exact point to be treated or it has been performed blind without being able to be sure of the area to which the therapy has been applied [8,9,10]. The positioning of the injection in relation to the tendon and target point is considered of significant importance.
When using injection treatment for proximal patellar tendinopathy, the proximal part of the dorsal side of the patellar tendon is the target point (Fig. 1). Ultrasound has been shown to be useful for diagnostic purposes and to guide injections (Fig. 2) [11].
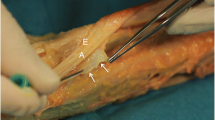
Image of the anatomical dissection of the patellar tendon where its close relationship with the paratenon and Hoffa fat pad can be observed, as well as the proximity of the lower patella of the patella with the deep fibers of the tendon. The deep and proximal part of the patellar tendon is the most frequently injured area (target point)
The aim of the present study was to evaluate the accuracy of ultrasound-guided versus blind injections positioned on the dorsal side of the proximal patellar tendon. As far as the authors know, this is the first work that analyzes the accuracy of ultrasound-guided versus blind punctures inside the injured area of the patellar tendon to provide guidelines for action in future interventions.
Material and methods
Specimens
Informed consent was obtained for all bodies donated to science (document approved by the Ethics and Experimentation Committee of the Faculty of Medicine, Universitat Autònoma Barcelona (UAB), as of 03/27/2015). The cadaveric material comes from the voluntary donation of the body to the Faculty, for teaching and research purposes, in accordance with procedure 2904 approved by the UAB Commission on Ethics in Animal and Human Experimentation on March 27, 2015.
A cadaveric study including 26 fresh knees was performed at the medical school. All the bodies were serologically tested to rule out infectious diseases (HIV, hepatitis B and hepatitis C) before starting the study. Of the 26 donors, 9 were women and 17 men. The average age was 82 years (range 69–98 years) and there were 14 right knees and 12 left knees. The limbs were stored frozen at − 40 °C until 2 h before use.
The authors declare no conflict of interest in this article.
Intervention
Twenty-six practitioners performed the infiltrations. The practitioners had an average of 3.8 years (range 1–15 years) of experience in the use of musculoskeletal ultrasound, and 2 years (range of 2 to 25 years) of experience from needle invasive therapies (needle injections).
The practitioners were fully informed about the objective of the study. The objective was to inject a volume into the deep side of the proximal patellar tendon at the level for the patellar tip (target point), with and without ultrasound-guidance (Fig. 3).
With the help of 5 ml luer-lock syringes, using 30 mm long 21G needles, 1 cc of colored natural latex was injected. The cadaver specimens and practitioners were randomized. Each practitioner conducted 6 ultrasound-guided and 6 blind (without ultrasound guidance) injections, each into a different specimen. After injection, the specimens were immediately frozen at − 40 °C. Altogether, 312 injections were included into a database. For analyses, serial cuts (1 cm thick in the sagittal plane) were made with the help of a vertical band saw—using a 4-mm blade (Medoc BR 400, Medoc SA, Logroño, Spain).
Image data analysis
Immediately after making the cuts, we proceeded to identify the two most significant serial cuts showing the location of the latex injection and photographed it (Canon Digital Camera G11 5x-6.1-30.5 mm 1: 2.8–4.5) with a ruler in the same photo. This information (26 practitioners × 12 infiltrations × 2 cuts) constituted a database of 624 measures (312 ultrasound-guided and 312 blind) for later statistical analysis.
For the computer analysis of the images, Fiji (Fiji Is Just ImageJ) software was used. It is an open source software focused on analyses of biological images [12]. Fiji is a distribution of the open sourced software ImageJ focused on biological image analysis. Using this software, the distance between the point to infiltrate (previously determined target point) and where the infiltration had been positioned, was calculated.
Reproducibility and statistical analysis
The statistical analysis was performed with SAS v9.4 software (SAS Institute Inc., Cary, NC, USA). Statistical decisions were based on 0.05 as the level of significance. A validation of the internal consistency of the database variables as well as out-of-range values and missing values was performed to fully ensure their reliability. The main responsible variable was analyzed, this being the average distance to the target point (target point) in the different sections, categorizing this mean as accuracy.
In the first place, since several punctures were made on the same tendon, the random effect was analyzed. Upon not obtaining statistically significant differences, the punctures were considered as independent. A Kolmogorov-Smirnov normality test for each study and section along with a descriptive analysis of the data was performed. The box diagram of the mean distance to the center of the tendon and the table with the basic descriptive statistics (n, medium, std., minimal, Q1, medium, Q3, maximums and missings) are presented according to whether the injection was ultrasound-guided or blind. The non-parametric contrast was performed for two independent samples.
For the evaluation of Accuracy, a descriptive analysis of the data was performed. It is presented with an Accuracy bar chart and a table with the basic descriptive statistics (N, Percentage) based on whether the injection was ultrasound-guided or not. The Chi-Square independence test was carried out to see whether there were differences in the distribution of the injection Accuracy based on whether it was ultrasound-guided or not. The indicator, accuracy = completely successful versus no accuracy = partially successful and not accurate, was calculated.
Results
There was a significant difference (p < 0.05) in the distance to the target point between the ultrasound-guided and blinded injections (Fig. 4). The median distance to the target point for the 156 blinded punctures was 4.64 mm (Q1 = 3.57, Q3 = 7.85) and for the ultrasound-guided 2.5 mm (Q1 = 1.9, Q3 = 3.11) (Table 1).
Statistically significant differences were observed (p < 0.0001) in the distance to the target point between those that were ultrasound-guided and those that were blinded. In addition, the median for the “non-guided” puncture group was 14 mm while for the “ultrasound-guided” group it was 9.5 mm. All the ultrasound-guided punctures were below the median of the “non-guided” punctures.
In ultrasound-guided punctures, an estimate of the mean distances to the target point of 2.6 mm was obtained, 95% CI = [2.05, 3.15]. In contrast, an estimate of the mean distances to the target point of 6.6 mm, 95% CI = [6.03, 7.13] was obtained in the non-guided punctures.
Statistically significant differences were detected (t value = −10.07; p value < 0.0001) between Ultrasound-guided and unguided (blinded) punctures. The difference between Ultrasound-guided/unguided punctures was − 10.3 mm (95% CI = [− 4.76, − 3.2]). This means that it was estimated that the unguided punctures are performed on average 3.9 mm farther from the target point than the ultrasound-guided punctures (Fig. 5).
Bar chart showing the distance to accuracy of the ultrasound-guided or blind infiltrations. Standing out in the target point is 100% infiltrations, 100% in green with the partially successful ones in blue and the wrong ones in red. Note the clear difference in the green bar of the ultrasound-guided infiltrations over the blind
A significant difference was observed when analyzing correct punctures versus incorrect punctures, depending on whether it is performed unguided or ultrasound-guided. Almost 90% of the unguided punctures are distributed in partially successful (48.1%) and not correct (40.4%) while most of the ultrasound-guided punctures (74.4%) are totally correct punctures and only have a ratio of 3.9% (6 punctures) incorrect (Table 2).
Discussion
The main finding of this study was that ultrasound-guided injections were significantly more accurate than blinded injections when injecting in the proximal patellar tendon. This information might be of significant information when addressing injection treatment to the patellar tendon.
The use of ultrasound-guidance for injections in knee disorders has been widely reported in the literature [8,9,10]. As far as we know, the present work demonstrates, for the first time, that injection treatments targeting the patellar tendon should also be ultrasound-guided.
Injection therapies are in focus for discussion considering their efficiency [1]. In some studies ultrasound-guidance is used, while in others no ultrasound guidance is used, and this difference might be of importance for the effect of the treatment. Therefore, it seems reasonable to establish the most accurate technique to use when injection to a specific target. Different studies show how the thickness and ultrasound characterization of the patellar tendon should be analyzed [13,14,15]. With the popularization of the ultrasound devices every day, more and more articles on ultrasound-guided techniques can be found, demonstrating the rise of these techniques [1, 3, 6, 16].
Ultrasound-guided electrolysis (USGET) has grown in scientific evidence and use in recent years [1, 3]. It has been positioned as an effective conservative technique in the treatment of refractory tendinopathies prior to the surgical option. The local inflammatory reaction produced by the passage of said galvanic current provides the start of the regenerative response of the injured tendon. Subsequently applied eccentric exercise provides an ideal mechanical stimulus for the remodeling of the treated tissue [3, 17]. Initially, 2–3 mA doses were applied but the use of larger doses (8–20 mA) under local anesthesia has been established until a total debridement of the lesion is achieved. The amount of energy provided in a treatment is estimated at between 1000 and 1500 millicoulombs. The application of high doses provides better results and allows for periods of time between major treatments and doing the necessary eccentric exercises between USGET treatments [1, 17].
For all the above, accuracy in the application of the USGET is of vital importance. Therefore, its use must be limited to trained personnel capable of performing it under ultrasound control.
The use of biological therapies such as PRP or mesenchymal stem cells (MSC) has grown in importance in recent years [1, 2, 6]. The importance of finding the target where to deposit the cells (platelets or mesenchymal) is likely crucial for maximal effects [10, 18]. As shown in the article of Abat et al. [8], the infiltration of PRP or MSC without ultrasound guidance could not place these cells in the target point. Hyaluronic acid has been proposed as a complement in the treatment of tendinopathies [7]. It is believed to improve tendon gliding functions and reduce adhesions between the paratenon and the tendon. The need to position the HA between the tendon and the paratenon, seems impossible without the use of ultrasound guidance.
The present study has limitations. We used cadavers from old donors, where the tissues are different to inject into compared to alive young humans.
In conclusion ultrasound-guidance for the positioning of injections on the correct spot of the proximal patellar tendon had a significantly higher accuracy compared to non-ultrasound-guided blind injections. This finding demonstrates the importance of using ultrasound guidance for patellar tendon treatments.
References
Abat F, Alfredson H, Cucchiarini M, Madry H, Marmotti A, Mouton C, et al. Current trends in tendinopathy: consensus of the ESSKA basic science committee. Part II: treatment options. J Exp Orthop. 2018;5(1):38. https://doi.org/10.1186/s40634-018-0145-5.
Di Matteo B, Filardo G, Kon E, Marcacci M. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy a systematic review. Musculoskelet Surg. 2015;99(1):1–9. https://doi.org/10.1007/s12306-014-0340-1.
Abat F, Sánchez-Sánchez JL, Martín-Nogueras AM, Calvo-Arenillas JI, Yajeya J, Méndez-Sánchez R, et al. Randomized controlled trial comparing the effectiveness of the ultrasound-guided galvanic electrolysis technique (USGET) versus conventional electro-physiotherapeutic treatment on patellar tendinopathy. J Exp Orthop. 2016;3(1):34.
Sunding K, Willberg L, Werner S, Alfredson H, Forssblad M, Fahlström M. Sclerosing injections and ultrasound-guided arthroscopic shaving for patellar tendinopathy: good clinical results and decreased tendon thickness after surgery-a medium-term follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2259–68. https://doi.org/10.1007/s00167-014-3028-z.
Alfredson H, Lorentzon R. Sclerosing polidocanol injections of small vessels to treat the chronic painful tendon. Cardiovasc Hematol Agents Med Chem. 2007;5(2):97–100.
Kumar Sahu A, Rath P, Aggarwal B. Ultrasound-guided injections in musculo-skeletal system-an overview. J Clin Orthop Trauma. 2019;10(4):669–73. https://doi.org/10.1016/j.jcot.2019.05.013.
Kumai T, Muneta T, Tsuchiya A, Shiraishi M, Ishizaki Y, Sugimoto K, et al. The short-term effect after a single injection of high-molecular-weight hyaluronic acid in patients with enthesopathies (lateral epicondylitis, patellar tendinopathy, insertional Achilles tendinopathy, and plantar fasciitis): a preliminary study. J Orthop Sci. 2014;19(4):603–11. https://doi.org/10.1007/s00776-014-0579-2.
Abat F, Campos J, Torras J, Madruga M, Planells G, Rodriguez-Baeza A. Comparison of ultrasound-guided versus blind interventions for supraspinatus tendinopathy: a cadaveric study. Muscles, Ligaments and Tendons Journal. 2019;9(3):328–37.
Daley EL, Bajaj S, Bisson LJ, Cole BJ. Improving injection accuracy of the elbow, knee, and shoulder: does injection site and imaging make a difference? A systematic review. Am J Sports Med. 2011;39(3):656–62. https://doi.org/10.1177/0363546510390610.
Sharpe RE Jr, Nazarian LN, Levin DC, Parker L, Rao VM. The increasing role of nonradiologists in performing ultrasound-guided invasive procedures. J Am Coll Radiol. 2013;10(11):859–63. https://doi.org/10.1016/j.jacr.2013.04.016.
Warden SJ, Kiss ZS, Malara FA, Ooi AB, Cook JL, Crossley KM. Comparative accuracy of magnetic resonance imaging and ultrasonography in confirming clinically diagnosed patellar tendinopathy. Am J Sports Med. 2007;35(3):427–36.
Schindelin J, Arganda-Carreras I, Frize E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. https://doi.org/10.1038/nmeth.2019.
Gellhorn AC, Morgenroth DC, Goldstein B. A novel sonographic method of measuring patellar tendon length. Ultrasound Med Biol. 2012;38(5):719–26. https://doi.org/10.1016/j.ultrasmedbio.2012.01.020.
Skou ST, Aalkjaer JM. Ultrasonographic measurement of patellar tendon thickness--a study of intra- and interobserver reliability. Clin Imaging. 2013;37(5):934–7. https://doi.org/10.1016/j.clinimag.2013.01.007.
Del Baño-Aledo ME, Martínez-Payá JJ, Ríos-Díaz J, Mejías-Suárez S, Serrano-Carmona S, de Groot-Ferrando A. Ultrasound measures of tendon thickness: intra-rater, inter-rater and inter-machine reliability. Muscles Ligaments Tendons J. 2017;7(1):192–9. https://doi.org/10.11138/mltj/2017.7.1.192.
Burke CJ, Adler RS. Ultrasound-guided percutaneous tendon treatments. AJR Am J Roentgenol. 2016;207(3):495–506. https://doi.org/10.2214/AJR.16.16089.
Abat F, Alfredson H, Cucchiarini M, Madry H, Marmotti A, Mouton C, et al. Current trends in tendinopathy: consensus of the ESSKA basic science committee. Part I: biology, biomechanics, anatomy and an exercise-based approach. J Exp Orthop. 2017;4(1):18. https://doi.org/10.1186/s40634-017-0092-6.
James SL, Ali K, Pocock C, Robertson C, Walter J, Bell J, et al. Ultrasound guided dry needling and autologous blood injection for patellar tendinosis. Br J Sports Med. 2007;41(8):518–21; discussion 522. https://doi.org/10.1136/bjsm.2006.034686.
Acknowledgments
To the department of anatomy of the UAB (Campus Bellaterra) for its collaboration in the project, especially to Manel Querol for his involvement and diligence. Special mention to donors who have made this study possible with their altruism. We must also make mention of all the colleagues who helped us during the infiltrations in the cadaverous parts. We are also grateful to Eric L. Goode for his help in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abat, F., Alfredson, H., Campos, J. et al. Ultrasound-guided versus blind interventions in patellar tendon lesions: a cadaveric study. Skeletal Radiol 50, 967–972 (2021). https://doi.org/10.1007/s00256-020-03635-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03635-1