Abstract
Objective
To describe the MRI features of paediatric conventional central chondrosarcoma (CC-CS) and correlate with histological grade.
Materials and methods
A retrospective review of children/adolescents with histologically confirmed CC-CS. Data collected included age, sex, skeletal location, and histology from needle biopsy or resection, which was classified as atypical cartilaginous tumours/grade 1 CS (ACT/Gd 1 CS), high-grade chondrosarcoma (HGCS), and dedifferentiated chondrosarcoma (DD-CS). MRI studies were reviewed independently by 2 radiologists blinded to the histology grade, who graded the tumours as ACT/Gd 1 CS, HGCS, and DD-CS based on MRI features.
Results
The study included 7 males and 10 females with mean age 13.9 years (range 6–18 years). Tumours were located in the femur (n = 6), humerus (n = 3), tibia, ilium, scapula, and ulna (n = 1 each), and the small bones of the hands or feet (n = 4). Final histology grade was ACT/Gd 1 CS in 15 cases and HGCS in 2 (both grade 1 CS with focal transition to grade 2), 15 based on surgical specimens, 1 based on open biopsy, and 1 on needle biopsy alone. Predicted MRI grade for the 2 readers was ACT/Gd 1 CS in 11 cases each and HGCS in 6 cases each, indicating a mismatch between predicted MRI grade and histological grade in 8 (47%) cases (4 cases with one reader mismatch and 4 cases with both).
Conclusions
MRI findings in paediatric CC-CS may be misleading, showing features suggestive of HGCS 7 of 17 (41.2%) of cases. This should be taken into consideration when planning surgical treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional central chondrosarcoma (CC-CS) typically presents in patients aged 40–60 years old and accounts for ~ 70% of all CS [1, 2]. Histologically, low-grade CS is sub-classified into atypical cartilaginous tumour (ACT) when involving the appendicular skeleton or grade 1 CS (Gd 1 CS) when involving the flat bones and axial skeleton, high-grade chondrosarcoma (HGCS; grades 2 and 3 CS), and dedifferentiated chondrosarcoma (DD-CS) [3]. CC-CS of the long bones in the paediatric population is rare, accounting for ~ 5% of all cases [4], and the literature to date describes only a few case series, the data from these papers focussing more on prognosis and survival rather than on radiological appearances [5,6,7,8].
Histological grade is one of the most important factors in determining optimal treatment of CC-CS [9, 10], and therefore, accurate pre-operative tumour grading is essential. ACT/Gd 1 CS is commonly managed with intra-lesional curettage (IC) with or without various adjuvant treatments followed by bone grafting or cementation [9, 10], while HGCS and DD-CS require wide local excision (WLE) with or without reconstruction, which may involve the use of an endoprosthetic replacement (EPR) [10]. Pre-operative diagnosis should be confirmed by needle biopsy following discussion at a specialist musculoskeletal sarcoma multidisciplinary team (MDT) meeting [11]. However, mismatch between histological tumour grade suggested by needle biopsy and that following curettage/resection is reported [12], which may potentially result in surgical mismanagement.
The grading of central chondral tumours is typically made on a combination of clinical and imaging findings. Certain MRI features aid in the differentiation between low-grade and high-grade appendicular CC-CS in adults, including bone expansion, active periostitis, cortical destruction, reactive bone and soft tissue oedema, and soft tissue mass [13,14,15,16]. Fayed et al. [17] described similar MRI features for HGCS of the hands and feet. For differentiation of low-grade from high-grade chondrosarcoma using MRI, Fritz et al. reported a diagnostic accuracy of 84.8% [16], while in a recent systematic review, Deckers et al. concluded that further studies were required to identify more reliable imaging features to differentiate ACT from HGCS [18].
The aim of the current study was to identify if the MRI criteria described to differentiate ACT from HGCS in adults could be applied to paediatric CC-CS.
Materials and methods
The study was approved by the local Research and Innovation Centre of The Institute of Orthopaedics under the Integrated Research Application System number 262826, with no requirement for informed patient consent.
The Histopathology Database was searched for all children (age 0–18 years) with a histologically proven diagnosis of enchondroma or CC-CS between January 2007 and May 2020 which yielded 75 results (43 enchondromas and 32 CC-CS). ACT/Gd 1 CS was differentiated histologically from enchondroma using the WHO 2020 criteria which include the presence of increased cellularity, irregular distribution of cells, the occurrence of bi-nucleate cells, and importantly the presence of host bone entrapment [3]. Cases of enchondroma were not further assessed. Of the 32 CC-CS, 7 were excluded since there was no clinical information available in the electronic patient record, 3 were excluded since they were recurrent tumours, and 5 were excluded since they were either surface or peripheral tumours. This left 17 patients with CC-CS for whom adequate imaging studies were available. Two cases had been referred purely for pathological review and had imaging available, but no clinical details or follow-up.
Pre-biopsy radiographs were available in 15 cases while all patients had pre-biopsy/surgery MRI studies, either from the referring hospital (n = 12) or performed at our centre following referral (n = 5; 4 at 1.5 T and 1 at 3 T). Studies performed prior to referral had a variety of sequences, but all had a combination of T1-weighted turbo spin echo (T1W TSE), T2-weighted fast spin echo (T2W FSE), or short tau inversion recovery (STIR) sequences. MRI studies performed following referral consisted of coronal T1W TSE, coronal STIR, sagittal T2W FSE, and axial proton density–weighted fast spin echo (PDW FSE) and spectral attenuated inversion recovery (SPAIR) sequences. Intravenous contrast was not used in any of the cases. MRI studies were reviewed independently by 2 consultant musculoskeletal radiologists with 21 and 8 years’ experience of bone tumour imaging who were blinded to the final histological diagnosis. The following criteria were assessed:
-
Tumour length: maximal tumour length in millimetres on coronal T1W TSE MRI
-
Cortical thickening: thickening of the cortical signal void
-
Cortical destruction: focal loss of the cortical signal void
-
Bone expansion: increase in axial dimensions of the medullary cavity
-
Active periostitis: increased SI around the cortical margin on fluid-sensitive sequences in the presence of an intact bone cortex
-
Bone marrow and soft tissue oedema: poorly defined increased SI in the medullary cavity adjacent to the tumour or in the soft tissues adjacent to the bone on fluid-sensitive sequences
-
Soft tissue mass: extension of tumour through the destroyed cortex into the adjacent soft tissue to any degree, differentiated from soft tissue oedema by its well-defined peripheral margin and identical signal characteristics to intra-osseous tumour
These definitions were as described by Douis et al. [14], the presence of any of these features favouring a diagnosis of HGCS or DD-CS. Based on the overall MRI features, each case was classified as ACT/Gd1 CS, HGCS, or DD-CS.
Twelve patients (70.6%) underwent image-guided needle biopsy with a total of 15 having a surgical procedure. Final histological grade of CC-CS was therefore based on surgical specimens in 15 cases (88.2%), open biopsy in 1 (5.6%), and needle biopsy alone in 1 (5.6%). For clinical purposes, all histological specimens were routinely reviewed in consensus by 2 experienced bone tumour consultant histopathologists following which all cases were classified as ACT/Gd1 CS, HGCS (all cases with at least focal transition to grade 2 CS but no dedifferentiation), and DD-CS. However, for the purposes of this study, cases were re-reviewed by a senior consultant histopathologist who had > 15 years’ experience of bone tumour pathology. The MRI grading for each tumour was then compared with the final histological grade.
Statistical methods
Descriptive statistics were used to assess all of the variables. In addition, sensitivity, specificity, and positive predictive value (PPV) were assessed for each MRI feature for the diagnosis of HGCS or DD-CS according to the following definitions:
True positive (TP): when the MRI feature resulted in diagnosis of a HGCS/DD-CS and the final histology was of HGCS/DD-CS
True negative (TN): when the MRI feature resulted in diagnosis of an ACT/Gd1 CS and the final histology was of ACT/Gd1 CS
False positive (FP): when the MRI feature resulted in diagnosis of a HGCS/DD-CS and the final histology was of ACT/Gd1 CS
False negative (FN): when the MRI feature resulted in diagnosis of an ACT/Gd1 CS and the final histology was of HGCS/DD-CS
Results
Of the 17 patients, 7 were males and 10 females with mean age of 13.9 years (range 6–18 years). No patients had a diagnosis of enchondromatosis. The details of tumour location, clinical and radiographic features, and treatment are presented in Table 1. Tumours were located in the femur in 6 cases; the humerus in 3 cases; the tibia, ilium, scapula, and ulna in 1 case each; and the small bones of the hands or feet in 4 cases. Mean maximal tumour length overall was 35.8 mm (range 10–68 mm). Mean maximal tumour length for ACT/HGCS was 35.3 mm (range 10–68 mm), while mean maximal tumour length for HGCS was 39 mm (range 29–49 mm). Based on MRI, both readers diagnosed 11 cases (64.7%) of ACT/Gd1 CS (Fig. 1) and 6 cases (35.3%) of HGCS (Figs. 2, 3, 4, and 5), with agreement between the 2 readers in 13 cases (76.5%). Final histological grade of CS was ACT/Gd1 CS in 15 cases (88.2%) and HGCS in 2 (11.8%). The HGCSs were both located in the femoral neck with surgical curettage specimens revealing grade 1 CS with focal transition to grade 2, high-grade tumour amounting to < 10% of the total tumour volume (Figs. 6 and 7). There were no cases of pure grade 2 CS, grade 3 CS, or DD-CS.
An 18-year-old girl who presented with painful swelling of the ulnar head (case 7). a DP radiograph demonstrates a lobular, mildly expansile lytic lesion (arrow) with a non-displaced pathological fracture (arrowhead). b Coronal T1W TSE and c axial SPAIR MR images show features consistent with a chondral tumour (arrows) which is resulting in mild bone expansion. Both readers diagnosed an ACT. Histological diagnosis from needle biopsy was ACT. The patient was treated conservatively
A 12-year-old girl who presented with a painful right thigh (case 17). a AP radiograph demonstrates a poorly defined lytic lesion (arrow) in the proximal femur. b Coronal T1W TSE MR image shows an intermediate SI lesion (arrow) resulting in minimal endosteal scalloping. c Axial SPAIR MR image shows circumferential periostitis (arrows) and minor soft tissue oedema (arrowhead). Both readers diagnosed a HGCS, but histological diagnosis from curettage was ACT
An 8-year-old girl who presented with a painful right shin (case 3). a Coronal T1W TSE MR image shows an intermediate SI lesion (arrow) with no endosteal scalloping. b Coronal STIR MR image shows prominent bone marrow oedema (arrows) and minor periostitis (arrowhead). Both readers diagnosed HGCS, but histological diagnosis from needle biopsy was ACT
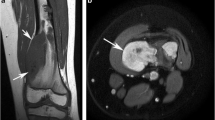
A 17-year-old girl being investigated for haematuria (case 4). CT-KUB showed a lesion in the let ilium. a Coronal T1W TSE and b axial PDW FSE MR images show an intermediate SI lesion (arrows) resulting in mild bone expansion and also a prominent extra-osseous mass (arrowheads—b). Diagnosed as Gd 1 CS by reader 1 and HGCS by reader 2, but histological diagnosis from curettage was Gd 1 CS
A 6-year-old boy with a left femoral tumour diagnosed as HGCS by both readers (case 1). a Lateral radiograph demonstrates deep endosteal scalloping and bone expansion (arrow). b Sagittal T1W TSE and c axial STIR MR images demonstrate a large lobular lesion (arrow—b) resulting in deep endosteal scalloping and a minor active periosteal response (arrows—c). Surgical histology confirmed an ACT. d Microphotograph showing host bone permeation, which is an unequivocal sign of malignancy allowing differentiation from enchondroma (H&E; × 10). e Microphotograph showing stellate-shaped, hyperchromatic, and enlarged nuclei set in a myxo-hyaline matrix (H&E; × 20). Histology confirmed an ACT
A 14-year-old boy who presented with a painful left hip (case 15). a AP radiograph demonstrates a well-defined lytic lesion (arrow) with a sclerotic margin in the femoral neck. b Coronal T1W TSE MR image shows an intermediate SI lesion (arrow). c Axial SPAIR MR image shows a small extra-osseous extension (arrow) and minor bone oedema (arrowhead). Both readers diagnosed an ACT. d Microphotograph showing increased cellularity with mild cytological atypia and myxo-hyaline nature of the matrix (H&E, × 4). e Microphotograph showing increased cellularity and cytological atypia. The neoplastic chondrocytes are spindle or stellate-shaped and are embedded within a myxoid matrix. The cytological atypia and the cellularity are higher when compared with those in Fig. 5d–e consistent with transition to chondrosarcoma grade 2 (H&E; × 10). Final surgical diagnosis from curettage was grade 1 CS with focal transition to grade 2
An 18-year-old boy who presented with a painful right hip (case 6). a Coronal T1W TSE MR image shows an intermediate SI lesion in the femoral neck (arrow) which has just extended into the soft tissues (arrowhead). b Axial SPAIR MR image shows the lesion extending through the cortex (arrow) with mild adjacent soft tissue oedema (arrowheads). Both readers diagnosed a HGCS and surgical histology confirmed grade 1 CS with focal transition to grade 2
Match between predicted MRI grade for both readers and final histological grade occurred in 8 cases (47%), a mismatch between MRI grade and histology for both readers in 4 cases (23.5%) and a mismatch between MRI and histological grade for 1 of the readers in 4 cases (23.5%) (Table 2). In 7 of the 8 cases (87.5%) of mismatch, MRI diagnosis was of HGCS while in only a single case did both readers diagnose an ACT/Gd1 CS while the histology was of a HGCS. Seven of the 8 cases (87.5%) of MR-histology mismatch involved the major long bones, while 1 case (12.5%) involved the ilium. Using the definitions for TP, TN, FP, and FN, the sensitivity, specificity, and PPV for each of the assessed MRI signs for making a diagnosis of HGCS/DD-CS are presented in Table 3.
Discussion
Paediatric CS is rare, a recent review of CS in children and adolescents identifying that between 4 and 7% of patients with CS are under 21 years of age, but it was unclear what proportion of these were central in location [19].
Several studies have reported specifically on CS in children. Wu et al. [5] reviewed the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) databases between 1973 and 2014, reporting on 247 cases of paediatric CS with the aim of trying to establish the effect of demographics and tumour characteristics on survival compared with adults. The limbs were involved in 120 cases, but it was not possible to determine what proportion was centrally located. Regarding histological sub-type, 151 were classified as CS not otherwise specified and these presumably represented CC-CS. Histological grade was only mentioned in 181 cases, 135 (74.6%) of tumours being low-grade and only 2 DD-CS. There was no reference to the imaging appearances of the tumours and therefore no information on how imaging features relate to histological grade or aid surgical decision-making. In 1987, Huvos et al. [6] published a series of 79 patients aged < 21 years presenting from 1928 to 1982 with CS, 77 of which arose in the skeleton (50 central CS). Just over half arose in the appendicular skeleton with the femur and humerus being the commonest sites. Regarding pathology, 55 were CC-CS, 41.8% grade 1, 18.2% grade 2, and 21.8% grade 3. The proportion of high-grade tumours varies very significantly from the current study, presumably due to a delayed presentation in this historic series. Young et al. [20] reported on 47 patients with CS under the age of 17 years, but it is unclear how many of these were central in location. Regarding histological grade, 83% were grade 1, 14.9% grade 2, and 2.1% grade 3, which is much closer to the findings in the current 17 cases. More recently, Gambarotti et al. reported on 17 paediatric patients with CS, 10 of which were centrally located (femur 3, tibia 3, humerus 2, ilium 1, and metacarpal 1): 5 were low-grade and 5 high-grade. Only a single MRI study was illustrated, showing a large humeral diaphyseal lesion causing marked bone expansion due to grade 2 CS [7]. Finally, Puri et al. [8] reported a series of 11 patients under the age of 21 years, 10 of which were CC-CS. The pelvis (n = 4), humerus (n = 3), and scapula, femur, and clavicle (n = 1 each) were involved, 2 cases being grade 1 CS and 8 cases grade 2 CS. Six of the tumours were described as secondary and 4 as primary, but it was not possible to determine which were centrally located and there were no details of imaging findings. Therefore, based on the above review, it is very unclear as to what proportion of conventional paediatric CS are central in location or what their histological grade is, and there is essentially no information on the MRI appearances of paediatric CS. A single detailed case report has described the development of a central low-grade CS from a presumed enchondroma after 2.75 years, the patient presenting with a pathological fracture but otherwise no aggressive features on MRI [4]. However, Bierry et al. did provide a detailed report on the imaging features of enchondromas in children [21]. Twelve enchondromas were described in 11 children with median age 14 years. Seven involved the phalanges or metacarpals, 4 the femur, and 1 the tibia. The mean lesion size was 28 mm and matrix mineralisation was noted in only 20% on CT. Bone expansion and endosteal scalloping were present in 75% of cases, while MRI in a lesion involving the femur demonstrated bone oedema and active periostitis. They concluded that enchondromas in children were frequently expansive with rare matrix mineralisation, potentially appearing more aggressive than enchondromas in adults [21].
The current study is the only detailed report of the MRI features in a relatively large group of paediatric patients with CC-CS. The MRI appearances were assessed according to the findings of Douis et al. [14] from a large group of adult patients (179 with mean age 51 years) with CC-CS of the major long bones. In that study, the authors described several features which were highly accurate for differentiating between low-grade and high-grade CC-CS. Features significantly associated with high-grade tumours included cortical thickening (p < 0.001), cortical destruction (p < 0.001; odds ratio 32.2), bone expansion (p < 0.001; odds ratio 12.9), active periostitis (p < 0.001; odds ratio 100.3), reactive bone marrow oedema (p < 0.001; odds ratio 4.5), reactive soft tissue oedema (p < 0.001; odds ratio 41), and soft tissue mass (p < 0.001; odds ratio 41). Multivariate analysis showed 4 features to be highly suggestive of high-grade CS: bone expansion (p = 0.001; odds ratio 8.8), active periostitis (p = 0.001; odds ratio 52.8), soft tissue mass (p < 0.001; odds ratio 21.1), and intra-osseous tumour length (p < 0.001; odds ratio 1.4) [14]. However, when these MRI features were applied to the 17 paediatric cases in the current study, there was a discrepancy in the MRI and histological grading of the tumours in 47% of cases for at least one reader and in 23.5% for both readers. Seven of 8 cases of mismatch were graded as HGCS, while histology revealed ACT/Gd1 CS, and in 1 case both readers diagnosed ACT/Gd1 CS on MRI, but histology following curettage revealed grade 1 CS with focal transition to grade 2. However, due to the microscopic nature of the high-grade component (< 10%), it is not surprising that the lesion was diagnosed as ACT/Gd1 CS on MRI. These findings indicate that the criteria for grading CC-CS established by Douis et al. [14] cannot be reliably applied to paediatric CC-CS: features such as cortical thickening, cortical destruction, bone expansion, active periostitis, bone and soft tissue oedema, and soft tissue mass, which were all highly significant for predicting HGCS in adults, had very variable sensitivity, specificity, and PPV in children (Table 3). The most concerning MRI feature to suggest HGCS in adults is active periostitis [14], while in the current study, this finding had sensitivity and PPV of 0% and specificity of only 60%. This is presumed to be due to the fact that the periosteum in children is more active and less adherent to the cortex than in adults, resulting in earlier and more aggressive periosteal reaction [22]. Therefore, it would appear that low-grade paediatric CC-CS has more ‘aggressive’ MRI features compared with final histology and this should be taken into account when planning surgery.
Seven of 8 cases of mismatch occurred in the major long bones (femur 5, tibia 1, and humerus 1), which is a particular problem with regard to surgical management, since low-grade CS can be safely treated with intra-lesional curettage whereas high-grade CS requires wide resection and reconstruction. The latter can be particularly challenging in the paediatric age group where there is still significant growth potential for the affected limb. Therefore, a very careful discussion needs to take place at the MDT when the initial management plan is decided incorporating both biopsy data as well as MRI findings. If a higher grade is assigned, then there will be a tendency to over treat and when long bones are involved, this will lead to resection of the affected segment of the bone (with or without sacrifice of the adjacent joint) and subsequent reconstruction with either biological options (allograft/autograft), EPR, or even ablative options in extreme cases. These procedures tend to be complex, with associated morbidity from growth disturbance and future surgical procedures, which are frequent if an endoprosthesis has been used. The findings of the current study are therefore crucial as they highlight that, particularly in the paediatric population, extreme caution is necessary when assigning a grade to central cartilaginous lesions at diagnosis and care needs to be taken when interpreting MRI findings.
The current study has several limitations. Its retrospective nature resulted in exclusion of some cases due to insufficient clinical or imaging data. Of the 17 cases reported, histological diagnosis was made purely on needle biopsy in 1, which is problematic since under-grading of CS based on needle biopsy compared with surgical histology is reported in approximately 20% of appendicular tumours [12]. This also becomes a major consideration when planning surgery for major long bone CS. In none of the cases was intravenous contrast material utilized. However, the role of contrast enhancement in the grading of chondral tumours is unclear. Choi et al. [23] and De Coninck et al. [24] suggested that conventional and/or dynamic contrast-enhanced MRI could aid the differentiation between enchondroma and grade 1 CS, while a more recent study by Douis et al. [25] found that administration of contrast material was of no additional value. The role of contrast in differentiating ACT/Gd 1CS from HGCS has received little attention. Yoshimura et al. reported that a ‘ring-and-arc’ pattern of enhancement had 100% specificity for ACT/Gd1 CS, but enhancement patterns for HGCS were not described [15]. Another limitation is that this group included no cases of pure grade 2 or 3 HGCS or DD-CS. However, this is in keeping with previous literature that has shown that the vast majority of paediatric CS cases are low-grade [5,6,7,8].
In conclusion, the current study describes the MRI features of 17 cases of paediatric CC-CS with histological correlation. HGCS is rare in children, and individual MRI features such as bone expansion, bone oedema, periostitis, and soft tissue oedema are not helpful in differentiating ACT/Gd 1CS from HGCS in the paediatric population. When applying MRI criteria used to differentiate low-grade CC-CS from high-grade CC-CS in adults, MRI over-graded 7 of 17 lesions. These findings must be considered when planning surgical management of paediatric CC-CS, particularly in the major long bones where the discrepancy between MRI and histology is most common.
References
Whelan JS, Davis LE. Osteosarcoma, chondrosarcoma, and chordoma. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36:188–93.
Francis M, Dennis N, Charman J, Lawrence G, Grimer R. Bone and soft tissue sarcomas. UK incidence and survival: 1996 to 2010. [Internet]. National Cancer Intelligence Network; 2013. Available from: http://www.ncin.org.uk/view?rid=2353
Soft Tissue and Bone Tumours. WHO classifications of tumours [Internet]. 5th ed. International Agency for Research on Cancer, World Health Organisation, International Academy of Pathology, WHO Classification of Tumours Editorial Board.; 2020. Available from: https://publications.iarc.fr/588
Mosier SM, Patel T, Strenge K, Mosier AD. Chondrosarcoma in childhood: the radiologic and clinical conundrum. J Radiol Case Rep. 2012;6:32–42.
Wu A-M, Li G, Zheng J-W, Chen C-H, Chen D, Qiao Z-G, et al. Chondrosarcoma in a paediatric population: a study of 247 cases. J Child Orthop. 2019;13:89–99.
Huvos AG, Marcove RC. Chondrosarcoma in the young. A clinicopathologic analysis of 79 patients younger than 21 years of age. Am J Surg Pathol. 1987;11:930–42.
Gambarotti M, Righi A, Picci P, Bertoni F, Manfrini M, Donati DM, et al. Paediatric chondrosarcomas: a retrospective review of 17 cases. Histopathology. 2016;68:1073–8.
Puri A, Gulia A, Kurisunkal VJ, Sukumar V, Rekhi B. Chondrosarcomas in adolescents: are they different? J Pediatr Orthop Part B. 2019;
Shemesh SS, Acevedo-Nieves JD, Pretell-Mazzini J. Treatment strategies for central low-grade chondrosarcoma of long bones: a systematic review of the literature and meta-analysis. Musculoskelet Surg. 2018;102:95–109.
Dierselhuis EF, Goulding KA, Stevens M, Jutte PC. Intralesional treatment versus wide resection for central low-grade chondrosarcoma of the long bones. Cochrane Database Syst Rev. 2019;3:CD010778.
Gerrand C, Athanasou N, Brennan B, Grimer R, Judson I, Morland B, et al. UK guidelines for the management of bone sarcomas. Clin Sarcoma Res. 2016;6:7.
Oliveira I, Chavda A, Rajakulasingam R, Saifuddin A. Chondral tumours: discrepancy rate between needle biopsy and surgical histology. Skelet Radiol. 2020;49:1115–25.
Yoo HJ, Hong SH, Choi J-Y, Moon KC, Kim H-S, Choi J-A, et al. Differentiating high-grade from low-grade chondrosarcoma with MR imaging. Eur Radiol. 2009;19:3008–14.
Douis H, Singh L, Saifuddin A. MRI differentiation of low-grade from high-grade appendicular chondrosarcoma. Eur Radiol. 2014;24:232–40.
Yoshimura Y, Isobe K, Arai H, Aoki K, Kito M, Kato H. Preoperative radiographic and histopathologic evaluation of central chondrosarcoma. Arch Orthop Trauma Surg. 2013;133:1225–31.
Fritz B, Müller DA, Sutter R, Wurnig MC, Wagner MW, Pfirrmann CWA, et al. Magnetic resonance imaging-based grading of cartilaginous bone tumors: added value of quantitative texture analysis. Investig Radiol. 2018;53:663–72.
Fayad LM, Ahlawat S, Khan MS, McCarthy E. Chondrosarcomas of the hands and feet: a case series and systematic review of the literature. Eur J Radiol. 2015;84:2004–12.
Deckers C, Steyvers MJ, Hannink G, Schreuder HWB, de Rooy JWJ, Van Der Geest ICM. Can MRI differentiate between atypical cartilaginous tumors and high-grade chondrosarcoma? A systematic review. Acta Orthop 2020;1–8.
Puri A. Chondrosarcomas in children and adolescents. EFORT Open Rev 2020;5:90–5.
Young CL, Sim FH, Unni KK, McLeod RA. Chondrosarcoma of bone in children. Cancer. 1990;66:1641–8.
Bierry G, Kerr DA, Nielsen GP, Rosenberg AE, Huang AJ, Torriani M, et al. Enchondromas in children: imaging appearance with pathological correlation. Skelet Radiol. 2012;41:1223–9.
Rana RS, Wu JS, Eisenberg RL. Periosteal reaction. AJR Am J Roentgenol. 2009;193:W259–72.
Choi B-B, Jee W-H, Sunwoo H-J, Cho J-H, Kim J-Y, Chun K-A, et al. MR differentiation of low-grade chondrosarcoma from enchondroma. Clin Imaging. 2013;37:542–7.
De Coninck T, Jans L, Sys G, Huysse W, Verstraeten T, Forsyth R, et al. Dynamic contrast-enhanced MR imaging for differentiation between enchondroma and chondrosarcoma. Eur Radiol. 2013;23:3140–52.
Douis H, Parry M, Vaiyapuri S, Davies AM. What are the differentiating clinical and MRI-features of enchondromas from low-grade chondrosarcomas? Eur Radiol. 2018;28:398–409.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the local Research and Innovation Centre of The Institute of Orthopaedics under the Integrated Research Application System number 262826, with no requirement for informed patient consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ardakani, A., Gikas, P., Khoo, M. et al. MRI-histopathological correlation in paediatric conventional central chondrosarcoma: a report of 17 cases. Skeletal Radiol 50, 711–721 (2021). https://doi.org/10.1007/s00256-020-03614-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03614-6