Abstract
Parosteal osteosarcomas and well-differentiated liposarcomas are both well-differentiated locally aggressive tumors. They both have simple karyotypes with amplification of the 12q13-15 regions including MDM2 and CDK4 genes. In this report, we describe the case of a parosteal osteosarcoma intertwined with a low-grade component similar to a well-differentiated liposarcoma. The association of a bone component with an adipose component was initially overlooked. We describe the histological, imaging, and molecular characteristics of this tumor stressing the importance of radio-pathological correlation. To our knowledge, this is the second report of a parosteal osteoliposarcoma. Awareness of this rare presentation may allow radiologists and surgeons to recognize the peripheral fatty component as an integral part of the tumor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parosteal osteosarcoma (OS) is a rare primary bone tumor, accounting for approximately 4% of all OSs [1]. Peak incidence is in the third and fourth decades. These tumors are located on the surface of the metaphyseal region of the distal femur (around 60% of cases), the proximal tibia, or proximal humerus.
Parosteal OS is a slowly growing tumor that may recur and rarely metastasizes [2,3,4].
In parosteal OSs, radiographs typically demonstrate an ossified cortically based mass arising from the surface of the metaphysis of a long bone. On axial computed tomography (CT), attachment to the cortex may be broad or narrow. A partial radiolucent cleavage plane, representing the periosteum, may be apparent between the ossified mass and the cortex. Magnetic resonance imaging (MRI) is useful to assess the soft-tissue extent and identify any non-osseous component.
Microscopically, parosteal OS is characterized by trabeculae of woven bone surrounded by a paucicellular spindle cell proliferation in a fibrous background. Atypia is usually minimal or mild. Mitoses are absent or scarce. In dedifferentiated parosteal OS, the dedifferentiated component is, by definition, always high-grade and consists in high-grade osteoblastic OS, fibroblastic OS, giant cell-rich OS, chondroblastic OS, fibrosarcoma, and malignant fibrous histiocytoma [2, 3]. In the literature, no liposarcoma component was found in a dedifferentiated parosteal OS.
Furthermore, no low-grade dedifferentiated component has been described hitherto.
Parosteal OSs are characterized by simple karyotypes with amplification of 12q13-15 regions [5] including MDM2 and CDK4 genes [6].
Use of MDM2 and CDK4 immunohistochemistry for the diagnosis of low-grade OS helps differentiate parosteal OS from benign mimics [7] although demonstration of MDM2 amplification by in situ hybridization is recommended [1].
Several features of parosteal OS mirror those of well-differentiated liposarcoma/atypical lipomatous tumor (WDLPS/ALT). Like parosteal OS, WDLPS is a slow-growing, locally aggressive tumor that can recur locally and may present de novo as a high-grade tumor or transform upon recurrence.
Unlike parosteal OS, WDLPS and DDLPS liposarcomas are not uncommon. They occur in adults and are most commonly found in the retroperitoneum and thigh [8].
Microscopically, WDLPS is an adipose tumor resembling normal fat. The diagnosis is based on the presence of scattered large atypical hyperchromatic spindle cells in fibrous septa. In WDLPS, foci of low-grade OS similar to parosteal OS or central low-grade OS overexpressing MDM2 and CDK4 were described [9].
In bone, WDLPS and DDLPS have not been described to our knowledge.
A few years ago, we described the first case [10] of a low-grade parosteal sarcoma composed of intermixed components of low-grade OS and WDLPS. The name “parosteal osteoliposarcoma” was proposed for this tumor.
Here we present a second case with similar features.
Case report
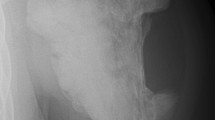
In 1999, a 23-year-old female patient was referred to the Department of Orthopedic Surgery for evaluation of a popliteal mass. There was no relevant medical history and no episode of trauma. On plain radiographs, a juxtacortical ossified mass of the posterior aspect of the proximal right tibia was found. An incisional biopsy was performed, and the pathologist concluded that the ossified mass represented a benign metaplastic process. The radiographs and histopathological slides from 1999 are no longer available. The patient was followed up using our standard guidelines. In 2010, plain radiographs showed a slight increase of the mass (Fig. 1).
In 2011, progressive onset of mild discomfort during physical exercise and knee flexion limitation motivated a resection. A partial resection of the ossified mass was performed, complete resection being deemed impossible because of encasement of femoropopliteal vessels (Fig. 2).
At the same time, a 4-cm adipose mass anterior to the gastrocnemius muscle was removed as an incidental lipoma. Histological examination of the bony specimen showed well-formed, anastomosing or parallel bone trabeculae with a hypocellular mildly atypical spindle cell component (Fig. 3). These histological features were not consistent with bone metaplasia but reminiscent of a parosteal OS.
Immunohistochemistry using antibodies against MDM2 (clone IF2, dilution 1:200, Zymed) and CDK4 (clone DCS-31, 1:100 dilution, Invitrogen) showed nuclear staining of spindle cells for MDM2 (Fig. 4) and CDK4 (not shown). Considering acid decalcification of this ossified specimen, in situ hybridization in search for MDM2 amplification could not be performed. MDM2 and CDK4 protein overexpression in tumor cells and morphological features was in accordance with the diagnosis of parosteal OS.
The adipose nodule sent as a lipoma consisted of sheets of mature adipocytes with moderate anisocytosis. Thick fibrous septa and atypical spindle cells were not seen (Fig. 5).
In light of our past experience, we investigated whether the adipose component was part of the tumor or was an incidental neoplasm.
To this end, we performed chromogenic in situ hybridization on formalin-fixed, paraffin-embedded tissue with a MDM2 probe (Zyto Dot SPEC MDM2 Probe, ZytoVision Gmbh). It demonstrated MDM2 gene amplification (Fig. 6) consistent with the tumoral nature of the adipocytic mass. To further analyze its genomic characteristics, pangenomic microarray comparative genomic hybridization (NimbleGen DNA microarrays, 72,000 probes) on a frozen tumor sample of the adipose mass showed no gain or loss of chromosome material but two independent amplicons at 12q14.1-q15 containing the CDK4 and MDM2 genes and an additional amplification of the 11q13.1 region (66,392,340–66,996,044) (Fig. 7).
Chromogenic in situ hybridization (CISH) performed on formalin-fixed, paraffin-embedded (FFPE) tissues with a MDM2 probe in the adipose component. A normal cell (arrowhead) shows two dots, consistent with two normal MDM2 alleles. MDM2 amplified tumor cells (arrow) show clusters of multiple dots in nuclei, corresponding to multiples copies of the MDM2 gene
This case, appearing as a parosteal OS associated with an adipose component similar to WDLPS/ALT, fits the criteria for the entity we previously described [11] as parosteal osteoliposarcoma.
A limb-sparing procedure with femoropopliteal artery bypass was performed.
Gross examination of the resection specimen showed a large ossified mass with a broad implantation on the tibial cortex, encircling the fibula. Two pale yellow adipose small masses were clearly visible at the periphery of the mass: one in anterior position and the other in posterior position (see Fig. 8 for radiologic-pathologic comparison).
A 37-year-old female with a long-standing juxtacortical ossified mass of the posterior surface of the proximal right tibia with a bifocal well-circumscribed adipose component. Radiologic-pathologic comparison of the CT scan and of cut sections of the corresponding gross specimen. a, c CT scan, axial sections. b, d Gross specimen. The tumor is made of two components: an ossified part, thickening and destroying the cortex, extending to the fibula, and a bifocal adipose part (white arrows). Anterior adipose mass is best seen on sections a and b and posterior mass on sections c and d. Encased artery (black arrow a)
The procedure was complicated by thrombosis of the graft on day 2, leading to acute distal limb ischemia, and an above-knee amputation had to be performed. The patient was alive and well 4 years after surgery, without any recurrence or metastasis.
Discussion
In 1999, the diagnosis of parosteal OS on the initial incisional biopsy was challenging, the tumor being very well differentiated and akin to metaplastic bone.
On radiographs, the very slow growing surface bone forming lesion was evocative of a parosteal osteosarcoma. However, a melorheostosis could also be discussed, given the flowing candle wax appearance on AP and lateral views.
On the resected specimen in 2012, the pitfall was to overlook the adipocytic component.
Few primary bone tumors combining OS and liposarcoma features [11] have been described in the literature. In most cases, they were composed of a high-grade OS and a liposarcoma variant and were named “malignant mesenchymoma” or “primary osteoliposarcoma of the bone.” As these cases are relatively old, no molecular data are available. However, none of these cases fit our description.
The only published case [12] of primary parosteal WDLPS is unconvincing as provided images favor a WDLPS originating in the deep soft tissues of the thigh.
There have been numerous cases of soft tissue liposarcomas with dedifferentiation into an OS. Some of them included cases with low-grade OS as described in a series of 9 cases of WDLPS/DDLPS with distinct areas of fibro-osseous tissue similar to low-grade OS [9].
Apart from our previous reported case, we are not aware of similar cases in the English literature, which is surprising. This entity might be underdiagnosed or underreported, as the adipose component may be inconspicuous.
Our case also raises intriguing questions about the pathogenesis of this tumor. The WDLPS component may be viewed as a dedifferentiated component of a parosteal OS, although this has never been described hitherto. Since dedifferentiation usually implies a higher grade component, the term “transdifferentiation” (change of a cell or tissue from one differentiated state to another differentiated state) would be more appropriate in our opinion.
In our case, a well-differentiated malignant osteogenic tumor switches phenotype and transforms into a well-differentiated adipocytic tumor. Several hypotheses could explain this change in phenotype.
-
First hypothesis: Additional genetic alterations may trigger a phenotype switch. An interesting feature in our case was that the adipocytic component arose simultaneously at two distinct locations in the tumor which suggests that the molecular events leading to phenotype switch must have happened in two populations of tumor cells.
-
Second hypothesis: Epigenetic changes may trigger a phenotype switch, potentially under the control of the tumor microenvironment. A same tumor might display an osteogenic phenotype in a specific microenvironment (e.g., bone) and an adipocytic phenotype in another microenvironment (e.g., soft tissue). A striking feature in our case was the peripheral location of the two adipose masses. This was also the case in the previously published parosteal osteoliposarcoma [10].
-
Third hypothesis: Both tumor components might originate from a common precursor derived from a human mesenchymal stem cell (hMSC), which retained multilineage differentiation potential. This potential has been demonstrated by experimentally induced in vitro tumorigenesis in hMSC leading to distinct sarcoma cell lines with osteogenic, adipogenic, or chondrogenic differentiation [13].
Conclusion
We present here the second description of a parosteal OS associated with a component that is morphologically, immunophenotypically, and molecularly indistinguishable from a WDLPS.
Whether it should be considered as a variant of parosteal OS (transdifferentiated parosteal OS) or as a distinct entity (parosteal osteoliposarcoma) is open for debate. Knowledge of this entity is of interest for radiologists, surgeons, and pathologists so as not to overlook or misdiagnose the tumoral nature of the adipose component and provide adequate treatment.
References
World Health Organization, International Agency for Research on Cancer. WHO classification of tumours of soft tissue and bone. 5th ed. Cree, I editor. Lyon: IARC Press; 2020.
Okada K, Frassica FJ, Sim FH, Beabout JW, Bond JR, Unni KK. Parosteal osteosarcoma. A clinicopathological study. J Bone Joint Surg Am. 1994;76:366–78.
Bertoni F, Bacchini P, Staals EL, Davidovitz P. Dedifferentiated parosteal osteosarcoma: the experience of the Rizzoli Institute. Cancer. 2005;103:2373–82.
Sheth DS, Yasko AW, Raymond AK, Ayala AG, Carrasco CH, Benjamin RS, et al. Conventional and dedifferentiated parosteal osteosarcoma. Diagnosis, treatment, and outcome. Cancer. 1996;78:2136–45.
Szymanska J, Mandahl N, Mertens F, Tarkkanen M, Karaharju E, Knuutila S. Ring chromosomes in parosteal osteosarcoma contain sequences from 12q13-15: a combined cytogenetic and comparative genomic hybridization study. Genes Chromosomes Cancer. 1996;16:31–4.
Gamberi G, Ragazzini P, Benassi MS, Ferrari C, Sollazzo MR, Molendini L, et al. Analysis of 12q13-15 genes in parosteal osteosarcoma. Clin Orthop. 2000;377:195–204.
Yoshida A, Ushiku T, Motoi T, Shibata T, Beppu Y, Fukayama M, et al. Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Mod Pathol Off J U S Can Acad Pathol Inc. 2010;23:1279–88.
Coindre J-M, Pédeutour F, Aurias A. Well-differentiated and dedifferentiated liposarcomas. Virchows Arch Int J Pathol. 2010;456:167–79.
Yoshida A, Ushiku T, Motoi T, Shibata T, Fukayama M, Tsuda H. Well-differentiated liposarcoma with low-grade osteosarcomatous component: an underrecognized variant. Am J Surg Pathol. 2010;34:1361–6.
Larousserie F, Chen X, Ding Y, Kreshak J, Cocchi S, Huang X, et al. Parosteal osteoliposarcoma: a new bone tumor (from imaging to immunophenotype). Eur J Radiol. 2013;82:2149–53.
De Padua M, Bhandari TPS, Pingle J. Primary osteoliposarcoma of the bone. Indian J Pathol Microbiol. 2009;52:80–2.
Szuhai K, IJszenga M, Knijnenburg J, Dijkstra S, de Schepper A, Tanke HJ, et al. Does parosteal liposarcoma differ from other atypical lipomatous tumors/well-differentiated liposarcomas? A molecular cytogenetic study using combined multicolor COBRA-FISH karyotyping and array-based comparative genomic hybridization. Cancer Genet Cytogenet. 2007;176:115–20.
Li N, Yang R, Zhang W, Dorfman H, Rao P, Gorlick R. Genetically transforming human mesenchymal stem cells to sarcomas: changes in cellular phenotype and multilineage differentiation potential. Cancer. 2009;115:4795–806.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This case was awarded the Corinne Farrell Best Case Report Presentation at the 2013 ISS Members’ Meeting.
Rights and permissions
About this article
Cite this article
Sohier, P., Rodrigues, M., Anract, P. et al. Parosteal osteosarcoma associated with a low-grade component mimicking well-differentiated liposarcoma: a case report. Skeletal Radiol 50, 243–248 (2021). https://doi.org/10.1007/s00256-020-03509-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03509-6