Abstract
Objective
Determine if differences in T1ρ would be detected in specific regions or layers of patellofemoral cartilage between patients with symptomatic patellofemoral pain syndrome and asymptomatic control subjects.
Materials and methods
Ten subjects diagnosed with patellofemoral pain syndrome were compared with ten age-, gender-, and BMI-matched control subjects with no knee pain or prior trauma. Conventional turbo (fast) spin echo sequences and T1ρ-weighted imaging were performed on the symptomatic knee in each of the ten subjects. At the patella and distal femur, cartilage regions of interest were divided into medial and lateral sub-regions, each then further sub-divided by layer (superficial, middle, or deep). Two-tailed t test and chi-squared tests were used to analyze demographic data. A mixed effect model was run for each sub-region of T1ρ imaging. Statistical significance was determined using the likelihood ratio test against reduced models without patellofemoral pain syndrome symptomatic status as a fixed effect.
Results
There was no difference in age, sex, or BMI between symptomatic and control patients. T1ρ values were significantly higher among patellofemoral pain syndrome patients when compared with controls in the superficial zone of the lateral patella (58.43 vs. 50.83, p = 0.03) and the middle zone of the lateral patella (52.67 vs. 43.60, p = 0.03). T1ρ was also higher in the superficial zone of the medial femur (50.94 vs. 46.70, p = 0.09) with a value approaching statistical significance.
Conclusion
We report statistically significant differences in the T1ρ value in the superficial and middle zones of the lateral patella in patients with patellofemoral pain syndrome who had no abnormalities seen on conventional MRI sequences, suggesting an alteration the macromolecular structure of the cartilage in this population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis is a degenerative process of articular cartilage that is characterized by loss of proteoglycans, increases in water content of articular cartilage, and changes in the composition and arrangement of collagen macromolecules [1]. It is a painful and debilitating disease that affects 60% of the population over the age of 70. Although conventional MRI techniques such as 2D turbo (fast) spin echo sequences have been able to provide information about later stages of cartilage degeneration in which structural defects are present, they are unable to detect early biochemical changes [1].
T1rho (T1ρ) has emerged as a method of quantitative MRI used to detect early cartilage degeneration. T1ρ values are heavily dependent on the proteoglycan content of cartilage [2, 3], although there is some contribution from collagen matrix as well [4,5,6]. Sodium MRI [7] and delayed gadolinium-enhanced proton MRI [8] have also been shown to be useful in measuring glycosaminoglycan content of cartilage; however, they require exogenous contrast administration and/or are not readily available in the clinical setting. T1ρ has been found to increase linearly with the depletion of proteoglycan [2, 3] and glycosaminoglycan content in cartilage [1]. As T1ρ does not require additional hardware or administration of contrast, it is a desirable choice for imaging to detect changes in cartilage structure and early osteoarthritis. Measurements of T2 values have also been used to assess cartilage structure, but are primarily dependent on changes in the collagen matrix [9].
T1ρ may also have utility in the evaluation of patellofemoral pain syndrome (PPS). Patellofemoral pain is characterized by diffuse pain on the anterior aspect of the knee, often found in young, physically active individuals [10]. Symptoms are worsened by activities that increase patellofemoral joint compressive forces, including squatting, ascending and descending stairs, and prolonged sitting [10]. It presents without evidence of arthritis on radiographs, and conventional MRI has been unable to show a difference between those with PPS and controls. Although there are many proposed causes of PPS, there is evidence that abnormal structure or alignment in the patellofemoral joint such as patella alta, high tibial-tubercle-trochlear groove (TT-TG) distance, and abnormal trochlea morphology may cause focal loading and patella maltracking [11]. One challenge to studying the development and progression of PPS is the lack of imaging criteria reflecting possible biochemical changes in articular cartilage of patients with PPS.
T1ρ-weighted imaging permits high resolution, quantitative detection of subtle biochemical changes in soft tissue, including cartilage [12,13,14]. Previous work by our group and others has demonstrated a reproducibility for T1ρ measurements in vivo of 5–10% [15,16,17,18]. Although early studies used T1ρ-weighted imaging to characterize proteoglycan changes in patients with patellofemoral pain [19], the local distribution of these changes with the patellofemoral joint is unknown. The purpose of this study was to determine if differences in T1ρ would be detected in specific regions or layers of patellofemoral cartilage between patients with symptomatic patellofemoral pain syndrome and asymptomatic control subjects.
Materials and methods
All human subject research was carried out in accordance with protocols approved previously by the Institutional Review Board at The University of Pennsylvania. A consecutive series of ten subjects was recruited from the orthopaedic patient population between 2015 and 2016 after having been diagnosed on clinical grounds as having PPS. Patients age 18–45 with a clinical diagnosis of PPS scheduled for an MRI were included in the study. Those with history of prior injury or surgery to the affected knee, radiographic evidence of osteoarthritis, or structural abnormalities on conventional MRI were excluded. The control group was comprised of asymptomatic volunteers who were age- and gender-matched to the symptomatic patients in our cohort.
Conventional turbo (fast) spin echo sequences and T1ρ imaging were performed on the symptomatic knee in each of the ten symptomatic subjects. The same sequences were performed on control subjects. Among the patients with PPS, conventional MRI examinations revealed normal appearing patellar and trochlear cartilage based on the formal interpretation made by one of a number of musculoskeletal radiologists on the Radiology faculty, all of whom are fellowship trained with more than 2 years of clinical experience.
The conventional turbo (fast) spin echo sequences consisted of T2W axial sequences with TR/TE = 5990/78 ms, 16 cm FOV, 384 × 307 acquisition matrix, and 3 mm section thickness; T1W and fat saturated T2W coronal sequences with TR/TE = 685/18 ms and 3800/62 ms, respectively, and the same acquisition matrix and section thickness, and intermediate-weighted sagittal sequences with TR/TE = 1650/48 ms, with the same acquisition matrix and section thickness. 3D GRE-based T1ρ sequences were acquired in the axial plane with spin-lock times of 0, 10, 20, 30, and 40 ms, spin-lock amplitude (B1 power) = 500 Hz, TR = 6 s, 0.5 × 0.5 × 3 mm3 resolution, and fat suppression. Manual shimming was performed on the region of interest including the patellofemoral compartment. The resulting data were then processed by a custom MATLAB script using a non-linear fit algorithm. After fitting, the script then mapped T1ρ values in a voxel-by-voxel fashion over manually segmented patellar and femoral regions of interest (ROIs). Voxels with R2 < 0.75 and T1ρ > 200 ms were excluded from mapping and analysis. Cartilage ROIs at the patella and distal femur were divided anatomically into medial and lateral sub-regions. These sub-regions were then further divided into thirds to generate superficial, middle, or deep layers based on depth of cartilage, using an image dilation and erosion script custom-written in MATLAB for the purposes of later analysis.
The six categories evaluated at both the patella and distal femur (12 categories in total) include the following: (1) medial region, superficial layer; (2) medial region, middle layer; (3) medial region, deep layer; (4) lateral region, superficial layer; (5) lateral region, middle layer; (6) lateral region, deep layer (Fig. 1).The 10 affected patients were compared with 10 asymptomatic control subjects.
Schematic illustrating the patellar and femoral regions of interest. Using MATLAB software, cartilage was divided into three regions—superficial (S), middle (M), and deep (D). Each layer was specified as 33% of the full thickness of the cartilage. These were then each split into medial and lateral halves using the normal to the line running between the medial and lateral femoral condyles on the posterior side of the knee
Statistical analysis was performed in JMP Pro 14 (SAS Inc., Cary NC) with two-tailed t test and chi-squared tests used to analyze demographic data. All segmented voxels in each cartilage sub-region of each subject were analyzed in this pilot study (81909 data points). Data was averaged for all 20 volunteers and 12 regions and normality assumption was verified by plotting Q-Q or normal quantile plots and fitting to a normal curve before modeling.
Twelve separate linear mixed effect models were created, one for each sub-region (2 gross regions of interest [patella/distal femur] * 2 sub-regions [medial/lateral] * 3 cartilage depth layers [superficial, middle, deep]), in order to test the effect of PPS symptomatic status, sex, age, and BMI values for their effect on T1ρ values. All models were controlled for inter-subject variance in T1ρ data by including a random effect based on subject ID number and were run with maximum likelihood estimation. Statistical significance was determined using the likelihood ratio test against a reduced model without PPS symptomatic status as a fixed effect. The Benjamini-Hochberg (B-H) post hoc test was used to reduce the false discovery rate—the proportion of significant results that were actually false positives from all positive test outcomes resulting from multiple testing [20]. Resultant p values < 0.05 were considered significant.
Results
Data averaging across patients and regions resulted in 207 data points since only six regions were observed each in three PPS and one control, and only three regions were observed in one PPS patient.
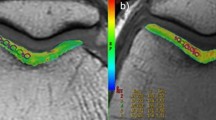
There was no difference in sex, age, or BMI between symptomatic and control patients (Table 1). Differences in T1ρ signal were most pronounced in the lateral patella but they could also be seen in the medial femoral trochlea (Fig. 2). T1ρ was significantly higher in the superficial layer of the lateral patella (58.43 vs. 50.83, p = 0.03) and the middle layer of the lateral patella (52.67 vs. 43.60, p = 0.03) among PPS patients (Table 2). Additionally, elevated T1ρ values trended towards significance in the superficial layer of the medial femur (50.94 vs. 46.70, p = 0.09). There was no difference in T1ρ values in the other sub-regions or layers analyzed.
Discussion
We report changes in T1ρ in several layers of articular cartilage in symptomatic PPS patients with normal appearing cartilage on conventional turbo spin echo sequences.
The superficial and middle layers of the lateral patella were the only sub-regions with statistically significant higher T1ρ values for the symptomatic patients compared with the controls. The superficial zone of the medial femur was also affected with statistics approaching significance. This finding is concordant with commonly reported symptoms in patellofemoral pain syndrome such as lateral facet pain. Notably, a prior study also demonstrated significantly higher values of T1ρ in the lateral patellar facet than the medial facet in patients with patellofemoral pain syndrome compared with controls, though these investigators did not segment the cartilage based on depth as we did [19]. However, the changes in the medial femur also indicate a global process at play.
It is well known that alterations in joint biomechanics affect cartilage metabolism. For example, static cartilage overload causes decreases in proteoglycan production [21] and cyclic loading at physiologic pressures causes anabolic production of proteoglycans [22, 23]. The variation of proteoglycan content identified in our study in areas outside of the overloaded lateral patellar facet indicates a possible alteration of global patellofemoral joint cartilage structure in patients with PPS that may be implicated in future patellofemoral disease progression.
We found T1ρ values to be most significantly higher in the superficial zone of cartilage; however, middle and deep zones also showed changes and each zone appears to be affected to a different extent. Zonal variations in cartilage structure play a role in its metabolism. Each zone has distinct biochemical content and organization that imparts its function [23]. The middle and deep zones provide load-bearing material properties and are high in proteoglycan content. The superficial zone is rich in collagens and provides low-friction sliding at the cartilage surface. In the superficial zone, collagen fibrils are parallel to the articular surface; in the middle zone, fibrils are random and less dense; and in the deep zone, collagen fibrils are arranged radially [24]. In osteoarthritis, zone-specific changes occur in different stages of the disease process [25]. For example, the earliest changes include a loss of cells in the superficial zone and abnormal cellular differentiation in the deep zone [26]. Additionally, in early stages of osteoarthritis, chondrocytes in the superficial zone are more catabolically active, which initiates collagen degeneration at the surface [24, 27]. Our study demonstrates the utility of T1ρ to detect these zone-specific changes before gross osteoarthritis is present.
This study has several potential limitations. First, the small sample size of both patients and controls (10 each) places some limitations on the validity of our findings. The small number of subjects is a result of the small number of patients with this disorder willing to participate, and the challenge of identifying age- and gender-matched controls. We plan to continue this work to enhance its validity, but we thought it important to publish these early and potentially significant results at this time.
Beyond sample size, the findings may also be impacted by having a T1ρ acquisition sequence for which the spin-lock times are all less than the actual T1ρ values in the cartilage. The maximum spin-lock time is determined by system software restrictions on SAR (measure of RF power deposition). Despite a maximum time that is less than the typical values of T1ρ in cartilage, over more than 20 years of both in vitro specimen imaging, where there is no SAR limitation and longer spin-lock times can be employed, and in vivo imaging, both in vitro and in vivo imaging sequences have yielded the same T1ρ values for cartilage [28,29,30]. The sequence used for in vivo studies has been shown to calculate accurate values of T1ρ up to 200 ms (5× the maximum spin-lock time) [28,29,30,31].
Conclusion
T1ρ MRI has been shown to demonstrate higher T1ρ values in the superficial and middle layers of the cartilage on the lateral patellar facet in patients with patellofemoral pain syndrome when compared with control subjects, even in a relatively small patient population. Higher T1ρ values that did not reach the level of statistical significance were observed in other facets and layers of patellar and femoral cartilage. Larger studies are needed to confirm this work and to determine if elevated T1ρ values will be found at other locations in the patellofemoral joint. Hopefully this technique will be used to better understand the changes in cartilage in this disorder and help guide early-intervention strategies in affected patients who are otherwise under-diagnosed and under-treated.
References
Regatte RR, Akella SVS, Borthakur A, Reddy R. Proton spin-lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. J Magn Reson Imaging [Internet]. 2003 [cited 2019 May 31];17:114–21. Available from: http://doi.wiley.com/10.1002/jmri.10228.
Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med [Internet]. 1997 [cited 2019 May 31];38:863–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9402184.
Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med [Internet]. 2001 [cited 2019 May 31];46:419–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11550230.
Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1ρ MRI in articular cartilage systems. Magn Reson Med [Internet]. John Wiley & Sons, Ltd; 2004 [cited 2020 Feb 9];51:503–9. Available from: http://doi.wiley.com/10.1002/mrm.10710
Van Tiel J, Kotek G, Reijman M, Bos PK, Bron EE, Klein S, et al. Is T1r mapping an alternative to delayed gadolinium-enhanced mr imaging of cartilage in the assessment of sulphated glycosaminoglycan content in human osteoarthritic knees? An in vivo validation study. Radiology. Radiological Society of North America Inc. 2016;279:523–31.
Duvvuri U, Kudchodkar S, Reddy R, Leigh JS. T1rho relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthr Cartil. 2002;10:838–44.
Reddy R, Insko EK, Noyszewski EA, Dandora R, Kneeland JB, Leigh JS. Sodium MRI of human articular cartilage in vivo. Magn Reson Med [Internet]. 1998 [cited 2019 May 31];39:697–701. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9581599.
Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, et al. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med [Internet]. 2001 [cited 2019 May 31];45:36–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11146483.
Eagle S, Potter HG, Koff MF. Morphologic and quantitative magnetic resonance imaging of knee articular cartilage for the assessment of post-traumatic osteoarthritis. J Orthop Res [Internet]. 2017 [cited 2020 Feb 5];35:412–23. Available from: http://doi.wiley.com/10.1002/jor.23345.
Witvrouw E, Callaghan MJ, Stefanik JJ, Noehren B, Bazett-Jones DM, Willson JD, et al. Patellofemoral pain: consensus statement from the 3rd International Patellofemoral Pain Research Retreat held in Vancouver, September 2013. Br J Sports Med [Internet]. 2014 [cited 2019 May 31];48:411–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24569145.
Stefanik JJ, Zhu Y, Zumwalt AC, Gross KD, Clancy M, Lynch JA, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the multicenter osteoarthritis study. Arthritis Care Res (Hoboken) [Internet]. 2010 [cited 2019 May 31];62:1258–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20506169.
Atkinson HF, Birmingham TB, Moyer RF, Yacoub D, Kanko LE, Bryant DM, et al. MRI T2 and T1ρ relaxation in patients at risk for knee osteoarthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord [Internet]. 2019 [cited 2020 Feb 5];20:182. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31039785.
Link TM, Li X. Establishing compositional MRI of cartilage as a biomarker for clinical practice. Osteoarthr Cartil [Internet]. 2018 [cited 2020 Feb 5];26:1137–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29550402.
Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthr Cartil [Internet]. 2013 [cited 2020 Feb 5];21:1474–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23896316.
Regatte RR, Akella SVS, Borthakur A, Kneeland JB, Reddy R. In vivo proton MR three-dimensional t1ρ mapping of human articular cartilage: initial experience. Radiology [Internet]. 2003 [cited 2020 Feb 5];229:269–74. Available from: http://pubs.rsna.org/doi/10.1148/radiol.2291021041.
Li X, Han ET, Ma CB, Link TM, Newitt DC, Majumdar S. In vivo 3T spiral imaging based multi-slice T1ρ mapping of knee cartilage in osteoarthritis. Magn Reson Med [Internet]. 2005 [cited 2020 Feb 5];54:929–36. Available from: http://doi.wiley.com/10.1002/mrm.20609.
Borthakur A, Mellon E, Niyogi S, Witschey W, Kneeland JB, Reddy R. Sodium and T1ρ MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed [Internet]. 2006 [cited 2020 Feb 5];19:781–821. Available from: http://doi.wiley.com/10.1002/nbm.1102.
Nemeth A, Di Marco L, Boutitie F, Sdika M, Grenier D, Rabilloud M, et al. Reproducibility of in vivo magnetic resonance imaging T1rho and T2 relaxation time measurements of hip cartilage at 3.0T in healthy volunteers. J Magn Reson Imaging. John Wiley and Sons Inc. 2018;47:1022–33.
Thuillier DU, Souza RB, Wu S, Luke A, Li X, Feeley BT. T1ρ imaging demonstrates early changes in the lateral patella in patients with patellofemoral pain and maltracking. Am J Sports Med [Internet]. 2013 [cited 2019 May 31];41:1813–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23845401.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Source J R Stat Soc Ser B. 1995.
Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res [Internet]. 2004 [cited 2019 May 31];17–26. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15232421.
Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev [Internet]. 2005 [cited 2019 May 31];33:195–200. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16239837.
Nugent GE, Schmidt TA, Schumacher BL, Voegtline MS, Bae WC, Jadin KD, et al. Static and dynamic compression regulate cartilage metabolism of PRoteoGlycan 4 (PRG4). Biorheology [Internet]. [cited 2019 May 31];43:191–200. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16912393.
Poole RA, Guilak F AS. Osteoarthritis: diagnosis and medical/surgical management [Internet]. 4th ed. In: Moskowitz RW, Altman RD, Hochberg MC, Buckwalter JA GV, editors. Philadelphia: Lippincott Williams & Wilkins; 2007 [cited 2019 May 31]. Available from: https://books.google.com/books?id=YfFj8Gbq5H0C&pg=PA27&source=gbs_toc_r&cad=3#v=onepage&q&f=false.
Fukui N, Miyamoto Y, Nakajima M, Ikeda Y, Hikita A, Furukawa H, et al. Zonal gene expression of chondrocytes in osteoarthritic cartilage. Arthritis Rheum [Internet]. 2008 [cited 2019 May 31];58:3843–53. Available from: http://doi.wiley.com/10.1002/art.24036
Lotz MK, Otsuki S, Grogan SP, Sah R, Terkeltaub R, D’Lima D. Cartilage cell clusters. Arthritis Rheum [Internet]. 2010 [cited 2019 may 31];62:2206–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20506158.
Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest [Internet]. 1995 [cited 2019 May 31];96:2859–69. Available from: http://www.jci.org/articles/view/118357.
Pedoia V, Gallo MC, Souza RB, Majumdar S. Longitudinal study using voxel-based relaxometry: association between cartilage T1ρ and T2 and patient reported outcome changes in hip osteoarthritis. J Magn Reson Imaging. John Wiley and Sons Inc. 2017;45:1523–33.
Wyatt C, Kumar D, Subburaj K, Lee S, Nardo L, Narayanan D, et al. Cartilage T1ρ and T2 relaxation times in patients with mild-to-moderate radiographic hip osteoarthritis. Arthritis Rheum. John Wiley and Sons Inc. 2015;67:1548–56.
Mosher TJ, Zhang Z, Reddy R, Boudhar S, Milestone BN, Morrison WB, et al. Knee articular cartilage damage in osteoarthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology Radiological Society of North America. 2011;258:832–42.
Li X, Wyatt C, Rivoire J, Han E, Chen W, Schooler J, et al. Simultaneous acquisition of T1ρ and T2 quantification in knee cartilage: repeatability and diurnal variation. J Magn Reson Imaging. John Wiley and Sons Inc. 2014;39:1287–93.
Acknowledgments
The authors would like to thank Dr. Hongzhe Li (Lee) , for his statistical assistance throughout the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human subjects were in accordance with the ethical standards or institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zelenski, N., Falk, D., D’Aquilla, K. et al. Zone- and layer-specific differences in proteoglycan content in patellofemoral pain syndrome are detectable on T1ρ MRI. Skeletal Radiol 49, 1397–1402 (2020). https://doi.org/10.1007/s00256-020-03418-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03418-8