Abstract
Objective
To determine whether a 3D magnetic resonance imaging (MRI) sequence with postprocessing applied to simulate computed tomography (CT) (“pseudo-CT”) images can be used instead of CT to measure acetabular version and alpha angles and to plan for surgery in patients with femoroacetabular impingement (FAI).
Materials and methods
Four readers retrospectively measured acetabular version and alpha angles on MRI and CT images of 40 hips from 20 consecutive patients (9 female patients, 11 male patients; mean age, 26.0 ± 6.5 years) with FAI. 3D models created from MRI and CT images were assessed by 2 orthopedic surgeons to determine the need for femoroplasty and/or acetabuloplasty. Interchangeability of MRI with CT was tested by comparing agreement between 2 readers using CT (intramodality) with agreement between 1 reader using CT and 1 using MRI (intermodality).
Results
Intramodality and intermodality agreement values were nearly identical for acetabular version and alpha angle measurements and for surgical planning. Increases in inter-reader disagreement for acetabular version angle, alpha angle, and surgical planning when MRI was substituted for CT were − 2.1% (95% confidence interval [CI], − 7.7 to + 3.5%; p = 0.459), − 0.6% (95% CI, − 8.6 to + 7.3%; p = 0.878), and 0% (95% CI, − 15.1 to + 15.1%; p = 1.0), respectively, when an agreement criterion ≤ 5° was used for angle measurements.
Conclusion
Pseudo-CT MRI was interchangeable with CT for measuring acetabular version and highly favorable for interchangeability for measuring alpha angle and for surgical planning, suggesting that MRI could replace CT in assessing patients with FAI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Femoroacetabular impingement (FAI) is a common cause of hip and groin pain in active young and middle-age adults, exacerbated by flexion and internal rotation, prolonged sitting, and stair climbing [1, 2]. FAI arises from abnormal development and configuration resulting in excess bone formation in the acetabulum, femur, or both [3]. Three distinct types of FAI have been identified: acetabular (pincer), femoral (cam), and mixed (Fig. 1) [4]. As the excess bone forms, abnormal contact occurs between the acetabulum and the femur, resulting in labral tears and articular cartilage damage and early development of hip osteoarthritis [5].
Illustration of cam and Pincer deformities resulting in femoroacetabular impingement (FAI). With cam deformity, asphericity of the femoral head with excess bone at the femoral head neck junction leads to abnormal contact between the femoral head and acetabulum in hip flexion. With pincer deformity, acetabular overcoverage with excess bone along the anterior margin of the acetabulum leads to abnormal contact between the femoral head and acetabulum in hip flexion
Magnetic resonance imaging (MRI) is considered the gold standard for evaluating labral and chondral abnormalities in the hip joint and is routinely performed when FAI is clinically suspected [4, 6]. Similarly, computed tomography (CT) with 3D reconstructions is considered the gold standard for evaluating the abnormal bone morphology that occurs with FAI and is also routinely performed to assess acetabular version (a measure of pincer deformity) and femoral alpha angle (a measure of cam deformity) to determine whether excess bone needs to be removed from the femur (femoroplasty) and/or the acetabulum (acetabuloplasty) [7, 8]. Thus, both MRI and CT are often performed as part of the preoperative evaluation for FAI patients. However, CT of the pelvis results in irradiation of the radiosensitive reproductive organs in these typically young patients. Additionally, the need to perform both MRI and CT leads to increased time to diagnosis and treatment of patients and increased costs to the health care system.
Previous work has shown that the 2D MRI sequences routinely obtained to evaluate FAI are not interchangeable with CT to evaluate bone morphology, specifically acetabular version [9]. Furthermore, 2D MRI sequences cannot be used to generate 3D bone models. As an alternative approach, 3D MRI sequences can be post-processed to produce an image similar to that seen with CT (“pseudo-CT MRI”). Pseudo-CT MRI imaging can be used to evaluate 2D bone morphology and generate 3D bone models [10,11,12]. Specifically, this technique was shown to be comparable with CT in a recent study designed to identify the presence of a cam deformity and other bone abnormalities in patients with FAI [12]. However, the ability of 3D MRI to replace CT for measuring acetabular version and alpha angles in patients with FAI has not been previously evaluated. These measurements, along with assessments of bone morphology, are ultimately used to determine whether an acetabuloplasty or femoroplasty needs to be performed, but the ability to use MRI instead of CT for surgical decision-making has also not been assessed.
Our primary aim in this study, therefore, was to determine the interchangeability of pseudo-CT MRI with CT for measurements of acetabular version in patients with FAI. We also assessed the potential for interchangeability with regard to measurements of alpha angle and with regard to surgeons’ decisions regarding whether an acetabuloplasty and/or a femoroplasty would be needed.
Materials and methods
Before any data were collected, permission was obtained from our Institutional Review Board to conduct this retrospective study with a waiver of informed consent. Twenty consecutive patients with clinical symptoms suggesting FAI who underwent both MRI with a 3D Dixon sequence and CT of the pelvis at our institution between September 2015 and January 2017 were included in this study. Patients with prior surgery and patients who did not have both imaging studies available for review were excluded. Patients in whom imaging did not include the entire pelvis, including both hips, or for whom the MRI scan did not include a 3D Dixon sequence were also excluded.
Imaging protocols
The CT scans were performed on multiple Somatom scanners (Siemens, Erlangen, Germany) using a standard hip protocol (120 kVP; 200 mAs; slice thickness, 0.75–2 mm), and 2-mm thick axial images were used for measurement of acetabular version and alpha angles. The MR scans were performed on a single 3 T MR scanner (Somatom Verio, Siemens) using a standard hip protocol (fat-saturated T2 fast spin-echo [FSE] coronal and axial scans and T1 FSE coronal scans of the pelvis; fat-saturated proton density weighted [PD] FSE coronal, sagittal, and oblique axial scans of the hip). With a 6-channel body matrix phased array coil, an additional 2-point Dixon sequence (axial 3D dual echo-time T1-weighted FLASH acquisition with Dixon-based water-fat separation) was performed using the following parameters: repetition time, 5.48 ms; echo time, 2.46/3.69 ms; field of view, 380 mm; voxel size, 1.2 × 1.2 × 1.2 mm; flip angle, 9°; acquisition time, 4 min 4 s [10]. The water-only images from the 3D Dixon sequence were then post-processed to accentuate the contrast difference between bone and adjacent soft tissues and create multiplanar images with CT-like tissue contrast (pseudo-CT MRI) with high intensity bony structures as described in previous work by Gyftopoulos et al. [10].
Image analysis
The CT and pseudo-CT MR images for each patient were anonymized and randomized before being evaluated. The acetabular version and femoral alpha angles were measured by two board-certified radiologists with more than 10 years of musculoskeletal experience, a musculoskeletal radiology fellow, and a CT technologist with 26 years of experience.
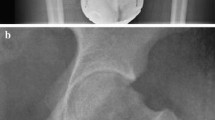
For measurements of acetabular version, any differences in patient positioning were corrected by choosing an axial plane in which the superior aspects of the femoral heads were aligned (Fig. 2). Differences in pelvic tilt were corrected by selecting a coronal plane in which the anterior margin of the ASIS (anterior superior iliac spine) and the anterior margin of the pubic symphysis were aligned on the sagittal images. Acetabular version was measured on the most superior axial slice passing through the physeal scar of the femoral head, with the coronal images used for cross-reference. The acetabular version angle was defined as the angle formed between a line joining the anterior and posterior acetabular rims and a line perpendicular to the posterior pelvic margins.
Pseudo-CT MR images of the pelvis demonstrating acetabular version angle measurement. For measurements of the acetabular version angle, a the tops of the femoral heads were first aligned in the axial plane. b, c The anterior margin of the ASIS and the anterior margin of the pubic symphysis were then aligned in the sagittal plane. d The most superior axial slice passing through the physeal scar of the femoral head was selected on the coronal plane. e On the selected axial slice, the acetabular version angle was defined as the angle formed between a line joining the anterior and posterior acetabular rims and a line perpendicular to the posterior pelvic margins
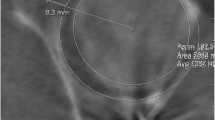
For measurements of the femoral alpha angle, femoral flexion was corrected by choosing a coronal plane parallel to the femoral shaft, with the sagittal images used for cross-reference (Fig. 3). On this coronal slice, an oblique axial plane parallel to and bisecting the femoral head and neck junction was selected. On the selected oblique axial slice, a circle encompassing the entire femoral head was drawn. The alpha angle was defined as the angle formed between a line connecting the center of the circle to the point at which the anterior cortex of the femoral head extended outside the circle and a line drawn parallel to the long axis of the femoral neck.
Pseudo-CT MR images of the pelvis demonstrating alpha angle measurement. For measurements of the femoral alpha angle, a femoral flexion was first corrected by choosing a coronal plane parallel to the femoral shaft, with the sagittal images used for cross-reference. b On this coronal slice, an oblique axial plane was selected parallel to and bisecting the femoral head and neck junction. c On the selected oblique axial slice, a circle encompassing the entire femoral head was drawn. The alpha angle was defined as the angle formed between a line connecting the center of the circle to the point at which the anterior cortex of the femoral head extended outside the circle and a line drawn parallel to the long axis of the femoral neck
3D bone models (Fig. 4) were created by a musculoskeletal radiology fellow on a stand-alone commercially available workstation (Leonardo, Siemens) using 3D volume-rendering tools and manual segmentation. The MR models were generated from the pseudo-CT MRI axial images, and CT models were generated from the axial CT images. The models were then rotated along the long axis of the femur to generate multiple screenshots and a 360° cine clip. The screenshots and cine clip were examined by two experienced hip surgeons to determine whether acetabuloplasty or femoroplasty was indicated in light of the angle measurements.
Statistical analysis
The frequency of inter-reader agreement using CT and MRI for acetabular version and alpha angle measurements was compared. The interchangeability of MRI with CT to measure acetabular version and alpha angles was also measured by estimating the frequency with which readers’ angle measurements on CT agreed with other readers’ angle measurements on CT (inter-reader intramodality agreement) and comparing this to the frequency with which readers’ measurements on MR agreed with readers’ measurements on CT (inter-reader intermodality agreement) [13]. Agreement was defined using 2 cutoff values: 1) if the difference between the intramodality and intermodality agreement was ≤ 5° and 2) if the difference between the intramodality and intermodality agreement was ≤ 10°.
Similarly, the interchangeability of CT and MRI to determine whether an acetabuloplasty and/or femoroplasty was needed was measured by estimating the frequency with which a surgeon using CT agreed with another surgeon using CT (inter-reader intramodality agreement) and comparing this to the frequency with which a surgeon using MR agreed with a surgeon using CT (inter-reader intermodality agreement). Agreement was defined as surgeons choosing the same operative plan (i.e., agreement for acetabuloplasty and femoroplasty).
Although interchangeability can be assessed with either intra-reader or inter-reader agreement, inter-reader agreement was used in this study as the more clinically relevant measurement; in clinical practice, when there is a question regarding the accuracy of a diagnostic finding or measurement, the clinician or radiologist is likely to ask the second opinion of another radiologist rather than reinterpreting the study themselves. Additionally, when patients are followed over time, a different reader than the one who interpreted baseline images will often interpret follow-up images.
To test for increased frequency of disagreement between readers when MRI was substituted for CT, logistic regression analysis was used with generalized estimating equations to account for multiple observations on the same subject; 95% confidence intervals (CIs) were constructed. A noninferiority margin of 5% excess disagreement when MRI was substituted for CT was used to define whether the modalities were interchangeable. The null hypothesis was that of inferiority of MRI; the alternative hypothesis was noninferiority. A significance level of 0.05 was used. The study was powered for the primary aim (i.e., to determine interchangeability for acetabular version) based on the results of previous work assessing acetabular version using 2D MRI and CT [9, 13].
Results
The study population consisted of 9 female patients and 11 male patients with a mean age of 26.0 ± 6.5 years (range, 17–36 years).
Agreement and interchangeability of acetabular version angle measurements
The inter-reader agreement for acetabular version angle measurements when both readers were using MRI was slightly higher than when both readers were using CT regardless of the agreement criterion (Fig. 5). In terms of interchangeability, using an agreement criterion of ≤ 5.0° for acetabular angle, the estimated proportion of disagreement between 2 readers both using CT was 27.9% (67/240 comparisons) compared with 25% (124/480 comparisons) between a reader using MRI and another reader using CT. The excess disagreement when using MRI instead of CT was − 2.1% (95% CI, − 7.7 to 3.5%; p = 0.459). The upper bound of the 95% CI was < 5%, suggesting that MRI can be interchanged with CT without a significant increase in disagreements between readers (Table 1).
The inter-reader agreement for acetabular version angle measurements when both readers were using MRI was slightly higher than the agreement when both readers were using CT regardless of the agreement criterion. The y-axis represents the proportion of cases in which 2 readers agreed on the angle measurement; the x-axis represents the definition of agreement, starting with a difference between measurements of 0° (far left) and moving toward a more lax definition
Using an agreement criterion of ≤ 10.0° for acetabular angle, the estimated proportion of disagreement between 2 different readers both using CT was 7.1% (17/240 comparisons) compared with 6.3% (30/480 comparisons) between a reader using MRI and another reader using CT. The excess disagreement when using MRI instead of CT was − 0.8% (95% CI, −3.5 to + 1.9%; p = 0.478). Again, the upper bound of the 95% CI was < 5%, suggesting that MRI is interchangeable with CT (Table 1).
Agreement and interchangeability of alpha angle measurements
The inter-reader agreement for alpha angle measurements when both readers were using MRI was nearly identical to the agreement when both readers were using CT regardless of the agreement criterion (Fig. 6). Using an agreement criterion of ≤ 5° for alpha angle, the estimated proportion of disagreement between 2 different readers using CT was 55.8% (134/240 comparisons). The estimated proportion of disagreement between a reader using MRI and a different reader using CT was 55.2% (265/480 comparisons). The excess disagreement when using MRI instead of CT was − 0.6% (95% CI, − 8.6 to + 7.3%; p = 0.878). Although the upper bound of the 95% CI was not < 5%, the trend was highly favorable for interchangeability with nearly identical agreement (Table 2).
The inter-reader agreement for alpha angle measurements when both readers were using MRI was nearly identical to the agreement when both readers were using CT regardless of the agreement criterion. The y-axis represents the proportion of cases in which 2 readers agreed on the angle measurement; the x-axis represents the definition of agreement, starting with a difference between measurements of 0° (far left) and moving toward a more lax definition
Using an agreement criterion of ≤ 10° for alpha angle, the estimated proportion of disagreement between 2 different readers using CT was 29.2% (70/240 comparisons). The estimated proportion of disagreement between a reader using MRI and a different reader using CT was 28.8% (138/480 comparisons). The excess disagreement when using MRI instead of CT was − 0.4% (95% CI, − 0.064 to + 0.055%; p = 0.890). Again, although the upper bound of the 95% CI was not < 5%, the trend was highly favorable for interchangeability with nearly identical agreement (Table 2).
Interchangeability of MRI and CT for surgical planning
When both surgeons used CT, they agreed regarding whether acetabuloplasty should be performed for 24 hips (60%) and whether femoroplasty should be performed for 29 hips (72.5%). They agreed regarding both procedures on a given hip for 18 hips (45%). When both surgeons used MRI, they agreed regarding whether acetabuloplasty should be performed for 28 hips (70%) and whether femoroplasty should be performed for 24 hips (60%). They agreed regarding both procedures on a given hip for 18 hips (45%).
The estimated proportion of disagreement regarding overall surgical plans between two surgeons using CT was 55% (22/40 comparisons). Similarly, the estimated proportion of disagreement between a surgeon using MRI and another surgeon using CT was 55% (44/80 comparisons). The excess disagreement when using MRI instead of CT was 0% (95% CI, − 15.1 to + 15.1%; p = 1.0). Although the upper bound of the 95% CI was not < 5%, the trend was highly favorable for interchangeability with identical agreement (Table 3).
Discussion
In this study, we demonstrated that 3D pseudo-CT MRI is interchangeable with CT for measurements of acetabular version. The inter-reader agreement when both readers were using CT was identical or nearly identical to inter-reader agreement when MRI was substituted for CT for 2D measurements of acetabular version. These findings are in contrast to previous work showing that 2D MRI was not interchangeable with CT [9, 13]. In addition, we found that the trend for interchangeability was highly favorable for both alpha angle measurements and surgical planning.
These results support and add to the findings from previously published studies that used 3D MRI to assess bone morphology and generate 3D bone models of the pelvis and hip, thus obviating the need for a separate CT examination. The 3D pseudo-CT MRI sequence that was used in this study was first described by Gyftopoulos et al. [10] in a cadaver study; the authors observed no statistical differences between MRI, 3D CT, and digital photographs in measurements of glenoid and humeral head morphology. This sequence was then used by the same group to evaluate FAI, and the authors observed 100% agreement between MRI and CT for the diagnosis and localization of cam deformities [12]. Our results are in concordance with these previous study results. Neither of these studies, however, specifically addressed our aims of determining whether MRI could be used in place of CT to measure acetabular version and alpha angles and whether using MRI is comparable to using CT to determine whether acetabuloplasty and/or femoroplasty was needed.
Other 3D MRI sequences have been used to evaluate bone morphology in the pelvis and hips and have also demonstrated good results. Yan et al. [14] compared measurements of acetabular version, alpha, center edge, and femoral-neck shaft angles using an isotropic 3D FSE sequence and CT and found good to excellent correlation for most measurements. Rathnayaka et al. [15] used a high-resolution 3D FLASH sequence to generate bone models of cadaver femurs and observed an average error that was only minimally higher (0.23 mm vs 0.15 mm) than the error seen with contact scanner-based reference models. Our results add further support to the conclusions of these previously published studies that 3D MRI can be used to assess bone morphology and generate 2D measurements in FAI and potentially eliminate the need for a CT scan. Additionally, this study demonstrates that there is much less variability in inter-reader measurements of acetabular version (26–28% disagreement) than in inter-reader measurements of alpha angle (55–56% disagreement) with both CT and MRI despite the use of a standardized measurement technique. Similarly, the agreement between surgeons for determining when to perform an acetabuloplasty or femoroplasty was also poor (55% disagreement) whether both surgeons were using CT or one was using MRI and the other was using CT.
The high rate of disagreement among surgeons despite the type of imaging modality used highlights the fact that surgeons consider multiple other factors when determining which type of procedure to perform. Specifically, clinical symptoms, physical examination findings, and other imaging findings such as labral tears and cartilage loss are taken into account when deciding on surgical management. In this study, however, we blinded the surgeons to these other factors to isolate the effect of imaging information that can be obtained from a CT scan (e.g., bone shape and bone measurements) in order to determine whether an MRI scan can be used to provide the same information. The high rate of disagreement also likely arises from the fact that there are no agreed-upon abnormal cutoff values for acetabular version or alpha angle. Although our study suggests that 3D MRI can provide information similar to that provided by CT regarding bone shape and bone measurements, it also demonstrates that neither modality provides all of the necessary information for surgical decision-making. With that in mind, however, the ability to use 3D MRI for surgical planning instead of CT offers the benefit of not exposing the patient to radiation and eliminating an additional test.
The small sample size of this study, which was based on the primary aim to test for interchangeability of MRI and CT for acetabular version, was underpowered to detect interchangeability for alpha angle and surgical planning because the inter-reader disagreement for these two measures was much larger than the disagreement for acetabular version; this reduced our ability to detect noninferiority. Although the strict threshold for interchangeability for alpha angle and surgical planning was not met, we observed near-identical agreement for these measures with a trend that was highly favorable to meet the threshold for interchangeability. Additional studies with larger samples are needed to validate interchangeability for these measures. This study was focused on the evaluation of pincer and cam morphologies, which are major contributing factors to FAI. Recently, femoral torsion, which is also typically measured using CT, has been shown to be another factor contributing to FAI [16]. While our study did not evaluate this factor, a recently published study demonstrated that femoral torsion can be evaluated with a 2D FSE MRI sequence [17]. Lastly, the commercially available CT tools used to automatically segment bone and generate 3D bone models were not designed to segment MRI data, requiring additional manual post-processing to generate 3D MRI bone models. After an initial learning curve, however, the additional time required to generate 3D MRI bone models was between 5 and 10 min on average, which is similar to what has been previously reported [12].
In conclusion, the results of this study suggest that a 3D MRI sequence post-processed to resemble a CT sequence (pseudo-CT MRI) could be used instead of CT for both 2D and 3D evaluation of bone morphology in patients with FAI and eliminate the need to perform an additional CT examination. Using this 3D pseudo-CT MRI sequence in lieu of CT has important clinical implications, including avoidance of ionizing radiation exposure in this young patient cohort, decreased preoperative assessment time, and lower overall costs.
References
Bredella MA, Ulbrich EJ, Stoller DW, Anderson SE. Femoroacetabular impingement. Magn Reson Imaging Clin N Am. 2013;21(1):45–64.
Ghaffari A, Davis I, Storey T, Moser M. Current concepts of femoroacetabular impingement. Radiol Clin N Am. 2018;56(6):965–82.
Leunig M, Beaule PE, Ganz R. The concept of femoroacetabular impingement: current status and future perspectives. Clin Orthop Relat Res. 2009;467(3):616–22.
Genovese E, Spiga S, Vinci V, et al. Femoroacetabular impingement: role of imaging. Musculoskelet Surg. 2013;97(Suppl 2):S117–26.
Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–20.
Riley GM, McWalter EJ, Stevens KJ, Safran MR, Lattanzi R, Gold GE. MRI of the hip for the evaluation of femoroacetabular impingement; past, present, and future. J Magn Reson Imaging. 2015;41(3):558–72.
Heyworth BE, Dolan MM, Nguyen JT, Chen NC, Kelly BT. Preoperative three-dimensional CT predicts intraoperative findings in hip arthroscopy. Clin Orthop Relat Res. 2012;470(7):1950–7.
Beaule PE, Zaragoza E, Motamedi K, Copelan N, Dorey FJ. Three-dimensional computed tomography of the hip in the assessment of femoroacetabular impingement. J Orthop Res. 2005;23(6):1286–92.
Yaddanapudi K, Subhas N, Polster J, Goodwin R, Rosneck J. Acetabular version measurements in native hips: comparison between MRI and 2D CT. Skelet Radiol. 2014;43(12):1795–6.
Gyftopoulos S, Yemin A, Mulholland T, et al. 3DMR osseous reconstructions of the shoulder using a gradient-echo based two-point Dixon reconstruction: a feasibility study. Skelet Radiol. 2013;42(3):347–52.
Gyftopoulos S, Beltran LS, Yemin A, et al. Use of 3D MR reconstructions in the evaluation of glenoid bone loss: a clinical study. Skelet Radiol. 2014;43(2):213–8.
Samim M, Eftekhary N, Vigdorchik JM, et al. 3D-MRI versus 3D-CT in the evaluation of osseous anatomy in femoroacetabular impingement using Dixon 3D FLASH sequence. Skelet Radiol. 2019;48(3):429–36.
Obuchowski NA, Subhas N, Schoenhagen P. Testing for interchangeability of imaging tests. Acad Radiol. 2014;21(11):1483–9.
Yan K, Xi Y, Sasiponganan C, Zerr J, Wells JE, Chhabra A. Does 3DMR provide equivalent information as 3DCT for the pre-operative evaluation of adult hip pain conditions of femoroacetabular impingement and hip dysplasia? Br J Radiol. 2018;91(1092):20180474.
Rathnayaka K, Momot KI, Noser H, et al. Quantification of the accuracy of MRI generated 3D models of long bones compared to CT generated 3D models. Med Eng Phys. 2012;34(3):357–63.
Sutter R, Dietrich TJ, Zingg PO, Pfirrmann CW. Femoral antetorsion: comparing asymptomatic volunteers and patients with femoroacetabular impingement. Radiology. 2012;263(2):475–83.
Fritz B, Bensler S, Leunig M, Zingg PO, Pfirrmann CWA, Sutter R. MRI assessment of supra- and Infratrochanteric femoral torsion: association with femoroacetabular impingement and hip dysplasia. AJR Am J Roentgenol. 2018;211(1):155–61.
Acknowledgments
We acknowledge Kavitha Yaddanapudi, MD, and Soterios Gyftopoulos, MD, MSc, for their help with study design and critical revision of the manuscript. We acknowledge Megan Griffiths, ELS, for help with manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guirguis, A., Polster, J., Karim, W. et al. Interchangeability of CT and 3D “pseudo-CT” MRI for preoperative planning in patients with femoroacetabular impingement. Skeletal Radiol 49, 1073–1080 (2020). https://doi.org/10.1007/s00256-020-03385-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03385-0