Abstract
Dedifferentiated liposarcoma can arise de novo or as a complication of a preexisting well-differentiated liposarcoma. We describe the radiologic and pathologic features of a long-standing liposarcoma with multiple recurrences in a 59-year-old male. Imaging demonstrated a heterogeneous fat-containing mass in the anterior thigh. The adjacent proximal femur showed irregular cortical new bone, eventually followed by intramedullary osteoblastic involvement and pathologic fracture. Histologic assessment at resection revealed dedifferentiated liposarcoma with low-grade osteosarcomatous component. The patient subsequently developed metastatic lesions in the lungs containing osteoid and osteoblastic bone metastases. We discuss the radiologic and pathologic features of this rare entity that, to our knowledge, has previously been reported to directly involve osseous structures in only one other case and discuss the potential pitfalls in diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liposarcoma is the most frequently encountered soft tissue sarcoma [1]. The World Health Organization (WHO) divides this tumor into two groups: atypical lipomatous tumor/well-differentiated liposarcoma (ALT/WDL), which are classified as intermediate (locally aggressive), and dedifferentiated liposarcoma, myxoid liposarcoma, pleomorphic liposarcoma, and liposarcoma not otherwise specified, which are classified as malignant [1]. Of these, well-differentiated liposarcoma is the most common subgroup; with 75% of these tumors occurring in the deep muscles of the extremities and 20% in the retroperitoneum [2]. Dedifferentiated liposarcoma is the third most common subgroup accounting for 18% of all liposarcomas, occurring three times more frequently in the retroperitoneum compared to the extremities [2]. Well-differentiated liposarcoma and dedifferentiated liposarcoma affect men and women equally, with peak incidence in the sixth decade [1]. Dedifferentiation of WDL is a well-documented but unpredictable occurrence, arising in approximately 10% of cases of well-differentiated liposarcomas; however, the rate of local recurrence, disease-related mortality, and dedifferentiation are strongly influenced by the location of the tumor [1, 2]. Rates of dedifferentiation of ALT/WDL range from 6% in the extremity, 17% in the retroperitoneum, to 28% in the groin [2]. Divergent osteosarcomatous dedifferentiation has been reported, typically to high-grade osteosarcoma [3–6]. Low-grade osteosarcomatous dedifferentiation has been infrequently reported in the literature and even less frequently reported in association with osseous structures, with only one other case reported to our knowledge [7]. We present the radiologic and pathologic findings of a long-standing ALT/WDL which, over a 17-year timeframe, evolved into a dedifferentiated liposarcoma with low-grade osteosarcomatous dedifferentiation and discuss the differential diagnosis of calcification in association with soft tissue tumors.
Case report
A 59-year-old male was initially referred to our institution in 1998 with a 2-year history of an enlarging right proximal thigh mass. Pathology upon excision revealed an 18 × 18 × 7-cm fat-containing mass, which microscopically showed sheets of mature adipose tissue separated by fibrous stroma containing atypical, spindled nuclei. A diagnosis of ATL/WDL was rendered. Post-operative MRI from September 1998 demonstrates a small residual fat-containing mass adjacent to the cortical surface of the subtrochanteric femur (Fig. 1).
In June 2002, at radical local resection of the proximal one-fourth of the vastus lateralis and vastus intermedius, gross pathology revealed two separate fat-containing masses measuring 2.3 × 1.5 × 1.4 cm and 3.0 × 3.2 × 1.7 cm, respectively. Histopathologic diagnosis of ATL/WDL was again rendered similar to the prior excision. In addition, a fragment of bone from the region of the greater trochanter was removed with a histopathologic diagnosis of myositis ossificans. Notably, the histopathologically diagnosed myositis ossificans was never appreciated radiographically. Between 2003 and 2013 only one follow-up radiograph was obtained and no CTs. Only MRI follow-up was performed for this WDL without suspected osseous involvement. MRI never identified soft tissue calcification identified at resection by pathology. In retrospect, this may be due to the ease at which one may overlook a small piece of bone or calcium by MRI because of its inherent signal characteristics. Alternatively, it is possible that cortical thickening appreciated on the surface of the femur beginning in 2003 was not initially recognized for what it truly was—tumor bone, and not heterotopic ossification (Fig. 2a–b).
a AP radiograph of the right hip from February 2003 demonstrates focal areas of cortical thickening at the lateral margin of the proximal femur in the subtrochanteric region. b Axial T1-weighted MR image of the right proximal thigh from June 2003. High signal intensity mass present anterior to the proximal femur, consistent with a fat-containing neoplasm. Mild low-intensity thickening of the adjacent cortex of the anterolateral proximal femur in the exact location of the residual fatty tumor in 1998
The patient was followed closely by MRI from 2003 to October 2006 where there was a slow increase in tumor size and cortical new bone over that time course (Fig. 3).
In October 2006, the patient presented with intermittent right thigh pain and underwent repeat excision. Pathology revealed two separate fat-containing masses measuring 15 × 7 × 4.2 cm and 9.0 × 5.8 × 1.6 cm, respectively. A fragment of bone was resected in addition to the lipomatous tumor; however, based upon surgical and pathology reports. the site of the resected bone is unclear. Margins were positive with a diagnosis of ALT/WDL and myositis ossificans. One year later, a follow-up MRI demonstrated a residual or recurrent fat-containing mass with involvement of the vastus lateralis. The patient was without symptoms and elected for MRI observation from 2007 to 2012, even with persistent increase in size of both the soft tissue mass and cortical new bone (Fig. 4a–c).
a Axial T1-weighted MR image of the right proximal femur from October 2007. High signal intensity mass is present anterior to the proximal femur. The mass contains thick low signal intensity septae. Increased low signal intensity bone formation along the adjacent anterolateral proximal femoral cortex is seen. b Axial T1-weighted MR image of the right proximal femur from June 2009. Minimal change in size of the proximal thigh fat-containing mass with adjacent cortical new bone of the anterolateral proximal femur is appreciated. c Axial T1-weighted MR image of the right proximal femur from June 2012 demonstrates increased size of both the fat-containing mass and the amount of cortical new bone of the proximal right femur
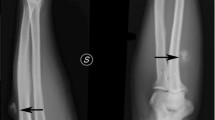
In April 2014, the patient presented with a pathologic fracture of the right femoral neck. Prior to presenting to the local emergency department, the patient reported a 2-week history of increased gluteal and trochanteric region pain. CT and radiographs demonstrated a nondisplaced basi-cervical right femoral neck fracture within an osteoid matrix forming right proximal femoral lesion (Fig. 5a–c).
a AP radiograph and b coronal oblique reformatted CT image [W: 1785 L: 343] of the right proximal femur of the right hip demonstrates a large osteoblastic lesion of the right proximal femur with an associated nondisplaced basi-cervical fracture of the femoral neck. The CT also demonstrates a heterogeneous soft tissue mass immediately adjacent to bone with both soft tissue and fat attenuation. c Axial CT image [W: 1785 L: 343] of the proximal right femur demonstrates the large osteoblastic lesion involving the cortex and medullary space. Heterogeneous adjacent soft tissue mass is also seen
Preoperative MRI, obtained 2 days after the CT, demonstrated an osteoid matrix-forming tumor involving the cortex and medullary space of the right proximal femur (Fig. 6a–d). The ossified component measured approximately 5.5 × 6.5 × 13 cm. The soft tissue component in the proximal thigh involved the vastus lateralis, vastus intermedius, and the distal iliopsoas muscles. It was multilobulated and heterogeneous, largely T1 hyperintense (Fig. 6a and b) with loss of signal on the STIR (Fig. 6b) and fat-saturated sequences, with thick septal enhancement (Fig. 6c) compatible with liposarcoma. The soft tissue component measured approximately 11 × 11 × 22 cm.
a Axial T1-weighted MR image of the right thigh demonstrates a large osteoid matrix-forming component of the tumor involving the cortex and medullary space of the proximal femur. In the adjacent soft tissues, there is a multi-lobulated T1 hyperintense mass consistent with fat-containing neoplasm. b Coronal T1-weighted MR image of the right thigh demonstrates the extent of the osteoblastic and fat-containing mass in the right proximal thigh. c Coronal STIR image of the right thigh demonstrates signal dropout within the soft tissue mass, confirming presence of fat. The STIR image also demonstrates fluid within the nondisplaced basi-cervical pathological fracture of the right femoral neck. d Coronal contrast-enhanced T1-weighted image with fat suppression demonstrates enhancement of the thick septations within the fat-containing mass compatible with liposarcoma
Biopsies were obtained of both the fatty and osseous components of the apparently large contiguous mass. Microscopic examination of the soft tissue and osseous components showed a liposarcoma exhibiting low-grade dedifferentiation (grade I-II) to include anastomosing trabecular-appearing bone separated by a hypocellular spindle cell stroma consistent with a variant of dedifferentiated liposarcoma exhibiting heterologous osteogenic differentiation (Fig. 7a). The following month the patient underwent radical resection of the femur with placement of a custom megaprosthesis. Histopathologic analysis of these specimens confirmed the presence of a dedifferentiated liposarcoma with areas of low-grade dedifferentiation (Fig. 7b); amongst the areas of dedifferentiation, there was mature woven bone trabeculae mostly without osteoblastic rimming and intervening spindle cell stroma lacking significant cytologic atypia.
a Right thigh biopsy (2014), anastomosing, woven bony trabeculae. Between the trabeculae are mildly atypical spindle cells separated by collagen fibers, consistent with a low-grade osteosarcomatous component (H&E stain at ×100). b Soft tissue excision (2014), transition between well-differentiated liposarcoma and foci with fibroblastic spindle cells exhibiting mild-to-moderate nuclear atypia consistent with low-grade dedifferentiation (H&E stain at ×100)
In addition, molecular cytogenetic (fluorescence in situ hybridization) studies were performed and amplification of the MDM2 (12q15) and CDK4 (12q14) loci was detected in both the liposarcomatous and osteosarcomatous components (Fig. 8a–d). The salient cytogenetic feature of well-differentiated liposarcoma is the presence of supernumerary ring or giant rod-shaped chromosomes. Molecular studies have shown that these aberrant chromosomes are composed mostly of amplified 12q13 ∼ q15 region to include most commonly the MDM2 gene locus at 12q15, but often other genes localized within this region such as CDK4 (12q14) are also co-amplified [1]. Notably, parosteal and low-grade central osteosarcomas harbor similar genetic alterations (supernumerary ring chromosomes with corresponding MDM2 and CDK4 amplification [8].
a, b MDM2-amplified cells, osteosarcomatous and liposarcomatous components, respectively (the probe for the MDM2 locus is labeled in spectrum orange and the copy number control probe for the centromeric region of chromosome 12 is labeled in spectrum green). c, d CDK4-amplified cells, osteosarcomatous and liposarcomatous components, respectively (similarly, the probe for CDK4 is labeled in spectrum orange and the probe for the centromeric region of chromosome 12 is labeled in spectrum green)
At follow-up, CT imaging of the chest in October 2014 demonstrated two indeterminate pulmonary nodules, the largest measuring 8 mm. The patient underwent CT of the chest in January 2015, which showed an increase in size of the pulmonary nodules, now containing ossification, concerning for osteosarcoma metastases. A CT-guided core biopsy was performed. Histopathologic examination was limited due to the small and fragmented nature of the involved portion of the needle core biopsy; however, metastatic tumor could be appreciated exhibiting greater intertrabecular cellularity and pleomorphism than witnessed in the thigh specimen, consistent with evolution to a higher grade of dedifferentiation. Mitotic figures, however, were not identified. Follow-up radiograph of the pelvis in August 2015 demonstrated new osteoblastic metastases in the bone and soft tissues of the pelvis, all of which were concerning for progression of metastatic disease.
Discussion
Dedifferentiation occurs in approximately 10% of well-differentiated liposarcomas and can be low- or high-grade [1]. Dedifferentiation in regards to a liposarcoma is defined as a region of tumor that lacks lipogenic differentiation with the degree of dedifferentiation based upon the cellularity, atypia, and mitotic activity [4]. Greater than 65% of these dedifferentiated tumors arise in the retroperitoneum while approximately 20% occur in the extremities. Dedifferentiation is time-dependent; the longer left in situ, the more likely the tumor will dedifferentiate with average time to dedifferentiation of 7–8 years [1, 4]. These tumors behave aggressively, with a local recurrence rate of 41%, metastasis rate of 17%, and disease-related mortality rate of 28% [4, 5].
Dedifferentiated liposarcomas may exhibit heterologous differentiation in approximately 5–10% of cases to include rhabdomyosarcomatous, leiomyosarcomatous, osteosarcomatous, and chondrosarcomatous elements [1, 9]. The osteosarcoma component in most dedifferentiated liposarcomas has been described as high-grade with immature osteoid, increased cellularity, and marked atypia often accompanied by brisk mitotic activity and/or necrosis (resembling conventional osteosarcoma). Mature bone formation or fibroosseous tissue histologically indistinguishable from low-grade osteosarcoma (i.e., parosteal osteosarcoma or low-grade central osteosarcoma) is much less common and in some reports has been considered reactive or metaplastic in nature rather than a true heterologous neoplastic component [10].
To our knowledge, only nine cases of liposarcoma with a low-grade osteosarcomatous component have been reported [3–6, 10, 11]; five of these cases were reported in the extremities but none were directly associated with osseous structures. Of note, one case arising as a parosteal mass of the upper humerus reported as a “parosteal osteoliposarcoma” composed of closely intermingled low-grade osteosarcomatous and well-differentiated liposarcomatous elements has been described [7]. This lesion has features overlapping with the above previously described cases lacking direct osseous involvement and our current case, which initially arose in the soft tissue without a heterologous osteosarcomatous component. The liposarcoma was always closely applied to the periosteal surface of the proximal femur and with disease persistence, disease recurrence and progression, the tumor dedifferentiated, with a heterologous osteosarcomatous component along the cortical surface and eventually extended into the intramedullary proximal femur. The osteosarcomatous component arose in the exact location of the longstanding residual liposarcoma (dating back to 1998).
Calcification and ossification within a lipomatous tumor is a documented occurrence and is most often the result of fat necrosis within a lesion [7]. It is seen in greater association with dedifferentiated or malignant lesions. Bone formation within soft tissues can also be seen in benign processes such as myositis ossificans and benign fat-containing lesions including conventional lipoma (osteolipoma) [1, 12]. In the cases of osteolipoma, histologically there is mature bone formation with osteoblastic rimming [12]. In myositis ossificans, there is a characteristic zonal maturation phenomenon with peripheral rim of mature bone [13]. In contrast, the fibroosseous component of the current case is morphologically indistinguishable from a low-grade osteosarcoma. Moreover, it represents the first case for which fluorescence in situ hybridization (FISH) studies were successfully conducted (as portions of the specimen were not subjected to decalcification solution, a known contraindication for nucleic acid studies) confirming the presence of MDM2 and CDK4 amplification not only in the adipose cells but also in the spindled cells in the stroma between the bony trabeculae and the osteocytes.
Collision tumor, or synchronous liposarcoma and parosteal osteosarcoma, is another diagnostic consideration. Collision tumors are neoplasms of the same organ or anatomical location, which are comprised of at least two different tumor components without mixed or transitional zone between the tumor components [14]. Given the clinical history in our case of an originally adipose only tumor with subsequent development of admixed osteoid after delayed surgical intervention, a dedifferentiated liposarcoma is more likely than collision liposarcoma and osteosarcoma.
The presence of MDM2 and CDK4 amplification in the osseous component would exclude the diagnosis of myositis ossificans or other reactive process and further supports the neoplastic nature of this heterologous low-grade osteosarcomatous component [15]. These findings also suggest that both the liposarcomatous and osteosarcomatous elements arose from the same abnormal clone and support the notion of a common primitive mesenchymal cell progenitor with the ability to differentiate or express features of more than one line of mesenchymal differentiation.
References
Fletcher CDM, Bridge JA, Hogendoorm PCW, Mertens F (eds) World Health Organization classification of tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon; 2013.
Goldblum JR, Weiss SW, Folpe AL. Enzinger and Weiss’s soft tissue tumors. 6th ed. Philadelphia: Saunders; 2014.
Fujii T, Arai T, Sakon M, et al. Retroperitoneal dedifferentiated liposarcoma with osteosarcomatous components: a case report. Int J Clin Exp Pathol. 2013;6(7):1427–31.
Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21(3):271–81.
Toms AP, White LM, Kandel R, Bell RS. Low-grade liposarcoma with osteosarcomatous dedifferentiation: radiologic and histological features. Skeletal Radiol. 2003;32:286–9.
Yu L, Jung S, Hojnowski L, Damron T. Dedifferentiated liposarcoma of soft tissue with high-grade osteosarcomatous dedifferentiation. Radiographics. 2005;25:1082–6.
Larousserie F, Chen X, Ding Y, et al. Parosteal osteoliposarcoma: a new bone tumor (from imaging to immunophenotype). Eur J Radiol. 2013;82:2149–53.
Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: osteosarcoma and related tumors. Cancer Genet Cytogenet. 2003;145(1):1–30.
Miettinen M. Atypical lipomatous tumor and liposarcomas. In: Miettinen M, editor. Modern soft tissue pathology. Cambridge: Cambridge University Press; 2010. p. 441–7.
Yoshida A, Ushiku T, Motoi T, Shibata T, Fukayama M, Tsuda H. Well-differentiated liposarcoma with low-grade osteosarcomatous component an underrecognized variant. Am J Surg Pathol. 2010;34:1361–6.
Aurello P, Edoardo V, Sirimarco D, Novi L, D’Angelo F, Ramacciato G. Dedifferentiated liposarcoma of the retroperitoneum with osteosarcomatous component. Int J Surg Pathol. 2013;21(3):314–5.
Fritchie KJ, Renner JB, Rao KW, Esther RJ. Osteolipoma: radiological, pathological, and cytogenetic analysis of three cases. Skeletal Radiol. 2012;41:237–44.
Kransdorf MJ, Meis JM, Jelinek JS. Myositis Ossificans: MR appearance with radiologic-pathologic correlation. AJR. 1991;157:1243–8.
Izumi H, Furukawa D, Yazawa N, et al. A case study of a collision tumor composed of cancers of the bile duct and pancreas. Surg Case Rep. 2015;1:40.
Yoshida A, Ushiku T, Motoi T, et al. Immunohistochemical analysis of MDM2 and CDK4 distinguishes low-grade osteosarcoma from benign mimics. Mod Pathol. 2010;23:1279–88.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zajicek, A.K., Bridge, J.A., Akers, J.W. et al. Dedifferentiated liposarcoma of the lower extremity with low-grade dedifferentiation and low-grade osteosarcomatous component. Skeletal Radiol 46, 265–271 (2017). https://doi.org/10.1007/s00256-016-2542-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-016-2542-0