Abstract
Ganglia are benign soft tissue masses that are found adjacent to joints and tendons. They can be multifocal but they are rarely more numerous than a few around any given joint. “Cystic ganglionosis” has been used to describe a condition in which multifocal and extensive ganglia are present. We present a rare case of cystic ganglionosis in a Caucasian girl with clinical symptoms detected at 6 months of age. To the authors’ knowledge, only a single other case report of cystic ganglionosis is documented in the English medical literature. The ganglia in this case are more extensive, manifested at an earlier age and caused erosions of multiple bones, a rarely observed complication of ganglia. Additionally, radiograph, MR and sonographic images collected over 9 years time allows for a detailed description of the imaging characteristics of this case of cystic ganglionosis, and offers unique insight into the natural history of this diagnosis. Extensive ganglia in multiple locations in a young child should alert clinicians to the possibility of cystic ganglionosis. Disease progression may lead to deleterious effects on bone warranting the use of maintenance imaging and possibly surgical resection of symptomatic lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A ganglion is a tumor-like, cystic mass that arises adjacent to joints and tendons. Less commonly, they can develop in an intra-articular location, in association with nerve sheaths or arise from the periosteum [1]. Juxta-articular ganglia are commonly found around the wrist and account for the majority of soft tissue masses encountered in the wrist. Ganglia are also often found in the soft tissues of the foot and ankle but in these locations more commonly arise from tendon sheaths [1]. While these are common locations, ganglia may arise adjacent to any joint or tendon sheath in the body [1]. Symptoms associated with ganglia include pain, tenderness, weakness, paraesthesias (if there is compression of adjacent nerves) or decreased range of motion around an affected joint. However, a painless mass is also a very common presentation [2, 3]. One MRI study documented 51 % of asymptomatic adult volunteers having one or more ganglia in a randomly selected wrist [4].
Ganglia most frequently appear between the ages of 25–45 years with females affected twice as commonly as males and typically only involve a single joint. Several studies report that children account for 8–10 % of cases [5, 6]. The majority of patients with symptomatic ganglia have a single lesion. It is unusual for a patient to develop many ganglia. Evaluating wrist MRIs in 103 asymptotic adults, only 12 wrist MRIs showed multiple ganglia, 10 having two ganglia and two having three ganglia [4, 5, 7–12].
The term “cystic ganglionosis” has been proposed to describe the condition when ganglia are numerous in multiple locations [13, 14]. We present an extensive case of cystic ganglionosis in a child in which dozens of ganglia are present in multiple locations. Although typically regarded as a nuisance, ganglia can be symptomatic and may infrequently cause osseous abnormalities, pressure erosions, as seen in this patient [15]. To our knowledge, no other reports have documented these changes in association with cystic ganglionosis. Additionally, radiologic, sonographic and MR images have been collected over 9 years in this patient, offering unique insight into the imaging findings and natural history of this rare condition.
Case report
The child being reported was born at 40 weeks gestation without complication. At the age of 6 months, her mother noted swelling of her bilateral ankles and wrists. Despite this, she continued to have normal growth and development, walking by 9 months. The swelling gradually increased with time, but would wax and wane in the short term, reportedly increasing with activity or excessive heat or humidity. The child’s condition was typically asymptomatic and any mild pain she did experience never altered her activity levels. Similar findings had never occurred in any family members and the family history was otherwise unremarkable. The child did not have any systemic symptoms such as fever, weight loss or fatigue. Laboratory values did not suggest an active infectious or inflammatory rheumatologic condition. There was a normal CBC, negative antinuclear antibody-HEp-2 titers (negative < 1:160 UNITS), erythrocyte sedimentation rate (ESR) = 6 (reference range 3–13 mm/h) and C-reactive protein (CRP) = 0 (reference value < 0.5 mg/dL). On physical examination at presentation, the patient had discrete and confluent deep and superficial subcutaneous nodules located on the bilateral hands, wrists, ankles and feet that ranged in size from a few millimeters to 2 cm. The nodules were soft and compressible with no overlying skin changes. They were non-tender to palpation. Peripheral joints had full range of motion and careful palpation of the metacarpophalangeal and proximal interphalangeal joints demonstrated no synovial thickening or joint effusions.
At 2 years of age, bilateral radiographs of the hands and feet were performed to evaluate the soft tissue swelling in those areas. These showed non-specific soft tissue swelling in both hands and feet, but no underlying osseous abnormalities. Subsequently, ultrasound of the soft tissues about the bilateral hands, wrists, feet and ankles demonstrated multiple well-defined anechoic oval or lobular masses, some containing thin septations (Fig. 1). These were located along multiple tendon sheaths (both in dorsal and palmar/plantar locations) of the hands, wrists, feet and ankles. Color Doppler imaging showed no internal flow in these lesions but some had minimal peripheral increased vascularity. The ultrasound findings were most consistent with ganglia or synovial cysts but were unusual in the size and number involving the bilateral hands, wrists, feet and ankles. Also in the differential diagnosis given, the ultrasound findings were—an inflammatory arthropathy, such as juvenile idiopathic arthritis (JIA) or a multifocal vascular anomaly, such as a lymphatic malformation given the lack of internal vascularity. However, the lack of apparent synovial inflammation made JIA less likely and the periarticular/peritendinous locations without other areas of involvement would be atypical for an extensive vascular anomaly.
At 2 years of age an ultrasound was performed to evaluate the soft tissue swelling of the left foot. Axial color Doppler ultrasound image of the dorsal soft tissues of the left foot in the region of the first metatarsal head (asterisk). There is a lobulated anechoic mass (arrowheads) within the soft tissues dorsal to the first metatarsal head. A few thin septations are present within the lesion (arrows). A peripheral artery and vein (dashed arrow) are seen along the lateral aspect of the lesion but there is no vascularity present within the lesion
The largest lesions at this time were located along the peroneal tendons of the ankles and measured up to 2.1 cm. Given the extensive periarticular soft tissue swelling, there was clinical concern that these findings could be related to synovial/tenosynovial inflammation associated with JIA or less likely an atypical presentation of multiple lymphatic malformations. This concern prompted referral to an orthopedic surgeon for surgical removal of the largest lesions in the lateral aspect of each ankle. Histologic analysis of the surgically resected lesions showed cystic spaces surrounded by fibrocollagenous tissue (Fig. 2). Features of a vascular or lymphatic malformation were not present. There was no evidence of synovial pannus formation and no villonodular or frond-like proliferation of synovium to suggest JIA or pigmented villonodular synovitis. Based on these findings, a pathologic diagnosis of multiple ganglia was made confirming the sonographic findings.
Hematoxylin and eosin stained low (a) and high-powered (b) photomicrographs of the surgically removed cystic lesion from the lateral ankle. There are variably collapsed cystic spaces (asterisks) that contain partially telescoping cyst walls (thick arrows), consisting of lamellarly arranged moderately dense collagen, which are lined by an irregular poorly defined and moderately plump reactive synovial layer. Scattered interposed adipocytes (dashed arrows) are also present. Delicate feeding capillaries (thin arrows) are appreciated at high power. The surrounding fibrocollagenous and adipose tissue, best appreciated at low power, is free of inflammation or other abnormalities. These findings are consistent with a ganglion
Due to increased swelling of the wrists and hands at 5 years of age, ultrasounds of the bilateral hands and wrists and bilateral hand radiographs were repeated. The ultrasound showed increased size and number of ganglia throughout the soft tissues of both hands and wrists (Fig. 3). The radiographs showed areas of early pressure erosions involving metacarpals of each hand, most notable the left fourth metacarpal (Fig. 4). Later that year she developed painful left wrist swelling prompting MRI of both hands and wrists. Extensive ganglia were present within the soft tissues of the hands and wrists in periarticular and peritendinous locations (Fig. 4). These showed mild peripheral and septal enhancement but no central enhancement. Pressure erosions of metacarpal bones in each hand were also present (Fig. 4). MRIs of both feet were performed at age 6 years. These also showed extensive bilateral ganglia that were periarticular and peritendinous in location (Fig. 5). No bony abnormalities were present in the feet at this age. Given the development of extensive ganglia in multiple areas, the patient was given the rare diagnosis of cystic ganglionosis.
Axial grey-scale ultrasound image of the dorsal soft tissues of the right wrist at 2 and 5 years of age shows progression of multiple ganglia. At 2 years of age (a) there are small anechoic ganglia along the extensor tendons (arrows). At 5 years of age, an ultrasound of the dorsal soft tissues of the right wrist was repeated (b), showing increase in the size and number of the ganglia around the dorsal extensor tendons (arrows)
At 5 years of age pressure erosions of the fourth metacarpal are present. Frontal radiograph of the left hand (a) shows pressure erosions of the fourth metacarpal (arrows). Coronal T1-weighted (TR/TE [repetition time/echo time, msec]) (450/17) MR image (b) and coronal short Tau inversion recovery (STIR) (3500/36) MR image (c) of the left hand show a pressure erosion along the proximal aspect of the fourth metacarpal (solid arrows) caused by a ganglion (arrowhead), which is low signal intensity on the T1-weighted image and increased signal intensity on the STIR image. The ganglion is extending from the intermetacarpal joint between the bases of the third and fourth metacarpals (open arrow). Multiple other ganglia are seen within the soft tissues of the wrist and hand (dashed arrows). Edema-like signal, low signal intensity on the T1-weighted (a) and increased signal on the STIR image (b) in the second metacarpal (curved arrows) without evident fracture is also present; at this time the patient had no signs of infection or pain localized to this area and this finding was thought to be related to pressure from adjacent ganglia or stress changes. Axial T2-weighted (3500/36) FS MR image of the left (d) wrist shows multiple ganglia along the extensor, flexor and abductor pollicis tendons
At 6 years of age, extensive ganglia are present within both feet and ankles. Sagittal T2-weighted (2517/70) fat-saturated (FS) MR image of the left foot (a) shows extensive hyperintense ganglia along the flexor digitorum longus and flexor hallicis longus tendons (solid arrows). Additionally, there are multiple other ganglia throughout the mid and fore foot along tendon sheaths and extending from joints (dashed arrows). Sagittal T2-weighted (3000/65) FS MR image of the right foot (b) shows similar hyperintense ganglia along the posterior tibial tendon (solid arrows) and smaller lesions throughout the right mid and forefoot (dashed arrows). Sagittal T1-weighted (667/8) FS post contrast MR image of the right foot (c) shows the ganglia along the posterior tibial tendon have peripheral enhancement (arrows) but no central enhancement
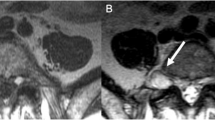
Over the next 5 years, this child was followed in clinic to the age of 11 years and remained relatively asymptomatic with conservative treatment, maintaining a normal activity level. At 7 years of age, she had a left hand and wrist MRI that showed an increase in the size and number of ganglia throughout the soft tissues and interval development of pressure erosions of multiple phalanges (Fig. 6). Follow up MRIs at age 11 years were performed of the bilateral feet and ankles (Fig. 7) as well as the right hand and wrist (Fig. 8). The size and number of cystic lesions in the feet had increased. Pressure erosions were seen in the left foot at the bases of the third, fourth and fifth metatarsals as well as the in the cuboid and talus bones (Fig. 7). A similar erosion was identified in the cuboid of the right foot with associated edema like signal and hyper-enhancement within the underlying bone marrow. As bony abnormalities of the feet were not previously visualized, these finding represented disease progression. The overall size and number of cystic lesions actually decreased in the right hand and wrist from previous MRI (Fig. 8), but their involvement was still extensive and pressure erosions of multiple phalanges were developing, as was previously seen in the left hand (Fig. 4).
At age 7 years there is thinning of the left proximal second and third phalanges with pressure erosions. a Axial T2-weighted (2970/70) MR image of the left hand shows thinning of the second and third phalanges (arrows) secondary to adjacent dorsal and volar ganglia (dashed arrows). The volar ganglia surround the flexor tendons (arrowheads). Sagittal T2-weighted (2800/64) FS (b) and T1-weighted (485/10) (c) MR images of the left wrist and hand at the level of the third digit show the thinning of the third proximal phalanx (arrows) secondary to adjacent ganglia, which are deep to the flexor and extensor tendons (dashed arrows). Other ganglia are seen throughout the hand and wrist (arrowheads)
Progression of ganglia in the left foot and ankle from 6 to 11 years of age. Sagittal T2-weighted (5050/56) short tau inversion recovery (STIR) MR image of the left foot at 6 years of age (a) and repeat imaging of the same foot at age 11 with a sagittal T2-weighted (4300/67) fat-saturated (FS) MR image (b). There is progression of in the size and number of the ganglia within the soft tissues anterior to the ankle/dorsal foot (solid arrows) and within the sinus tarsi (arrowheads). Increase in the size of ganglia extending posteriorly from the tibiotalar joint (open arrow) and along the peroneus longus tendon (dashed arrow) as it passes under the cuboid. Additionally, a pressure erosion has developed along the dorsal aspect of the talus (curved arrow)
Interval decrease in the size of multiple ganglia within the soft tissues of the right wrist from 5 to 11 years of age. Axial T2-weighted (4000/36) FS MR image of the right wrist (a) at 5 years of age shows extensive ganglia within the soft tissues around the wrist adjacent to the extensor tendons (solid arrows), flexor tendons (dashed arrows) and extensor pollicis brevis and abductor pollicis longus tendons (open arrows). Axial T2-weighted FS (3360/74) MR image at 11 years of age (b), shows decrease in the size of the ganglia along the extensor tendons (solid arrows) and extensor pollicis brevis and abductor pollicis longus tendons (open arrows). There is interval resolution of the previously seen ganglia adjacent to the flexor tendons (dashed arrows)
Discussion
A ganglion is a tumor-like, cystic mass that typically arises adjacent joints and tendons. The wall of a ganglion consists of a crisscrossing pattern of condensed hyalinized collagen. The inner surface is often partially lined by spindle cells, but lacks a continuous cellular lining differentiating it from synovial tissue where small, round synovial cells, one to four layers thick, line the entirety of the surface. The spindle cells in ganglia stain for markers of early muscle differentiation, a feature not noted in ligament or synovial tissue [16, 17]. Fluid within ganglia is a gelatinous material that contains higher concentrations of hyaluronic acid and other mucopolysaccharides than synovial fluid, accounting for its higher viscosity [18].
Despite advances in the description of ganglia and their contents, their etiology remains unclear. Previously, it was theorized that ganglia were simply a herniation of the synovium. However, this has been largely disproven given the lack of synovial lining cells. Others believe that the synovial fluid leaks from the joint through a stalk or pedicle leading to a fluid collection and cyst-like development [18]. Myxoid degeneration is a widely accepted mechanism in which the decomposition of collagen fibers into a liquid ground substance leads to ganglia formation [19]. Another possibility is that ligaments and joint capsules disrupted by chronic stress lead to mucin production by modified cells, which eventually coalesces into a ganglion [17, 20].
A ganglion often presents as a soft tissue mass and the differential diagnosis is made accordingly. The list varies with location, but usually includes various types of cysts (e.g., synovial, retinacular, and epidermal inclusion cysts), vascular lesions (e.g., vascular malformations and pseudoaneurysms) as well as benign (e.g., lipomas, glomus tumors, and giant cell tumors of the tendon sheath) and less commonly malignant (e.g., synovial cell sarcomas and fibrosarcomas) soft tissue tumors [21]. Typically ganglia are diagnosed with history and physical examination that will show a firm, tethered mass 1–4 cm in size with no overlying skin changes. They typically trans-illuminate. If no mass is present, an “occult ganglion” may still be causing symptoms. These are best diagnosed with ultrasound or MRI [2]. Radiographs are often the first imaging modality obtained in the workup of ganglia, particularly when the diagnosis is unclear or the clinician suspects an underlying bony abnormality [22]. A superficial ganglion may be seen on a radiograph as a nonspecific soft tissue mass [1]. Deeper ganglia are usually radiographically occult; however, a pressure erosion of an adjacent bone caused by a ganglia may be seen on radiographs [1].
For further characterization of suspected ganglia, ultrasound is generally preferred rather than MRI due to its lower cost and widespread availability, but both are effective in detecting ganglia. On ultrasound, a ganglion appears as a fluid-filled anechoic to hypoechoic mass with well-defined borders [1, 23]. Although ganglia are often thought of as simple cysts, they often do not meet ultrasound criteria of a simple cyst because the fluid within ganglia is commonly hypoechoic rather than anechoic. Septations, loculations and poor definition of walls are also common findings. A complex appearance was found to predominate over a simple cyst appearance in two ultrasound reviews of ganglia [9, 24]. A pedicle or stalk connecting to the joint can also be detected by ultrasound but rates vary between 25 and 70 % [9, 23, 24]. Lack of sonocompressibility is another feature on ultrasound that may help differentiate a ganglion from a synovial cyst [18]. Similarly, ganglia often have a complex appearance on MRI, with internal septations. The typical MRI appearance is high signal intensity on fluid sensitive sequences and low signal intensity on T1-weighted images. Rim enhancement is often seen after gadolinium administration, but more extensive enhancement is possible in septated or compressed cysts [1].
Ganglia are considered a benign condition with no reports of malignant transformation [20]. Spontaneous resolution is also commonly noted in their natural history. Meena and Gupta recently reviewed the literature on ganglia and found the average rate of spontaneous resolution to be 49 %, often within two years [2]. Reported rates of spontaneous resolution are even higher in children at 64–83 % [25–27]. For these reasons, conservative management is often the treatment of choice, especially in children. Various types of procedures are available to treat ganglia for those who are symptomatic or prefer definitive treatment. These include aspiration with or without injections, puncture procedures, and surgical excision. Success rates and recurrence rates vary drastically between studies and no gold standard has yet emerged.
Our review of the English medical literature revealed only a single case report of cystic ganglionosis. Shinawi et al. described multifocal ganglia in an 11-year-old boy. Foot swelling was the first physical manifestation of disease at 2 years of age. His first resection of a recurrent ganglion occurred at 5 years of age, confirming the diagnosis by pathology. The total number of ganglia reached over 20 and caused significant discomfort and inability to participate in athletic events. Failed aspiration and steroid injection of a wrist ganglion led to another excision for pain relief. A unique aspect of this case was ganglia involvement in rare sites including the temporomandibular joints bilaterally and the atlanto-axial synovial articulation [13].
Given the relationship of ganglia to connective tissue and joints, it is not surprising that associated osseous changes have been previously documented. Intraosseous ganglia have caused impending or confirmed pathologic fractures of the scaphoid, glenoid and ilium [28–31]. The characteristic radiographic finding of a periosteal ganglion is cortical bone scalloping due to pressure remodeling. More rarely, a soft tissue ganglion may cause resorption of adjacent bone due to pressure remodeling or a periosteal reaction [1, 15]. The prior report on cystic ganglionosis did not note bony changes as radiographs were normal [13]. However, in our patient, pressure erosions were first diagnosed in the bilateral metacarpals of both hands at age 5 years and were involving phalanges of the left hand by 7 years. By age 11, erosions also involved multiple phalanges, metatarsals and tarsal bones. The final set of MRIs in our patient also provides insight into the natural history of cystic ganglionosis. Disease progression was noted in the feet and ankles, where the size and number of ganglia increased. However, the opposite was true of the right hand and wrist where resolution or regression of cysts predominated. Shinawi et al. also described a waxing and waning course in their case report [13].
Both the case we report here and the prior case report of cystic ganglionosis in the literature, show an early age of onset, as foot and/or hand swelling was first noted in these two children at a very young age [13]. Swelling was first noted in our patient’s bilateral wrists and ankles at the age of 6 months, which is even earlier than the foot swelling reported at 2 years of age in the other reported case [13]. If these patients are typical of this condition, then extensive involvement of the joints of the distal extremities with ganglia by teenage years would be expected. Complications seen with this condition are similar to individual ganglia, but are expected to occur at higher rates due to the sheer number of ganglia. The patient described by Shinawi et al. required surgical excision of some ganglia for pain relief [13]. Our patient is being followed closely for symptomatic progression and surgical intervention remains a consideration if necessary, particularly in areas where the ganglia are increasing in size and causing osseous erosions. In regards to surgery, there remains the concern of scar development as well as recurrence. Follow-up imaging has also shown regression of cysts in certain areas. As a result, the child will continue with close observation and conservative management. Given that the best treatment option for an isolated ganglion in children is still uncertain, the treatment of ganglia in cystic ganglionosis needs to be considered carefully with more invasive treatments being reserved for those causing significant pain, loss of function or potentially harmful disruption of bony structures.
Current theories of the pathological mechanisms of ganglion formation likely play a role in disease process of cystic ganglionosis, however they cannot account for its widespread nature. As in the other case reported in the literature, our patient did not have inflammatory changes seen on microscopic evaluation of her ganglia and her laboratory work up was negative for an acute inflammatory process or autoimmune disorder. Shinawi et al. proposed that their patient with cystic ganglionosis must have an inherent susceptibility to develop ganglia, likely due to a genetic disorder affecting connective tissues surrounding joints and tendons [13]. Currently, with this being only the second reported case in the English medical literature the etiology of cystic ganglionosis remains unknown. Also unknown is the long-term natural history and prevalence of this disorder. More cases of children with cystic ganglionosis need to be documented before this information can be determined.
Based on the clinical exam of the patient we present here, differential diagnoses including JIA or a slow flow vascular or lymphatic malformation were considered. The ultrasound and MRI workup showed periarticular and peritendinous cystic lesions with thin peripheral and/or septal enhancement on post contrast MR images. Synovitis and Tenosynovitis in JIA could involve these locations; however, there was a lack of synovial inflammation on imaging, which would be expected with extensive involvement secondary to JIA. Lymphatic malformations typically are seen as a multicystic mass, or masses, with peripheral and septal enhancement on MRI with a similar appearance to some of the MR images in our patient (Figs. 5 and 7). Furthermore, lymphatic malformations within the soft tissues adjacent to joints or adherent to joint capsules can present with juxta-articular swelling similar to how the patient in this case initially presented [32]. However, the imaging findings in our patient would be atypical for a lymphatic malformation given that multiple cystic lesions were seen exclusively in periarticular/peritendinous locations without other areas of involvement, as is typically seen with diffuse lymphatic malformations. Given the rarity of extensive ganglia, excision and histologic evaluation of superficial lesions is recommended to confirm the diagnosis.
In conclusion, the case we present is important for several reasons. First, it contributes to the very limited available literature regarding cystic ganglionosis by describing a child with extensive ganglia in multiple locations followed over 9 years. Furthermore, multiple radiographs, ultrasounds and MRIs were available to correlate with the child’s clinical progression over time. This offers unique insight into the imaging findings and natural history of this rare condition, including the development of osseous changes that have not previously been reported with cystic ganglionosis.
References
Kransdorf M, Murphey M. In: Pine Jr J, editor. Imaging of soft tissue tumors. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2014.
Meena S, Gupta A. Dorsal wrist ganglion: current review of literature. J Clin Orthop Trauma [Internet]. 2014;5(2):59–64. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0976566214000319
Angelides AC, Wallace PF. The dorsal ganglion of the wrist: its pathogenesis, gross and microscopic anatomy, and surgical treatment. J Hand Surg [Am]. 1976;1(3):228–35. doi:10.1016/S0363-5023(76)80042-1.
Lowden CM, Attiah M, Garvin G, MacDermid JC, Osman S, Faber KJ. The prevalence of wrist ganglia in an asymptomatic population: magnetic resonance evaluation. J Hand Surg [Am]. 2005;30(3):302–6.
Satku K, Ganesh B. Ganglia in children. J Pediatr Orthop. 1985. Jan–Feb;5(1):13–5.
Nelson CL, Sawmiller S, Phalen GS. Ganglions of the wrist and hand. J Bone Joint Surg Am. 1972;54(7):1459–64.
Paivansalo M, Jalovaara P. Ultrasound findings of ganglions of the wrist. Eur J Radiol. 1991;13(3):178–80.
Latif A, Ansar A, Butt MQ. Treatment of ganglions: a five year experience. J Pak Med Assoc [Internet]. 2014;64(11):1278–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25831646. Accessed November 2015.
Teefey SA, Dahiya N, Middleton WD, Gelberman RH, Boyer MI. Ganglia of the hand and wrist: a sonographic analysis. Am J Roentgenol. 2008;191(3):716–20.
MacKinnon AE, Azmy A. Active treatment of ganglia in children. Postgrad Med J. 1977;53(621):378–81.
Bracken J, Bartlett M. Ganglion cysts in the paediatric wrist: magnetic resonance imaging findings. Pediatr Radiol. 2013;43(12):1622–8.
Coffey MJ, Fazlur Rahman M, Thirkannad SM. Pediatric ganglion cysts of the hand and wrist: an epidemiologic analysis. Hand. 2008;3(4):359–62.
Shinawi M, Hicks J, Guillerman RP, et al. Multiple ganglion cysts (‘cystic ganglionosis’): an unusual presentation in a child. Scand J Rheumatol. 2007;36(2):145–8.
Stocker JT, Dehner LP, Husain AN. Stocker and Dehner’s pediatric pathology. 3rd ed. Philadelphia: Wolters Kluwer Health; 2012. 1255 p.
Bauer JS, Müller D, Sauerschnig M, et al. Ganglia of the tarsal sinus: MR imaging features and clinical findings. Eur J Radiol. 2011;80(3):394–400.
O’Valle F, Hernández-Cortés P, Aneiros-Fernández J, et al. Morphological and immunohistochemical evaluation of ganglion cysts. cross-sectional study of 354 cases. Histol Histopathol. 2014;29(5):601–7.
Psaila JV, Mansel RE. The surface ultrastructure of ganglia. J Bone Joint Surg (Br). 1978;60-B(2):228–33.
Giard M-C, Pineda C. Ganglion cyst versus synovial cyst? Ultrasound characteristics through a review of the literature. Rheumatol Int. 2014;35(4):597–605. doi:10.1007/s00296-014-3120-1.
Soren A. Pathogenesis, clinic, and treatment of ganglion. Arch Orthop Trauma Surg. 1982;99(4):247–52.
Angelides A. Ganglions of the hand and wrist. In: Green D, Hotchkiss R, Pederson W, editors. Green’s operative hand surgery. 5th ed. New York: Churchill Livingstone; 1999. p. 2171–83.
Colon F, Upton J. Pediatric hand tumors: a review of 349 cases. Hand Clin. 1995;11(2):223–43.
Nahra ME, Bucchieri JS. Ganglion cysts and other tumor related conditions of the hand and wrist. Hand Clinics; 2004. p. 249–60.
De Flaviis L, Nessi R, Del Bo P, Calori G, Balconi G. High-resolution ultrasonography of wrist ganglia. J Clin Ultrasound. 1987;15(1):17–22.
Wang G, Jacobson JA, Feng FY, Girish G, Caoili EM, Brandon C. Sonography of wrist ganglion cysts: variable and noncystic appearances. J Ultrasound Med. 2007;26(10):1323–8.
MacCollum MS. Dorsal wrist ganglions in children. J Hand Surg [Am]. 1977;2(4):325. doi:10.1016/S0363-5023(77)80137-8.
Rosson JW, Walker G. The natural history of ganglia in children. J Bone Joint Surg (Br). 1989;71(4):707–8.
Calif E, Stahl S, Stahl S. Simple wrist ganglia in children: a follow-up study. J Pediatr Orthop B. 2005;14(6):448–50.
Castellanos J, Bertrán C, Pérez R, Roca J. Pathologic fracture of the scaphoid caused by intraosseous ganglion followed by regression after the healing of the fracture. J Trauma. 2001;51(1):141–3.
Tudisco C, Bisicchia S. Intraosseous ganglion with impending fracture of the glenoid. Orthopedics. 2011;956–9.
Sakamoto A, Oda Y, Iwamoto Y. Intraosseous ganglia: a series of 17 treated cases. Biomed Res Int. 2013;2013:3–6.
Murata K, Nakagawa Y, Suzuki T, Kobayashi M, Kotani S, Nakamura T. Intraosseous ganglion about to cause a fracture of the glenoid: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1261–3.
Jain D, Selhi HS, Yamin M. Lymphangioma presenting as juxta-articular swelling in children: a case series. APSP J Case Rep. 2013;4(2):30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The authors received no funding for this project.
Rights and permissions
About this article
Cite this article
Meyer, N.P., Meyers, A.B., Szabo, S. et al. A rare case of cystic ganglionosis in a child with associated imaging findings. Skeletal Radiol 45, 419–426 (2016). https://doi.org/10.1007/s00256-015-2294-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-015-2294-2