Abstract
Antibiotics have been shown to have a major impact on the composition and metabolism of gut microbiota, while unpredictable stress has been shown to have major influences on gut microbiota homeostasis. However, the impact of combination antibiotics (e.g. metronidazole and ciprofloxacin) on gut microbiota dysbiosis that is induced by stress remains unclear. Here, chronic unpredictable mild stress (CUMS) was used to simulate unpredictable stress, and Sprague–Dawley rats received antibiotics (metronidazole and ciprofloxacin) after CUMS. The weights and coefficients of the thymus, liver and spleen were analysed. 16S rRNA gene sequencing was performed to determine the gut microbiota in the colon, cecum and ileum. We found that antibiotics decreased the CUMS-induced increases in spleen coefficients. Short-term antibiotic exposure significantly increased the cecum and ileum microbiota richness and significantly increased the ileum microbiota diversity after CUMS. Long-term antibiotic exposure significantly decreased the colon microbiota diversity and significantly increased the ileum microbiota richness after CUMS. The impacts of antibiotic exposure on the microbiota compositions at the phylum and genus levels in different gut segments were different after CUMS. However, it is worth noting that the most differentially abundant bacteria in the colon, cecum and ileum were all Verrucomicrobia under long-term antibiotic exposure. Antibiotic exposure significantly increased the relative abundance of Lactobacillus in the colon and ileum and significantly increased the relative abundance of Akkermansia in the colon and cecum after CUMS. In conclusion, this study showed that metronidazole and ciprofloxacin differentially affected the CUMS-induced changes in the microbiota of the colon, cecum and ileum.
Key points
• The impacts of antibiotic exposure on the changes in microbiota that were induced by chronic unpredictable mild stress were analysed.
• We collected rat gut microorganisms from the colon, cecum and ileum.
• The diversities, compositions, functions and network structures were analysed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibiotics are not only widely used in clinical treatment but are also ubiquitous in food and the environment (Clardy et al. 2009; Wang et al. 2020). Antibiotics are a double-edged sword. On the one hand, antibiotics can cause a series of negative effects, such as Clostridium difficile infections, antibiotic resistance and microbiota imbalance. On the other hand, antibiotics have also saved countless lives. Moreover, the effect of antibiotics on bacteria depends not only on the chemical structure or combination of antibiotics but also depends on the duration of antibiotic exposure. In addition, people are currently faced with all types of chronic stress in their lives, such as chronic diseases, economic instability, separation and violence. Kuti et al. (2020) found that Clostridium species increased in the colon in chronically stressed mice, while rifaximin treatment decreased the Clostridium concentrations and increased the colonic expressions of the tight junction proteins. Yang et al. (2020) found that chronic minocycline treatment altered the gut microbiota species abundances and the imbalance of gut microbiota metabolites that were induced by chronic unpredictable mild stress (CUMS) in faecal samples.
Chronic stress has been shown to have a major influence on gut microbiota homeostasis (Siopi et al. 2020; Li et al. 2019a, b). There are more than 100 trillion microbiota in the human gastrointestinal tract, and most of them are bacteria (Rooks and Garrett 2016; Cryan et al. 2019). The gut microbiota is considered to be an important “organ” for maintaining host immune and metabolic homeostasis (Clarke et al. 2014; Cani 2017). Gut microbiota dysbiosis is usually defined as disorders of diversity or composition of the gut microbiota, which are often associated with inflammatory bowel disease and nervous system diseases (Carding et al. 2015; Lavelle and Sokol 2020). Gut-associated lymphoid tissue (GALT) is one of the largest masses of lymphoid tissue in the body (Claud and Walker 2008). Although the number of genes that are encoded by the gut microbiota is greater than 150 times more numerous than those encoded by the human genome and is highly involved in metabolic reactions, they have been neglected in disease diagnosis and treatment (Zhu et al. 2010). Moreover, studies have shown that the gut microbiota may have a great influence on brain development and function (Cryan et al. 2019; Heijtz et al. 2011). Li et al. (2019a, b) found that the overall gut microbiota compositions were significantly different between the CUMS group and control group, and CUMS increased anxiety-like and depression-like behaviour in mice. Marin et al. (2017) reported that the gut microbiota compositions were altered by CUMS. However, the current understanding focuses on colon microbiota dysbiosis. Thus, it is of great relevance to explore the impact of antibiotics on the CUMS-induced microbiota dysbiosis of different gut segments.
Metronidazole is mainly used in infection treatment or prevention that are caused by anaerobic bacteria (Freeman et al. 1997). Ciprofloxacin, as the third generation of quinolones, has broad-spectrum antibacterial activity (Campoli-Richards et al. 1988). Metronidazole and ciprofloxacin are often used in combination in clinical practice (Werk and Schneider 1988). However, the impact of metronidazole and ciprofloxacin on gut microbiota dysbiosis induced by stress remains unclear. Therefore, in this study, CUMS was used to simulate unpredictable stress to explore the effects of metronidazole and ciprofloxacin on the CUMS-induced microbiota dysbiosis of different gut segments.
Materials and methods
Experimental design
Male Sprague–Dawley (SD)-specific pathogen-free (SPF) rats were obtained from Beijing SPF Biotechnology Co. Ltd. (Beijing, China). All rats were kept under a 12-h light/dark cycle at a constant temperature (21–22℃) and humidity (55 ± 5%). All rats received the same sterile standard food (Beijing SFB Biotechnology Co. Ltd., Beijing, China) and tap water. Three rats were fed in each cage. After efficacy analysis, 6-week-old (n = 24) rats were allowed to adapt to the environment prior to the experiment in the 1st week. The rats were randomly assigned to one of four groups of 6 animals each: the CTL group rats (n = 6) did not receive any treatment, the CUMS group rats (n = 6) were exposed to chronic unpredictable mild stress from the 2nd to 5th week, and the CUMS + ABX-s group rats (n = 6) were exposed to chronic unpredictable mild stress from the 2nd to 5th week as well as to metronidazole and ciprofloxacin in drinking water (metronidazole, 1 g/L, Sangon Biotech, Shanghai, China; ciprofloxacin hydrochloride, 0.2 g/L, Solarbio, Beijing, China) in the 6th week. The rats in the CUMS + ABX-l group (n = 6) were exposed to chronic unpredictable mild stress from the 2nd to 5th week as well as to metronidazole and ciprofloxacin in drinking water (metronidazole, 1 g/L, Sangon Biotech, Shanghai, China; ciprofloxacin hydrochloride, 0.2 g/L, Solarbio, Beijing, China) from the 6th to 9th week. All rats were euthanized (carbon dioxide) in the 9th week. The thymus, liver, spleen, colon (cn), cecum (cm) and ileum (lm) contents were collected and stored rapidly at − 80 °C until analysis. “cn” in figures represent the colon contents of rats, “cm” in figures represent the cecum contents of rats, and “lm” in figures represent ileum contents of rats.
Chronic unpredictable mild stress
The studied rats were exposed to chronic unpredictable mild stress from the 2nd to 5th week: (1) food deprivation for 24 h, (2) water deprivation for 24 h, (3) rotation on a shaker for 1 h, (4) tail pinch for 1 min, (5) forced swimming in (cold) water for 5 min, (6) modified light/dark cycle, or (7) heat stress at 50 °C for 15 min (Nollet et al. 2013). Two types of chronic unpredictable mild stress on each day were randomly assigned. The rats in the CTL group were fed according to routine feeding and were not affected by the other groups of rats.
Organ coefficients
The thymus, liver and spleen were removed from the sacrificed rats. All organs were washed in saline, sucked dry and weighed. The organ coefficients (e.g. thymus coefficient, liver coefficient and spleen coefficient) were calculated by dividing the weight of the individual organs by the weight of a specific rat. A larger organ coefficient indicates organ congestion, oedema or hypertrophy, and a smaller organ coefficient indicates organ atrophy and degenerative changes.
16S rRNA gene sequencing
At least 1 g of homogeneous fresh samples (e.g. colon contents, cecum contents and ileum contents) was collected and stored quickly at − 80 °C until analysis. When all samples had been collected, DNA extraction was performed. First, the gut contents were thawed on ice, and the total genomic DNA was extracted by phenol/trichloromethane/isoamyl alcohol. The DNA quality and concentrations were determined with a NanoDrop spectrophotometer (Thermo Scientific Inc., Waltham, MA, USA). The DNA was diluted to 1 ng/µL using sterile water based on the concentration.
The 16S rRNA genes (V3–V4) were amplified using 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′) primers. The sequencing libraries were generated using a DNA PCR-Free Sample Preparation Kit (Illumina Co., Ltd., San Diego, CA, USA) following the manufacturer’s recommendations. The DNA library was sequenced on an Illumina NovaSeq platform, and 250-bp paired-end reads were generated. High-throughput sequencing was performed on an Illumina NovaSeq platform at Novogene Technologies Co., Ltd. (Beijing, China). These data were uploaded as a bioproject to the National Center for Biotechnology Information (NCBI) database (PRJNA745052).
Microbiota analysis
The 16S rRNA gene sequences were processed using R software (Version 4.0.0, https://www.r-project.org/), USEARCH10.0 (www.drive5.com/usearch/) and in-house scripts. The sequences with ≥ 97% similarity were assigned to the same operational taxonomic units (OTUs). Representative sequences of the OTUs were selected and analysed with the USEARCH (http://www.drive5.com/usearch/) and Greengenes databases (http://greengenes.secondgenome.com/) to annotate the taxonomic information: kingdom, phylum, class, order, family, genus and species. The OTU table was standardized to the sample with the lower number of sequences and was subjected to the following analyses. The taxonomy was visualized by ImageGP (http://www.ehbio.com/ImageGP). The results of the constrained principal coordinate analysis (CPCoA) were visualized by RStudio (https://rstudio.com/), and the reshape2, ggplot2, vegan, digest, ggrepel and ggpubr packages were used in the CPCoA.
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) (http://picrust.github.io/picrust/) was performed using the online version of Galaxy (http://huttenhower.sph.harvard.edu/galaxy/) and was visualized by STAMP (Version 2.1.3) (Parks et al. 2014). The bacterial networks were constructed using Gephi (Version 0.9.2, https://gephi.org/). Moreover, the significantly different species between the CUMS and the CUMS + ABX groups were generated by the LEfSe method (Segata et al. 2011). The threshold of the linear discriminant was set to 2.0.
Statistical analyses
All statistical analyses were performed using Prism 6.0 software (GraphPad Software, https://www.graphpad.com/) and OriginPro 2018C (Origin Software, https://www.originlab.com/). The histograms are presented as the means ± standard error (SE). The differences between the groups were evaluated with one-way ANOVA with Duncan’s test. P < 0.05 was considered to indicate a statistically significant difference. The differences were noted as significant for *P < 0.05, **P < 0.01 and ***P < 0.001.
Results
Changes in body weights and organ coefficients
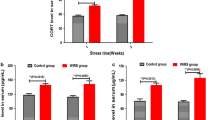
To explore the effect of CUMS and antibiotic exposure on the body weights and organ coefficients, the body and organ weights were measured. Compared with the CTL group, the body weight (Fig. 1b, P < 0.01, F (3, 20) = 7.78) and weight gain (Fig. 1c, P < 0.01, F (3, 20) = 9.74) decreased significantly in the CUMS group. There were no significant differences between the CUMS group and CUMS + ABX group, which indicated that the antibiotics used had no significant effects on the CUMS-induced decreases in body weights and weight gains.
The effect of antibiotic exposure on the growth state. a Experimental design and procedures. b–c Body weight and weight gain were monitored during the experiment (n = 6). d–f Thymus, liver and spleen weights and coefficients (n = 6) were measured. ABX, antibiotics; CUMS, chronic unpredictable mild stress; CUMS + ABX-s, short-term antibiotic exposure; and CUMS + ABX-l, long-term antibiotic exposure. *P < 0.05; **P < 0.01
Compared with the CTL group, the thymus coefficients (Fig. 1d, P < 0.05, F (3, 20) = 3.35) and spleen coefficients (Fig. 1f, P < 0.01, F (3, 20) = 4.84) significantly increased, while the liver weights significantly decreased (Fig. 1e, P < 0.01, F (3, 20) = 5.25) in the CUMS group. In addition, we found that when compared to the CUMS group, the CUMS + ABX-s group displayed significantly lower spleen coefficients (Fig. 1f, P < 0.05, F (3, 20) = 4.84), while the CUMS + ABX-l group exhibited a trend toward decreased spleen weights (Fig. 1f, P = 0.08, F (3, 20) = 1.301), which indicated that the antibiotics reduced the CUMS-induced increases in the spleen coefficients.
Effects on the diversity of gut microbiota in different segments
To explore the effects of antibiotic exposure on the microbiota of different gut segments after CUMS, different gut segment contents were analysed by sequencing the bacterial 16S rRNA V3 + V4 region. A total of 3,452,508 high-quality sequences were obtained from 24 samples (ranging from 32,845 to 72,112 reads per sample). The low-abundance OTUs were discarded, and we obtained 6,089 OTUs that could be used for further analysis. The greater the value of Chao1, the greater the community richness, the greater the Shannon_e value, the higher the community diversity, and the greater the Simpson value, the lower the community diversity.
Compared with the CTL group, the microbiota richness (Chao1) increased significantly (Fig. 2b, P < 0.01, F (3, 20) = 12.75) in the CUMScm group, and the microbiota diversities (Shannon_e and Simpson) significantly increased in the CUMScm group (Fig. 2b, middle, P < 0.01, F (3, 20) = 8.28) and CUMSlm group (Fig. 2c, middle, P < 0.05, F (3, 20) = 20.46; Fig. 2c, right, P < 0.01, F (3, 20) = 9.77). Compared with the CUMS group, the CUMS + ABXcm-s (Fig. 2b, left, P < 0.01, F (3, 20) = 12.75) and CUMS + ABXlm-s (Fig. 2c, left, P < 0.001, F (3, 20) = 29.44) groups exhibited significantly increased microbiota richness, while the CUMS + ABXlm-s group exhibited significantly increased microbiota diversity (Fig. 2c, middle, P < 0.001, F (3, 20) = 20.46; Fig. 2c, P < 0.05, right, F (3, 20) = 9.77). Compared to the CUMS group, the CUMS + ABXcn-l group displayed significantly decreased microbiota diversity (Fig. 2a, middle, P < 0.001, F (3, 20) = 7.29; Fig. 2a, right, P < 0.01, F (3, 20) = 10.0029), while the CUMS + ABXlm-l group displayed significantly increased microbiota richness (Fig. 2c, left, P < 0.01, F (3, 20) = 29.44).
Antibiotic exposure alters the richness and diversity of different gut segment microbiota. a–c Dot plots showing the α-diversity indices (e.g. Chao1, Shannon_e and Simpson) of different gut segment microbiota (n = 6). d–f Constrained principal coordinate analysis (CPCoA) of different gut segment microbiota (n = 6). “CTLcn” represents the colon contents of rats in CTL group, “CTLcm” represents the cecum contents of rats in CTL group, “CTLlm” represents ileum contents of rats in CTL group, “CUMScn” represents the colon contents of rats in CUMS group, “CUMScm” represents the cecum contents of rats in CUMS group, “CUMSlm” represents the ileum contents of rats in CUMS group, “CUMS + ABXcn-s” represents the colon contents of rats in CUMS + ABX-s group, “CUMS + ABXcm-s” represents the cecum contents of rats in CUMS + ABX-s group, “CUMS + ABXlm-s” represents the ileum contents of rats in CUMS + ABX-s group, “CUMS + ABXcn-l” represents the colon content of rats in CUMS + ABX-l group, “CUMS + ABXcm-l” represents the cecum content of rats in CUMS + ABX-l group, and “CUMS + ABXlm-l” represents the ileum content of rats in CUMS + ABX-l group
In addition, the beta diversity analysis showed that the microbiota in the colon, cecum and ileum were significantly affected by antibiotic exposure after CUMS (Fig. 2d–f).
Effects on the gut microbiota composition at the phylum level
Compared with the CTLcn group, Proteobacteria significantly (Fig. 3a, P < 0.05, F (3, 20) = 14.29) decreased in the CUMScn group. Compared with the CUMScn group, the CUMS + ABXcn-s group exhibited a trend toward a decreased relative abundance of Proteobacteria (Fig. 3a, P = 0.068, F (3, 20) = 14.29), while the Actinobacteria/Proteobacteria (A/P) ratio significantly increased (Fig. 3b, P < 0.05, F (3, 20) = 7.79). Compared with the CUMScn group, Bacteroidetes (Fig. 3a, P < 0.001, F (3, 20) = 12.98) and Proteobacteria (Fig. 3a, P < 0.05, F (3, 20) = 14.29) significantly decreased, Firmicutes (Fig. 3a, P < 0.05, F (3, 20) = 3.34) and Verrucomicrobia (Fig. 3a, P < 0.001, F (3, 20) = 8.95) significantly increased, while the A/P (Fig. 3b, P < 0.01, F (3, 20) = 7.79) and Firmicutes/Bacteroidetes (F/B) (Fig. 3c, P < 0.001, F (3, 20) = 8.19) ratios significantly increased in the CUMS + ABXcn-l group.
Antibiotic exposure alters the composition of gut microbiota at the phylum level. a–c Relative abundances of the top 8 phyla in each sample, Actinobacteria/Proteobacteria ratios and Actinobacteria/Proteobacteria ratios of each group in the colon. d–f Relative abundances of the top 8 phyla in each sample, Actinobacteria/Proteobacteria ratios and Actinobacteria/Proteobacteria ratios of each group in the cecum. g–i Relative abundances of the top 8 phyla in each sample, Actinobacteria/Proteobacteria ratios and Actinobacteria/Proteobacteria ratios of each group in the ileum. “*” represents the CUMS group versus the CTL group, “#” represents the CUMS + ABX-s group versus the CUMS group, “a” represents the CUMS + ABX-l group versus the CUMS group, one symbol represents P < 0.05, two symbols represent P < 0.01, and three symbols represent P < 0.001
Compared with the CTLcm group, Bacteroidetes (Fig. 3d, P < 0.01, F (3, 20) = 6.26) significantly decreased, Firmicutes (Fig. 3d, P < 0.05, F (3, 20) = 5.17) significantly increased, and the A/P (Fig. 3e, P < 0.05, F (3, 20) = 4.47) and F/B (Fig. 3f, P < 0.01, F (3, 20) = 5.75) ratios significantly increased in the CUMScm group. There were no significant differences between the CUMScm group and CUMS + ABXcm-s group at the phylum level. Compared with the CUMScm group, Firmicutes (Fig. 3d, P < 0.05, F (3, 20) = 5.16) significantly decreased, Bacteroidetes (Fig. 3d, P < 0.05, F (3, 20) = 6.26) and Verrucomicrobia (Fig. 3d, P < 0.001, F (3, 20) = 9.82) significantly increased in the CUMS + ABXcm-l group, while the F/B ratio (Fig. 3f, P < 0.01, F (3, 20) = 5.75) significantly decreased in the CUMS + ABXcm-l group.
There were no significant differences between the CUMSlm group and CTLlm group at the phylum level. Compared with the CUMSlm group, Bacteroidetes (Fig. 3g, P < 0.01, F (3, 20) = 5.61) significantly increased, Firmicutes (Fig. 3g, P = 0.06, F (3, 20) = 1.51) exhibited a decreasing trend, while the A/P (Fig. 3h, P < 0.05, F (3, 20) = 2.804) and F/B (Fig. 3i, P < 0.05, F (3, 20) = 4.06) ratios significantly decreased in the CUMS + ABXlm-s group. Compared with the CUMSlm group, Bacteroidetes (Fig. 3g, P = 0.09, F (3, 20) = 5.61) and Verrucomicrobia (Fig. 3g, P = 0.07, F (3, 20) = 1.77) significantly increased, while the A/P (Fig. 3h, P = 0.09, F (3, 20) = 2.804) and F/B (Fig. 3i, P = 0.08, F (3, 20) = 4.06) ratios exhibited decreasing trends in the CUMS + ABXlm-l group.
Effects on the gut microbiota compositions at the genus level
To investigate the effects of antibiotics on the gut microbiota compositions at the genus level in different segments after CUMS, we analysed the genera with the top ten highest relative abundances (Fig. 4a–c). As shown in Fig. 4a, compared with the CUMScn group, Turicibacter (P < 0.05, F (3, 20) = 4.21) significantly increased, while Peptococcaceae (P < 0.05, F (3, 20) = 6.35) and Ruminoccaceae (P < 0.001, F (3, 20) = 12.03) significantly decreased in the CUMS + ABXcn-s group. Compared with the CUMScn group, Prevotella (P < 0.001, F (3, 20) = 9.66), Clostridiales (P < 0.001, F (3, 20) = 9.96), SMB53 (P < 0.05, F (3, 20) = 5.25), Peptococcaceae (P < 0.01, F (3, 20) = 6.35) and Ruminoccaceae (P < 0.01, F (3, 20) = 12.03) significantly decreased, and Akkermansia (P < 0.001, F (3, 20) = 8.95) significantly increased in the CUMS + ABXcn-l group.
Antibiotic exposure alters the gut microbiota compositions at the genus level. a–c Relative abundances of the top 10 genera in each sample. d–f Bacterial network of the colon, cecum and ileum contents. The nodes represent OTUs, node sizes represent connectivity, and colours represent different genus levels. Edges (connections): red represents a positive correlation, and green represents a negative correlation (Spearman, | r |> 0.6, P < 0.05). “*” represents the CUMS group versus the CTL group, “#” represents the CUMS + ABX-s group versus the CUMS group, “a” represents the CUMS + ABX-l group versus the CUMS group, one symbol represents P < 0.05, two symbols represent P < 0.01, and three symbols represent P < 0.001
As shown in Fig. b, when compared with the CTLcm group, SMB53 (P < 0.05, F (3, 20) = 14.49) significantly decreased, and Lachnospiraceae (P < 0.01, F (3, 20) = 3.68) and Ruminoccaceae (P < 0.05, F (3, 20) = 15.14) significantly increased. Compared with the CUMScm group, Ruminoccaceae (P < 0.001, F (3, 20) = 15.14) significantly increased, and Turicibacter (P < 0.01, F (3, 20) = 8.83), SMB53 (P < 0.05, F (3, 20) = 14.49) and Lachnospiraceae (P < 0.05, F (3, 20) = 3.68) significantly decreased in the CUMS + ABXcm-s group. Compared with the CUMScm group, Turicibacter (P < 0.001, F (3, 20) = 8.83), Clostridiales (P < 0.05, F (3, 20) = 2.37), SMB53 (P < 0.01, F (3, 20) = 14.49), Lachnospiraceae (P < 0.05, F (3, 20) = 3.68) and Peptococcaceae (P < 0.001, F (3, 20) = 12.25) significantly decreased, while S24–7 (P < 0.001, F (3, 20) = 18.44) and Akkermansia (P < 0.001, F (3, 20) = 9.82) significantly increased in the CUMS + ABXcm-l group.
As shown in Fig. 4c, compared with the CUMSlm group, Prevotella (P < 0.001, F (3, 20) = 9.036), Lachnospiraceae (P < 0.01, F (3, 20) = 6.65), Peptococcaceae (P < 0.01, F (3, 20) = 4.52) and Ruminoccaceae (P < 0.001, F (3, 20) = 18.71) significantly increased, while Turicibacter (P < 0.05, F (3, 20) = 5.33) and SMB53 (P < 0.01, F (3, 20) = 13.39) significantly decreased in the CUMS + ABXlm-s group. Compared with the CUMScm group, Turicibacter (P < 0.01, F (3, 20) = 5.33) and SMB53 (P < 0.001, F (3, 20) = 13.39) significantly decreased, while Lactobacillus (P < 0.001, F (3, 20) = 8.15) and Ruminoccaceae (P < 0.05, F (3, 20) = 18.71) significantly increased in the CUMS + ABXlm-l group.
The microbial interaction networks were constructed by using the Spearman correlation coefficients (Fig. 4d–f). Table 1 shows the structural characteristics of the microbial networks for the different treatment groups. The numbers of edges and average degrees for the cecum were lower than those for the colon and cecum, and the number of edges for cecum was the highest. The average path length and modularity of the cecum were higher than those of the colon and ileum.
In the CUMS + ABX-s group compared with the CUMS group, the most differentially abundant bacteria from the colon belonged to the phyla Verrucomicrobia and Actinobacteria (supplementary Fig. S1a), the most differentially abundant bacteria from the cecum belonged to the phylum Firmicutes (supplementary Fig. S1b), and the most differentially abundant bacteria from the ileum belonged to the phylum Proteobacteria (supplementary Fig. S1c). It is worth noting that under long-term antibiotic exposure, the most differentially abundant bacteria in the colon, cecum and ileum all belonged to the phylum Verrucomicrobia (Fig. 5a–c).
Variations in different gut segment microbiota under long-term antibiotic exposure. a–c Cladogram plot showing the taxonomic levels represented by rings in the colon, cecum and ileum samples from the CUMS group and CUMS + ABX-l group, with phyla in the outermost ring and genera in the innermost ring. Each circle represents a member within that level. The taxa at each level were coloured according to their abundances (P < 0.05; LDA score > 3)
Effects on the metabolism functions of gut microbiota
To determine the changes in microbiome metabolites that were induced by the changes in microbiota abundances, the 16S rRNA gene data were annotated with metabolic pathways from the KEGG database using PICRUSt prediction analysis (Fig. 6a–c and Fig. 7a–c). In the colon, compared with the CUMScn group, six metabolic pathways were significantly affected by short-term antibiotic exposure, and sixteen metabolic pathways were significantly affected by long-term antibiotic exposure. Compared with the CUMScm group, two metabolic pathways in the cecum were significantly affected by short-term antibiotic exposure, and eighteen metabolic pathways were significantly affected by long-term antibiotic exposure. Compared with the CUMSlm group, thirty metabolic pathways in the ileum were significantly affected by short-term antibiotic exposure, and eighteen metabolic pathways were significantly affected by long-term antibiotic exposure.
Differential microbial functions in different gut segments under short-term antibiotic exposure. a–c The metagenomic analysis was based on 16S rRNA gene sequencing data and was performed using PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States), which was followed by annotation with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and visualization using STAMP. The statistical differences between the two groups were screened by Welch’s t test with P < 0.05
Differential microbial functions in different gut segments under long-term antibiotic exposure. a–c The metagenomic analysis was based on 16S rRNA gene sequencing data and conducted using PICRUSt, which was followed by annotation with the KEGG database and visualization using STAMP. The statistical differences between the two groups were screened by Welch’s t test with P < 0.05
Discussion
In the present study, we observed some interesting changes in the gut microbiota that were related to antibiotic exposure after CUMS. For example, Lactobacillus significantly increased in the colon, which has been reported to ferment sugars to produce lactic acid and increase anti-inflammatory factors such as IL-4 and IL-10 (Partrick et al. 2021; Angelis and Gobbetti 2011). Turicibacter also significantly increased in the colon, which was reported to be involved in fermentation metabolism, and lactic acid was the main metabolite (Bosshard et al. 2002; Lee et al. 2018). However, Turicibacter significantly decreased in both the cecum and ileum. Akkermansia, which was significantly elevated in the colon and cecum in this study, has been reported to have an anti-inflammatory effect (Derrien et al. 2017; Zhao et al. 2017). Dubourg et al. (2013) and Li et al. (2020) both observed an increase in Akkermansia bacteria after treatment with broad-spectrum antibiotic or compound of antibiotic activity. In addition, the effects of metronidazole and ciprofloxacin exposure on the A/P and F/B ratios of different gut segments were different in the CUMS-treated rats. The A/P ratios significantly increased in the colon, but no significant differences were observed in the cecum and ileum, while the changes in the F/B ratios in the colon were opposite to those in the cecum and ileum. These results suggested that the gut microbiota from different gut segments influenced the host physiological and psychological health by shaping different changes in the gut microbiota compositions.
Moreover, Liu et al. (2018) reported that chronic minocycline treatment inhibited the activation of microglia through neuroinflammation regulation. Yang et al. (2020) found that chronic minocycline treatment inhibited neuroinflammation. Our study found that the activities of the nervous system pathway in the colon and cecum were significantly upregulated under long-term antibiotic exposure after CUMS but that the activity in the ileum was significantly downregulated under antibiotic exposure. These results suggested that the gut microbiota from different gut segments influenced the host physiological and psychological health by shaping different metabolic functions of the gut microbiota changes.
Hemmings et al. (2017) reported that Verrucomicrobia decreased in patients with PTSD. Tenorio-Jiménez et al. (2019) found that Verrucomicrobia in the gastrointestinal microbiota increased in metabolic syndrome patients after probiotic Lactobacillus reuteri V3401 treatment. We found that under long-term metronidazole and ciprofloxacin exposure, the most differentially abundant bacteria in the colon, cecum and ileum were all Verrucomicrobia when compared to the CUMS-treated rats, which suggested that Verrucomicrobia may play a critical role in metronidazole and ciprofloxacin regulating the host physiological and psychological health.
This study not only emphasized how antibiotic exposure significantly affects the microbiota in the colon, cecum and ileum after CUMS but also emphasized the importance of studying the microbiota in a location-specific manner. In conclusion, our results showed that antibiotics promoted compositional changes in the gut microbiota in different gut segments, which provide unexpected clues for the roles of different gut segment microbiota in diseases. The driving factors of antibiotic upregulation and the mechanism that induces imbalances remain to be determined in complex immune and inflammatory diseases.
Data Availability
The data of this study was uploaded as a bioproject to the National Center for Biotechnology Information (NCBI) database (PRJNA745052).
References
Angelis MDD, Gobbetti M (2011) Lactic acid bacteria | Lactobacillus spp.: general characteristics. In: Fuquay JW (ed) Encyclopedia of dairy sciences. Academic Press, San Diego, pp 78–90
Bosshard PP, Zbinden R, Altwegg M (2002) Turicibacter sanguinis gen. nov., sp. nov., a novel anaerobic, gram-positive bacterium. Int J Syst Evol Microbiol 52:1263–1266. https://doi.org/10.1099/00207713-52-4-1263
Campoli-Richards DM, Monk JP, Price A, Benfield P, Todd PA, Ward A (1988) Ciprofloxacin Drugs 35:373–447. https://doi.org/10.2165/00003495-198835040-00003
Cani PD (2017) Gut microbiota - at the intersection of everything? Nat Rev Gastroenterol Hepatol 14:321–322. https://doi.org/10.1038/nrgastro.2017.54
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ (2015) Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis 26:26191. https://doi.org/10.3402/mehd.v26.26191
Clardy J, Fischbach MA, Currie CR (2009) The natural history of antibiotics. Curr Biol 19:R437–R441. https://doi.org/10.1016/j.cub.2009.04.001
Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG (2014) Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol 28:1221–1238. https://doi.org/10.1210/me.2014-1108
Claud EC, Walker WA (2008) The intestinal microbiota and the microbiome. In: Polin RA and Neu J (eds), Gastroenterology and nutrition: neonatology questions and controversies. W.B. Saunders, pp 73–92
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O’Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, Wouw MVD, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG (2019) The microbiota-gut-brain axis. Physiol Rev 99:1877–2013. https://doi.org/10.1152/physrev.00018.2018
Derrien M, Belzer C, Vos WMD (2017) Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 106:171–181. https://doi.org/10.1016/j.micpath.2016.02.005
Dubourg G, Lagier JC, Armougom F, Robert C, Audoly G, Papazian L, Raoult D (2013) High-level colonisation of the human gut by Verrucomicrobia following broad-spectrum antibiotic treatment. Int J Antimicrob Agents 41:149–155. https://doi.org/10.1016/j.ijantimicag.2012.10.012
Freeman CD, Klutman NE, Lamp KC (1997) Metronidazole. a therapeutic review and update. Drugs 54:679–708. https://doi.org/10.2165/00003495-199754050-00003
Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 108:3047–3052. https://doi.org/10.1073/pnas.1010529108
Hemmings SMJ, Malan-Müller S, Heuvel LLVD, Demmitt BA, Stanislawski MA, Smith DG, Bohr AD, Stamper CE, Hyde ER, Morton JT, Marotz CA, Siebler PH, Braspenning M, Criekinge WV, Hoisington AJ, Brenner LA, Postolache TT, McQueen MB, Krauter KS, Knight R, Seedat S, Lowry CA (2017) The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med 79:936–946. https://doi.org/10.1097/psy.0000000000000512
Kuti D, Winkler Z, Horváth K, Juhász B, Paholcsek M, Stágel A, Gulyás G, Czeglédi L, Ferenczi S, Kovács KJ (2020) Gastrointestinal (non-systemic) antibiotic rifaximin differentially affects chronic stress-induced changes in colon microbiome and gut permeability without effect on behavior. Brain Behav Immun 84:218–228. https://doi.org/10.1016/j.bbi.2019.12.004
Lavelle A, Sokol H (2020) Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 17:223–237. https://doi.org/10.1038/s41575-019-0258-z
Lee YS, Kim TY, Kim Y, Lee SH, Kim S, Kang SW, Yang JY, Baek IJ, Sung YH, Park YY, Hwang SW, O E, Kim KS, Liu S, Kamada N, Gao N, Kweon MN, (2018) Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe 24:833-846.e836. https://doi.org/10.1016/j.chom.2018.11.002
Li JG, Jia XY, Wang C, Wu CX, Qin XM (2019a) Altered gut metabolome contributes to depression-like behaviors in rats exposed to chronic unpredictable mild stress. Transl Psychiatry 9:40. https://doi.org/10.1038/s41398-019-0391-z
Li L, Chang L, Zhang X, Ning Z, Mayne J, Ye Y, Stintzi A, Liu J, Figeys D (2020) Berberine and its structural analogs have differing effects on functional profiles of individual gut microbiomes. Gut Microbes 11:1348–1361. https://doi.org/10.1080/19490976.2020.1755413
Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y, Li X (2019b) Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress 22:592–602. https://doi.org/10.1080/10253890.2019.1617267
Liu HY, Yue J, Hu LN, Cheng LF, Wang XS, Wang XJ, Feng B (2018) Chronic minocycline treatment reduces the anxiety-like behaviors induced by repeated restraint stress through modulating neuroinflammation. Brain Res Bull 143:19–26. https://doi.org/10.1016/j.brainresbull.2018.08.015
Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, Wu M, Overall CC, Kipnis J, Gaultier A (2017) Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep 7:43859. https://doi.org/10.1038/srep43859
Nollet M, Guisquet AML, Belzung C (2013) Models of depression: unpredictable chronic mild stress in mice. Curr Protoc Pharmacol Chapter 5: Unit 5.65. https://doi.org/10.1002/0471141755.ph0565s61
Parks DH, Tyson GW, Hugenholtz P, Beiko RG (2014) STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30:3123–3124. https://doi.org/10.1093/bioinformatics/btu494
Partrick KA, Rosenhauer AM, Auger J, Arnold AR, Ronczkowski NM, Jackson LM, Lord MN, Abdulla SM, Chassaing B, Huhman KL (2021) Ingestion of probiotic (Lactobacillus helveticus and Bifidobacterium longum) alters intestinal microbial structure and behavioral expression following social defeat stress. Sci Rep 11:3763. https://doi.org/10.1038/s41598-021-83284-z
Rooks MG, Garrett WS (2016) Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16:341–352. https://doi.org/10.1038/nri.2016.42
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. https://doi.org/10.1186/gb-2011-12-6-r60
Siopi E, Chevalier G, Katsimpardi L, Saha S, Bigot M, Moigneu C, Eberl G, Lledo PM (2020) Changes in gut microbiota by chronic stress impair the efficacy of fluoxetine. Cell Rep 30:3682-3690.e3686. https://doi.org/10.1016/j.celrep.2020.02.099
Tenorio-Jiménez C, Martínez-Ramírez MJ, Castillo-Codes ID, Arraiza-Irigoyen C, Tercero-Lozano M, Camacho J, Chueca N, García F, Olza J, Plaza-Díaz J, Fontana L, Olivares M, Gil Á, Gómez-Llorente C (2019) Lactobacillus reuteri V3401 reduces inflammatory biomarkers and modifies the gastrointestinal microbiome in adults with metabolic syndrome: the PROSIR study. Nutrients 11:1761. https://doi.org/10.3390/nu11081761
Wang Q, Duan YJ, Wang SP, Wang LT, Hou ZL, Cui YX, Hou J, Das R, Mao DQ, Luo Y (2020) Occurrence and distribution of clinical and veterinary antibiotics in the faeces of a Chinese population. J Hazard Mater 383:121129. https://doi.org/10.1016/j.jhazmat.2019.121129
Werk R, Schneider L (1988) Ciprofloxacin in combination with metronidazole. Infection 16:257–260. https://doi.org/10.1007/bf01650774
Yang Q, Luo L, Sun T, Yang L, Cheng LF, Wang Y, Liu QQ, Liu A, Liu HY, Zhao MG, Wu SX, Feng B (2020) Chronic minocycline treatment exerts antidepressant effect, inhibits neuroinflammation, and modulates gut microbiota in mice. Psychopharmacology 237:3201–3213. https://doi.org/10.1007/s00213-020-05604-x
Zhao S, Liu W, Wang J, Shi J, Sun Y, Wang W, Ning G, Liu R, Hong J (2017) Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol 58:1–14. https://doi.org/10.1530/jme-16-0054
Zhu B, Wang X, Li L (2010) Human gut microbiome: the second genome of human body. Protein Cell 1:718–725. https://doi.org/10.1007/s13238-010-0093-z
Funding
This work was financially supported by the grant from the National Natural Science Foundation of China (Grant No. 81871520).
Author information
Authors and Affiliations
Contributions
CM performed experiments, analysed data and wrote original draft. CD performed experiments, collected data and revised the article. HL designed experiments and revised the article.
Corresponding authors
Ethics declarations
Ethics approval
All procedures were performed according to the National Institutes of Health Guide for the care and use of laboratory animals and were approved by the ethics committee of Beihang University (BM20210108).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meng, C., Dong, C. & Liu, H. Metronidazole and ciprofloxacin differentially affect chronic unpredictable mild stress-induced changes in the colon, cecum and ileum microbiota. Appl Microbiol Biotechnol 106, 369–381 (2022). https://doi.org/10.1007/s00253-021-11685-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11685-1