Abstract
Recently, thermophilic Thermoanaerobacterium species have attracted increasing attentions in consolidated bioprocessing (CBP), which can directly utilize lignocellulosic materials for biofuels production. Compared to the mesophilic process, thermophilic process shows greater prospects in CBP due to its relatively highly efficiency of lignocellulose degradation. In addition, thermophilic conditions can avoid microbial contamination, reduce the cooling costs, and further facilitate the downstream product recovery. However, only few reviews specifically focused on the microbial applications of thermophilic Thermoanaerobacterium species in lignocellulosic biorefinery. Accordingly, this review will comprehensively summarize the recent advances of Thermoanaerobacterium species in lignocellulosic biorefinery, including their secreted xylanases and bioenergy production. Furthermore, the co-culture can significantly reduce the metabolic burden and achieve the more complex work, which will be discussed as the further perspectives.
Key points
• Thermoanaerobacterium species, promising chassis for lignocellulosic biorefinery.
• Potential capability of hemicellulose degradation for Thermoanaerobacterium species.
• Efficient bioenergy production by Thermoanaerobacterium species through metabolic engineering.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biorefinery is a sustainable multi-step process, which can achieve the conversion of lignocellulosic materials into bioenergy and biochemicals (Calvo et al. 2018). As one of the most abundant and renewable resources in the world, lignocellulose can be widely found in agricultural and industrial waste residues, such as bagasse, corn stalks, wheat stalks, and wood chips. Besides, as a ubiquitous energy source, lignocellulose not only provides energy security but also minimizes greenhouse gas emissions, which makes it an attractive material in biorefinery (Chatalova and Balmann 2017; Yadav et al. 2019). The major compositions of lignocellulose are cellulose, hemicellulose, and lignin. Among them, cellulose is a glucose polymer linked by β-(1 → 4)-glycosidic bonds, which usually accounts for 40–50% of the total lignocellulose. Hemicellulose accounts for 15–35% of plant biomass, which is a heterogeneous polymer composed of five different types of pentose or hexose, including D-xylose, L-arabinose, D-galactose, D-mannose, and D-glucose. Lignin is a complex natural polymer, which is widely present in plant cells (Menon and Rao 2012; Ravindran and Jaiswal 2016). However, due to the cross-linked structure, lignocellulosic materials are difficult to be hydrolyzed by hydrolases (Mirmohamadsadeghi et al. 2021; Ravindran and Jaiswal 2016). Pretreatment before enzymatic hydrolysis is a key step to improve the biodegradability of lignocellulose, including physical pretreatment (grinding, etc.), chemical pretreatment (acid, alkali, oxidant), enzymatic hydrolysis, and the combination of these pretreated methods (ammonia fiber explosion etc.) (Frigon et al. 2012; Zheng et al. 2017). However, the high cost hinders their large-scale applications (Sołowski et al. 2020).
Consolidated bioprocessing (CBP) is considered as a promising method for biofuels production from lignocellulose, which combines hydrolytic enzymes production, lignocellulose hydrolysis, and microbial fermentation in one tank (Jiang et al. 2020a). The combination of these steps into one process configuration is encouraging; however, adoption of appropriate chassis microorganisms is still the prime requirement. Thermophilic anaerobes refer to those whose optimal growth temperature is higher than 50 °C, which will be beneficial to increase reaction kinetics and lignocellulose degradation efficiency (Fu et al. 2015; Singh et al. 2020). Compared with mesophilic microorganisms, higher cultural temperature can also reduce the risk of microbial contamination (Jiang et al. 2017). Additionally, the thermophilic process can reduce cooling costs and further promote downstream product recovery (Bhalla et al. 2013; Tukacs-Hajos et al. 2014).
Thermoanaerobacterium species are a group of endospores that form swimming, rod-shaped, and obligate anaerobic prokaryotes (Jiang et al. 2018). This genus can directly utilize hemicellulose, some of which can even degrade cellulose (Jiang et al. 2019; O-Thong et al. 2008). Thus, Thermoanaerobacterium species show promise as potential chassis microorganisms for achievement of lignocellulosic biorefinery. Accordingly, this review will comprehensively investigate the hydrolases and potential functions of Thermoanaerobacterium species as industrial workhorses for lignocellulosic material degradation and chemicals production, and the analysis of key enzymes and metabolic pathways will provide guidance for subsequent engineering and applications. Additionally, the perspective applications via CBP by using microbial consortia will also be discussed, which is beneficial to further improve the efficiency of lignocellulosic biorefinery.
Hydrolases of hemicellulose degradation secreted by thermophilic Thermoanaerobacterium species
The efficient hydrolysis of lignocellulosic biomass into fermentable sugars is the primary step for biorefinery. However, due to the complex structure of lignocellulose, a large number of hydrolytic enzymes are required for the effective saccharification process. Glycoside hydrolases play the synergistical roles in lignocellulose degradation, including cellulase, hemicellulase, and pectinase, which can be produced by various bacteria and fungi to hydrolyze the glycosidic bonds between carbohydrates or carbohydrates and non-carbohydrates (Singh et al. 2020).
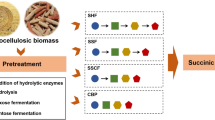
As known, Thermoanaerobacterium species can quickly decompose lignocellulosic materials especially hemicellulose. Xylan is the main constituent of hemicellulose, and xylanase and β-xylosidase are the two crucial xylanolytic enzymes involved in the biodegradation of xylan. Xylanase can randomly cleave the β-1,4 bonds in the backbone, transforming xylan into xylo-oligosaccharides with different lengths. The mixture of xylo-oligosaccharides is then hydrolyzed by β-xylosidase into xylose molecules (Chadha et al. 2019; Lee et al. 1993b). Generally, xylanases are divided into several glycoside hydrolase (GH) families (5, 7, 8, 10, 11, 26, 30, and 43), which can be produced by a variety of microorganisms such as extremophiles, bacteria, and yeast (Chadha et al. 2019). In terms of Thermoanaerobacterium species, the major secreted xylanases belong to GH10 and contain carbohydrate-binding module (CBM) and S-layer homology (SLH) domain (Fig. 1).
CBM is defined as a continuous amino acid sequence with discreet folds and carbohydrate binding activity, which was once classified as cellulose binding domain (CBD). Based on bioinformatics and molecular biology technology, a new viewpoint called the “modular structure” of xylanase is proposed. This view points out that these xylanases include at least one catalytic module and/or CBM. Different CBMs act as targeting agents to attach the catalytic module of enzymes to the corresponding polysaccharide component of lignocellulosic biomass. The complexity of enzymes depends on the number of catalytic modules and CBMs on each polypeptide chain (Singh et al. 2020). Furthermore, the xylanases secreted by Thermoanaerobacterium species also have the S-layer homology (SLH) domain, which is a C-terminal triplet sequence related to bacterial cell surface proteins. Sequence analysis indicated that the SLH domain can fixes the protein on the cell surface to promote the binding with enzyme complex (Lemaire and Ohayon 1995).
A newly obtained Thermoanaerobacterium sp. M5 is a thermophilic and butanogenic bacterium with the ability of xylan degradation and butanol production. The final metabolic products of strain M5 contain butyrate, acetate, ethanol, butanol, and H2. According to the phylogenetic analysis, Thermoanaerobacterium sp. M5 shows 99% similarity with T. thermosaccharolyticum KKU19 (Jiang et al. 2018). The xylanase (D9TMZ9) that was annotated in strain M5 contains a GH10 domain, three CBMs, and three SLH domains. The GH10 domain is responsible for the random hydrolysis of β-(1,4)-xylosidic bonds within the insoluble xylan backbone. And the two domains of CBMs and SLHs facilitate the binding between the enzyme and xylan, contributing to the efficient xylan degradation (Jiang et al. 2018). T. thermosaccharolyticum DSM 571 is a rigorous anaerobe that can grow on various hexose and pentose sugars at temperatures between 37 and 75 °C (Li et al. 2014), and the detected major metabolic products are H2 and biofuels. XYN is a heat-resistant, cellulase-free xylanase secreted by the strain DSM 571, which is an endoxylanase with the capability of xylan degradation instead of xylobiose. The catalytic domain of XYN belongs to the GH10 family, and three CBMs strengthen the connection between the enzyme and insoluble substrates. Additionally, the anchoring of the enzyme on bacterial surface layer is mediated by the three SLH domains. It has been found that 65 °C and pH 6.5 are the most suitable conditions for the enzyme activity of XYN and the half-life is 1 h at 71 °C, indicating the good thermal stability of XYN (Li et al. 2014).
T. saccharolyticum is another extensively studied species for xylan degradation and form ethanol and/or lactic acid as major end products, as well as lower levels of H2-CO2 and acetate (Lee et al. 1993a). It is a potential chassis microorganism for ethanol production. For example, T. saccharolyticum JW/SL-YS485 is a strain that came from a site in Yellowstone National Park, which can grow at a wide range of pH from 3.85 to 6.35 and a temperature within the scope of 30 to 66 °C (Shao et al. 1995). TsXynA is a cell-associated endoxylanase extracted from T. saccharolyticum JW/SL-YS485, which belongs to the GH10 family (Han et al. 2013). The enzyme has a large molecular weight, which is composed of two subunits (24 and 180 kDa). The 180-kDa subunit was deemed as a glycoprotein containing 6% of carbohydrates. The 24-kDa one is a breakdown product of the 180-kDa protein which has an N-terminal sequence and was considered to be necessary for maximum enzymatic activity (Liu et al. 1996). A catalytic domain, two CBMs, and three S-layer repeats at the C-terminal constitute the TsXynA. This enzyme also exhibited excellent thermotolerance, which the optimal temperature was 80 °C, and the half-life at 77 °C was 1 h (Han et al. 2013). Similar with TsXynA, XynFCB, an endo-1,4-β xylanase generated by strain T. saccharolyticum NTOU1 also belongs to the GH10 family. Especially, the thermophilic marine bacterium T. saccharolyticum NTOU1 was acquired from an oceanic hydrothermal vent, resulting in XynFCB was also proven to be a salt-tolerant enzyme (Hung et al. 2011). In details, the activity of XynFCB was shown an increase in 0–12.5% (w/v) NaCl, and maintained 67% of activity in 15% (w/v) NaCl after a 48-h incubation. Resemble with the overall growth conditions of strain NTOU1 (45–78 °C and pH 5.0–9.0), XynFCB operated well between 55 and 65 °C and pH within the extent of 6.0 to 6.5, and its half-life was 55 min at 65 °C (Hung et al. 2011).
In addition to T. thermosaccharolyticum and T. saccharolyticum that are commonly reported anaerobic species with the xylanases secretion, T. aotearoense is another studied one for xylan degradation and their xylanases also have such a modular structure. T. aotearoense SCUT27 is a Gram-positive, strictly anaerobic, thermophilic bacterium that was recently isolated from a hot spring in China and developed as a biocatalyst for the production of ethanol, H2, and lactic acid (Ai et al. 2014). It possesses the capability of utilization xylan as the sole carbon source by secretion of a xylanase: XynA, which was attested containing one GH10 domain, four CBMs, and three SLH domains (Huang et al. 2015; Yang et al. 2013).
Taken together, the xylanases produced by the Thermoanaerobacterium species have a similar “modular structure” and is generally with good heat resistance (Table 1), which renders them to effectively hydrolyze xylan in lignocellulose and shows great potential for applications in lignocellulosic biorefinery.
Application of Thermoanaerobacterium species in bioenergy production
As discussed above, the bioenergy production from the lignocellulosic biomass is a potential way to reduce the demand for carbon-intensive fossil fuels (Herring et al. 2016). Currently, microbial catalysts that can directly convert cellulose and xylan into biofuels are being developed. Owing to the capability of hydrolyzing and fermenting soluble cellodextrins and xylan, Thermoanaerobacterium species is a promising chassis microorganism in cellulosic biofuel industry (Shaw et al. 2011). Unlike Saccharomyces cerevisiae, Zymomonas mobilis, and thermophilic Clostridium thermocellum, which have only been used hexose sugars for bioenergy production, but not the pentose sugars, Thermoanaerobacterium species are hemicellulolytic thermophilic anaerobes that can utilize pentose sugars derived from hemicellulose for hydrogen and ethanol production. Thus, the relatively high growth and metabolic rates of Thermoanaerobacterium species growing on both cellulose and hemicellulose indicate they are the potential microorganisms for lignocellulosic biorefinery (Barnard et al. 2010).
Biohydrogen production from lignocellulose by Thermoanaerobacterium species
On account of the high energy content (142 kJ/g) and the environmentally friendly feature as its combustion results only in water vapors, hydrogen is being received an attention as the fuel of future. Due to the high conversion efficiency, recyclability and nonpolluting nature, sustainable biological hydrogen has enormous potential as an alternative energy, and the development of its production process is of great significance (Sinha and Pandey 2011). Lignocellulosic biohydrogen is a promising renewable energy that might be an ideal substitute for unsustainable fossil fuel-based energy and can be produced by anaerobic bacteria degrading carbohydrate-rich substrates (O-Thong et al. 2008; Wang et al. 2016). Among these H2 producers, mesophilic bacteria are the most extensively studies ones. However, it is reported that the yields of H2 were enhanced when using thermophiles or extreme thermophiles in the fermentation process (Kadar et al. 2004; van Niel et al. 2002). The reason why is that a higher temperature can provide favorable thermodynamics conditions which will not only speed up the production rate of H2, but also minify the variety of end products in fermentation (Schonheit and Schafer 1995). Therefore, compared with mesophilic bacteria, thermophilic bacteria are considered to have a greater development prospect in the production of H2 (O-Thong et al. 2008). In recent years, there are many researches of using the thermophilic Thermoanaerobacterium species in H2 manufacturing that attract widespread attention ascribed to its direct exploit of lignocellulose.
T. saccharolyticum KKU-ED1 is a thermophilic hydrogen producing strain which was isolated from elephant dung (Saripan and Reungsang 2013b). It has a broad substrates spectrum for hydrogen production including glucose, xylose, fructose, starch, xylan, and mixed xylose/arabinose (Saripan and Reungsang 2013a). As described above, xylanase is the key enzyme in the depolymerization of xylan into xylose, and the highly activity of xylanase by T. saccharolyticum KKU-ED1 contributes to the hemicellulosic hydrogen production. After the optimization of the cultural conditions, the initial pH of 7.0, temperature of 55 °C, and xylan concentration of 15 g/L were the best conditions for strain KKU-ED1 to produce hydrogen from xylan. Finally, the hydrogen yield, productivity, and xylanase activity were achieved at 120.05 mL H2/g xylan, 11.53 mL H2/L•h, and 0.41 U/mL, respectively (Table 2). Considering as the excellent utilization capability of xylan in T. saccharolyticum KKU-ED1, the sugarcane bagasse (SCB) riched hemicellulose was attempted as the carbon source for hydrogen fermentation. While due to the more complex structure of the SCB, the hydrogen production has slightly declined to some extent (Saripan and Reungsang 2013b). Compared to 0.41 U/mL, the xylanase activity decreased to 0.26 U/mL, and the hydrogen yield and productivity were decreased at 5.77 mL H2/g xylan (1.39 mL H2/g SCB) and 0.66 mL H2/L•h, respectively (Table 2).
A newly isolated thermophilic bacterium Thermoanaerobacterium sp. strain F6 showed relatively high hydrogen generation capability from wide substrate spectrums, including hemicellulosic materials and cellulosic materials (Jiang et al. 2019). Compared with cellulosic feedstock, strain F6 produced higher hydrogen production from hemicellulosic substrates, such as xylan. When the concentration of xylan increased to 60 g/L, the maximum cumulative hydrogen production increased at 370.7 mM (Table 2). Considering as the better hemicellulose degradation capability, corn cob, a kind of agricultural residues whose contents of hemicellulose can reach 38% was investigated as the carbon source to produce hydrogen (Mullen et al. 2010; Shen et al. 2015). From 30 to 60 g/L of corn cob, the accumulated hydrogen was 41.7 and 66.7 mM, respectively (Table 2). Despite the final hydrogen production from lignocellulosic biomass is much less than xyaln, these researches demonstrated the feasibility of hydrogen production via CBP by using lignocellulosic materials without any hydrolysis pretreatment (Jiang et al. 2019).
Bioethanol production from lignocellulose by Thermoanaerobacterium species
Ethanol is the first commercialized cellulosic biofuel, which produces water and carbon dioxide when fully burned (Herring et al. 2016). Using the thermophilic bacteria to produce ethanol though biological fermentation shows several advantages: (i) elevated temperatures can prevent the contamination of mesophilic bacteria; (ii) ethanol may self-distill to avoid the generally low tolerance of these bacteria to ethanol and reduce the cost of separation; (iii) the high cultivation temperature can also reduce the pressure of the cooling system and reduce the cooling cost (Almarsdottir et al. 2012). Thermoanaerobacterium species with the capability of hemicellulose degradation are the promising candidate for ethanol production from lignocellulosic biomass. However, the natural Thermoanaerobacterium species generally produce low level of ethanol. Therefore, it is very necessary to improve the final ethanol titer through genetic engineering, and classical mutagenesis/selection et al.
T. saccharolyticum possesses natural susceptibility and recombination ability, making gene manipulation relatively easy (Herring et al. 2016). These engineering attempts in T. saccharolyticum can be divided into two categories: the first mainly focuses on the elimination of natural competitive metabolic pathways (such as organic acid production), usually combined with adaptive evolution to accelerate the growth of engineered strains (Shaw et al. 2008b). The second type of engineering attempts focus on the introduction of heterologous genes into T. saccharolyticum (Shaw et al. 2012).
T. saccharolyticum has the capability to degrade lignocellulose and produce bioethanol. However, accompanied with the production of ethanol, some organic acids have also been produced which lessen the yield of the primary product (Shaw et al. 2008a). Analysis of the central metabolic pathways in T. saccharolyticum (Fig. 2), it can be noticed that ethanol is converted from pyruvate. From pyruvate to ethanol, there are two main branched fermentation pathways leading to lactic acid and acetic acid, separately, which are the major hurdles for the efficient ethanol production. In terms of lactic acid, it is formed from pyruvate by lactate dehydrogenase, while with regard to acetic acid, its synthesis from pyruvate is through a pathway containing pyruvate:ferredoxin oxidoreductase (POR), phosphate acetyltransferase, and acetate kinase (Shaw et al. 2008b). Based on the understanding of these pathways, the researchers have come up with gene knockout. By knocking out genes involved in the formation of lactic acid and acetic acid, the two branched fermentation pathways can be blocked. And at the same time, new metabolic flux distribution might be constructed, which may lead more carbon flux available for ethanol synthesis. Three genes (L-ldh, pta, ack) related to the by-products production were knockout, and three mutants of T. saccharolyticum were obtained, including ΔL-ldh, Δack Δpta, and Δack Δpta ΔL-ldh one which was designated as strain ALK1. Analysis the fermentation products of these strains in xylose-grown cultures, the ΔL-ldh mutant did not produce detectable lactic acid, and the Δack Δpta mutant did not produce acetic acid, while both of them have an increased yield of ethanol from pyruvate. As to strain ALK1, the results showed that it produced ethanol as the only detectable organic product. After continuously cultivating strain ALK1 for near 3000 h with the progressively enhanced fed xylose concentrations, a strain that have better xylose consumption ability in both batch and continuous culture have been acquired and was named ALK2. The strain ALK2 was used for ethanol fermentation in both of continuous culture and fed-batch culture. In terms of continuous culture, the mean ethanol yield of strain ALK2 at pH 5.2–5.4 was 0.46 g ethanol/g xylose, and the maximum ethanol concentration and volumetric productivity was 33 g/L and 2.2 g/L•h, respectively (Table 3). Compared with some other xylose-utilizing recombinant organisms such as S. cerevisiae and Z. mobilis, whose ethanol concentration that finally harvested was under 25 g/L (Kuyper et al. 2005; Lawford and Rousseau 1999; Sedlak and Ho 2004), T. saccharolyticum ALK2 exhibited favorably ethanol productivity. When it comes to fed-batch culture, strain ALK2 was cultivated in a 1-L fed-batch fermenter from 50 g/L of mixed sugars including glucose, xylose, galactose, and mannose. Differs from some mesophilic strains which use glucose preferentially to xylose, strain ALK2 can utilize glucose and xylose simultaneously and co-ferments mannose and galactose. The final ethanol concentration obtained from the mixed sugars reached 37 g/L, and the maximum ethanol production rate was 2.7 g/L•h, with the average of 1.5 g/L•h (Table 3) (Shaw et al. 2008b).
The metabolic pathway for H2 and ethanol synthesis in Thermoanaerobacterium species. A The H2 formation pathway in Thermoanaerobacterium species. B The ethanol formation pathway in T. saccharolyticum. L-ldh, L-lactate dehydrogenase; Fdox, pyruvate:ferredoxin oxidoreductase oxidized; Fdred, pyruvate:ferredoxin oxidoreductase reduced; HydA, hydrogenase; pta, phosphotrans acetylase; ack, acetate kinase; adhE, acetaldehyde dehydrogenase; bdh, butanol dehydrogenase; adh, alcohol dehydrogenase
Because lignocellulosic raw materials are relatively lack of nitrogen to support the growth of fermenting organisms, low-cost, easy-to-use nitrogen sources are particularly important for lignocellulose conversion. In industrial fermentation, nitrogen including ammonia, ammonium salts, and amino acids can be supplied to support the growth and metabolism of microorganisms. The nitrogen cost of urea is lower than other nitrogen sources, while organisms using urea must have urease (EC 3.5.1.3) or urea amidolyase to catalyze urea into carbon dioxide and ammonia. The urease gene ureABCDEFG from C. thermocellum ATCC 27405 (Cthe_1812-Cthe_1818) can be heterologously expressed in T. saccharolyticum strain. After amplified by PCR, this 5.1-kb fragment was cloned into a chromosomally integrating expression vector in T. saccharolyticum. Finally, one strain which was re-isolated after 2–3 transfers in TSD1 minimal medium had been verified for the existence of the urease genes through PCR, and was named M1051. The strain M1051 was cultured in a complex medium containing 8.5 g/L yeast extract and produced 15 g/L of ethanol. After addition of urea or ammonium sulfate to the complex medium, the ethanol production of strain M1051 was increased, indicating that the yeast extract itself is not sufficient as a nitrogen source for cells. When using 90 mM urea (5.4 g/L) as the main nitrogen source, the cultured strain produced 54.3 g/L of ethanol, and the fermentation ended at pH 6.7. In contrast, when 90 mM ammonium sulfate was used as the nitrogen source, 25.9 g/L of ethanol was obtained, and the fermentation pH ended at 4.0 (Table 3). In response to the difference in the obtained ethanol titers, the researchers pointed out that this may have to do with the movement of pH in the medium. It is believed that the non-metabolized ammonium ions which are hydrolyze from urea could elevate the medium pH, combined with the ability of urea to provide metabolizable nitrogen without decreasing the medium pH that leads to higher ethanol titers. However, as for ammonium sulfate, its metabolism drops the pH of the medium to a level which is not conducive to the continued ethanol fermentation by T. saccharolyticum. In conclusion, the introduction of heterologous urease genes into the T. saccharolyticum strain not only allows it to utilize low-cost nitrogen sources, but also achieve higher ethanol titers (Shaw et al. 2012).
T. aotearoense SCUT27 exhibits the capability of utilization a wide spectrum of carbohydrates including glucose, xylose, and xylan and can produces biofuels such as hydrogen and ethanol. This ability renders the strain an attractive bacterium for the conversion of lignocellulosic biomass to ethanol (Cai et al. 2011). Although SCUT27 can co-utilize some sugars derived from lignocellulosic biomass, the existence of carbon catabolite repression (CCR) cannot be neglected. For example, it was reported that to some extent, the utilization of xylose by SCUT27 was repressed by the presence of glucose (Zhu et al. 2015). Since xylose approximately accounts for 30% of the total carbohydrates of most lignocellulose biomass, it is crucially important to heighten the ability of the use of xylose in lignocellulosic biorefinery (Qiu et al. 2018). ATP is essential in both the uptake and the catabolism of xylose, so higher energy level in the cell can be conducive to the utilization of xylose (Qu et al. 2020). Besides, the redox balance also plays a considerable role in the substrate utilization (Salusjarvi et al. 2013). Arginine is regarded as a precursor of energy since it can provide ATP through the arginine deiminase (ADI) pathway (De Angelis et al. 2002). Arginine repressor (ArgR), the homohexamer which activates various arginine catabolic pathways and suppress the biosynthesis of arginine, had been originally reported to involved in several key metabolic pathways such as amino acid metabolism, carbohydrate, nucleotide, and energy metabolisms (Cho et al. 2012), all of which have vital impacts on the redox balance. Owing to the multifunctional roles that ArgR shows in the growth of microorganisms, it is considered to be a global regulator in microorganisms and has been used as a target for genetic engineering by researchers (Qu et al. 2020).
Through analysis of the T. aotearoense SCUT27 genome, three genes (V518_0585, V518_1870, V518_1864) were annotated as arginine repressor gene. Using homologous recombination, these three genes had been knocked out separately, and mutants of SCUT27/ΔargR0585, SCUT27/ΔargR1870, and SCUT27/ΔargR1864 were obtained. Among these mutants, only SCUT27/ΔargR1864 exhibited the ideal changes, and the specific activities of xylose isomerase and xylulokinase in SCUT27/ΔargR1864 enhanced about 51.52% and 13.90% respectively, compared with that of SCUT27. These results in the increasement of xylose consumption rate. In comparison with SCUT27, it raised by 32.35% under xylose and 76.90% under glucose/xylose mixture. The deletion of argR1864 not only facilitated sugar metabolism, but also lead to the re-establishment of a new redox balance. Higher cellular concentration of ATP and NAD(H) had positive influence in cell growth and stress response (Wu et al. 2019), which endowed the mutant a better viability of for ethanol production from lignocellulosic hydrolysates. Rice straw, corn cob, and sugarcane bagasse were chosen as the carbon sources in the fermentation broth for ethanol production. The ethanol titer and yield of SCUT27/ΔargR1864 was achieved at 5.59, 5.40, 5.24 g/L, and 0.35, 0.37, 0.40 g/g, respectively, which had been greatly promoted about 660.56–760.00% and 344.44–400.00% each (Table 3). These results indicated that knocking out argR1864 is a convincing strategy to promote the ethanol production by using lignocellulosic hydrolysates (Qu et al. 2020). This research showed that in addition to the relevant genes in the corresponding metabolic pathways, global regulation genes are also essential to the whole efficiency for ethanol production.
Conclusion and future perspectives
As described as above, Thermoanaerobacterium species possess enormous potential to achieve the efficient lignocellulosic biorefinery. The final titers of products, especially ethanol exhibited significant improvement after the metabolic engineering. However, achievement of complex products, such as biobutanol from lignocellulose in one single microbe is still impossible due to the overwhelming metabolic stress. Construction of microbial consortia offers a promising alternative approach, which can reduce metabolic burden through labor division and metabolic cooperation in different microbial species (Jiang et al. 2020a).
Generally, thermophilic microbes exhibit relatively high degradation rates of cellulose owing to its higher growth temperature, which is close to the optimum degradation temperature for cellulase. As the thermophilic bacteria with the capability of hemicellulose degradation capability, Thermoanaerobacterium species can take the responsibility of lignocellulose degradation in co-culturing systems. One typical example for butanol production from lignocellulose via CBP was the combination of thermophilic T. thermosaccharolyticum M5 and C. acetobutylicum NJ4. Among the two-species microbial consortium, T. thermosaccharolyticum M5 plays the role in lignocellulose degradation, and the efficient sugars utilization capability can relieve the inhibition to hydrolytic enzymes secreted by strain M5. Additionally, butyrate generated by strain M5 also induced the expression of butanol synthetic genes in strain NJ4. The mutual interaction of these two species exhibited 7.61 g/L of butanol from corn cobs via CBP. While two bottlenecks still present in this microbial consortium. The first one is the limitation of cellulose degradation capability for T. thermosaccharolyticum M5 (Jiang et al. 2020b). On the other hand, sequential inoculation was required in the fermentation due to the different temperature demands for these two strains.
To further improve the efficiency of lignocellulosic biorefinery, another well-known anaerobic cellulosic microbe C. thermocellum with the efficient cellulose degradation capability is considered as a promising partner for Thermoanaerobacterium species. Importantly, T. saccharolyticum and C. thermocellum show a complementary function to achieve ethanol production from lignocellulose with the similar growth conditions. To eliminate the by-products, organic acids formation can be deleted through genetic modification. Finally, 38 g/L of ethanol could be produced from 92 g/L of crystalline cellulose, achieving nearly 80% of the theoretical maximum concentration (Argyros et al. 2011). The successful example indicates that the suitable chassis microorganisms and combination of complementary functions in synthetic microbial consortia can achieve substantial improvement of lignocellulosic biorefinery. However, until now, the exploitation and application of microbial consortia are still emerging and developing, and the researches were still restricted at the substrate communications, such as fermentable sugars. The insightful and systematical mechanisms of cooperation in microbial consortia still should be explored, which can further guide the design and construction of synthetic microbial consortia.
References
Ai H, Zhang J, Yang M, Yu P, Li S, Zhu M, Dong H, Wang S, Wang J (2014) Draft genome sequence of an anaerobic, thermophilic bacterium, Thermoanaerobacterium aotearoense SCUT27, isolated from a hot spring in China. Genome Announcements 2(1). https://doi.org/10.1128/genomeA.00041-14
Almarsdottir AR, Sigurbjornsdottir MA, Orlygsson J (2012) Effect of various factors on ethanol yields from lignocellulosic biomass by Thermoanaerobacterium AK17. Biotechnol Bioeng 109(3):686–694. https://doi.org/10.1002/bit.24346
Argyros DA, Tripathi SA, Barrett TF, Rogers SR, Feinberg LF, Olson DG, Foden JM, Miller BB, Lynd LR, Hogsett DA, Caiazza NC (2011) High ethanol titers from cellulose by using metabolically engineered thermophilic, anaerobic microbes. Appl Environ Microbiol 77(23):8288–8294. https://doi.org/10.1128/Aem.00646-11
Barnard D, Casanueva A, Tuffin M, Cowan D (2010) Extremophiles in biofuel synthesis. Environ Technol 31(8-9):871–888. https://doi.org/10.1080/09593331003710236
Bhalla A, Bansal N, Kumar S, Bischoff KM, Sani RK (2013) Improved lignocellulose conversion to biofuels with thermophilic bacteria and thermostable enzymes. Bioresour Technol 128:751–759. https://doi.org/10.1016/j.biortech.2012.10.145
Cai YH, Lai CF, Li SA, Liang ZX, Zhu MJ, Liang SZ, Wang JF (2011) Disruption of lactate dehydrogenase through homologous recombination to improve bioethanol production in Thermoanaerobacterium aotearoense. Enzyme Microb Tech 48(2):155–161. https://doi.org/10.1016/j.enzmictec.2010.10.006
Calvo MV, Colombo B, Corno L, Eisele G, Cosentino C, Papa G, Scaglia B, Pilu R, Simmons B, Adani F (2018) Bioconversion of giant cane for integrated production of biohydrogen, carboxylic acids, and polyhydroxyalkanoates (PHAs) in a multistage biorefinery approach. ACS Sustain Chem Eng 6(11):15361–15373. https://doi.org/10.1021/acssuschemeng.8b03794
Chadha BS, Kaur B, Basotra N, Tsang A, Pandey A (2019) Thermostable xylanases from thermophilic fungi and bacteria: current perspective. Bioresour Technol 277:195–203. https://doi.org/10.1016/j.biortech.2019.01.044
Chatalova L, Balmann A (2017) The hidden costs of renewables promotion: the case of crop-based biogas. J Clean Prod 168:893–903. https://doi.org/10.1016/j.jclepro.2017.09.031
Cho B-K, Federowicz S, Park Y-S, Zengler K, Palsson BØ (2012) Deciphering the transcriptional regulatory logic of amino acid metabolism. Nat Chem Biol 8(1):65–71. https://doi.org/10.1038/nchembio.710
De Angelis M, Mariotti L, Rossi J, Servili M, Fox PF, Rollan G, Gobbetti M (2002) Arginine catabolism by sourdough lactic acid bacteria: purification and characterization of the arginine deiminase pathway enzymes from Lactobacillus sanfranciscensis CB1. Appl Environ Microbiol 68(12):6193–6201. https://doi.org/10.1128/Aem.68.12.6193-6201.2002
Frigon JC, Mehta P, Guiot SR (2012) Impact of mechanical, chemical and enzymatic pre-treatments on the methane yield from the anaerobic digestion of switchgrass. Biomass Bioenergy 36:1–11. https://doi.org/10.1016/j.biombioe.2011.02.013
Fu SF, Wang F, Yuan XZ, Yang ZM, Luo SJ, Wang CS, Guo RB (2015) The thermophilic (55 degrees C) microaerobic pretreatment of corn straw for anaerobic digestion. Bioresour Technol 175:203–208. https://doi.org/10.1016/j.biortech.2014.10.072
Han X, Gao J, Shang N, Huang CH, Ko TP, Chen CC, Chan HC, Cheng YS, Zhu Z, Wiegel J, Luo WH, Guo RT, Ma YH (2013) Structural and functional analyses of catalytic domain of GH10 xylanase from Thermoanaerobacterium saccharolyticum JW/SL-YS485. Proteins 81(7):1256–1265. https://doi.org/10.1002/prot.24286
Herring CD, Kenealy WR, Joe Shaw A, Covalla SF, Olson DG, Zhang J, Ryan Sillers W, Tsakraklides V, Bardsley JS, Rogers SR, Thorne PG, Johnson JP, Foster A, Shikhare ID, Klingeman DM, Brown SD, Davison BH, Lynd LR, Hogsett DA (2016) Strain and bioprocess improvement of a thermophilic anaerobe for the production of ethanol from wood. Biotechnol Biofuels 9(1):125. https://doi.org/10.1186/s13068-016-0536-8
Huang XL, Li Z, Du CY, Wang JF, Li S (2015) Improved expression and characterization of a multidomain xylanase from Thermoanaerobacterium aotearoense SCUT27 in Bacillus subtills. J Agric Food Chem 63(28):6430–6439. https://doi.org/10.1021/acs.jafc.5b01259
Hung KS, Liu SM, Tzou WS, Lin FP, Pan CL, Fang TY, Sun KH, Tang SJ (2011) Characterization of a novel GH10 thermostable, halophilic xylanase from the marine bacterium Thermoanaerobacterium saccharolyticum NTOU1. Process Biochem 46(6):1257–1263. https://doi.org/10.1016/j.procbio.2011.02.009
Jiang YJ, Xin FX, Lu JS, Dong WL, Zhang WM, Zhang M, Wu H, Ma JF, Jiang M (2017) State of the art review of biofuels production from lignocellulose by thermophilic bacteria. Bioresour Technol 245:1498–1506. https://doi.org/10.1016/j.biortech.2017.05.142
Jiang YJ, Guo D, Lu JS, Durre P, Dong WL, Yan W, Zhang WM, Ma JF, Jiang M, Xin FX (2018) Consolidated bioprocessing of butanol production from xylan by a thermophilic and butanologenic Thermoanaerobacterium sp. M5. Biotechnol Biofuels 11:ARTN89. https://doi.org/10.1186/s13068-018-1092-1
Jiang YJ, Lu JS, Lu Y, Wu RF, Dong WL, Zhou J, Jiang M, Xin FX (2019) Efficient hydrogen production from lignocellulosic feedstocks by a newly isolated thermophlic Thermoanaerobacterium sp. strain F6. Int J Hydrogen Energ 44(28):14380–14386. https://doi.org/10.1016/j.ijhydene.2019.01.226
Jiang Y, Dong W, Xin F, Jiang M (2020a) Designing synthetic microbial consortia for biofuel production. Trends Biotechnol 38(8):828–831. https://doi.org/10.1016/j.tibtech.2020.02.002
Jiang YJ, Lv Y, Wu RF, Lu JS, Dong WL, Zhou J, Zhang WM, Xin FX, Jiang M (2020b) Consolidated bioprocessing performance of a two-species microbial consortium for butanol production from lignocellulosic biomass. Biotechnol Bioeng 117(10):2985–2995. https://doi.org/10.1002/bit.27464
Kadar Z, De Vrijek T, van Noorden GE, Budde MAW, Szengyel Z, Reczey K, Claassen PAM (2004) Yields from glucose, xylose, and paper sludge hydrolysate during hydrogen production by the extreme thermophile Caldicellulosiruptor saccharolyticus. Appl Biochem Biotechnol 113:497–508
Kuyper M, Hartog MMP, Toirkens MJ, Almering MJH, Winkler AA, van Dijken JP, Pronk JT (2005) Metabolic engineering of a xylose-isomerase-expressing Saccharomyces cerevisiae strain for rapid anaerobic xylose fermentation. FEMS Yeast Res 5(4-5):399–409. https://doi.org/10.1016/j.femsyr.2004.09.010
Lawford HG, Rousseau JD (1999) The effect of glucose on high-level xylose fermentations by recombinant Zymomonas in batch and fed-batch fermentations. Appl Biochem Biotechnol 77(9):235–249. https://doi.org/10.1385/Abab:77:1-3:235
Lee YE, Jain MK, Lee CY, Lowe SE, Zeikus JG (1993a) Taxonomic distinction of saccharolytic thermophilic anaerobes - description of Thermoanaerobacterium-Xylanolyticum Gen-Nov, Sp-Nov, and Thermoanaerobacterium-Saccharolyticum Gen-Nov, Sp-Nov - reclassification of Thermoanaerobium-Brockii, Clostridium-Thermosulfurogenes, and Clostridium-Thermohydrosulfuricum E100-69 as Thermoanaerobacter-Brockii Comb-Nov, Thermoanaerobacterium-Thermosulfurigenes Comb-Nov, and Thermoanaerobacter-Thermohydrosulfuricus Comb-Nov, Respectively - and Transfer of Clostridium-Thermohydrosulfuricum 39e to Thermoanaerobacter-Ethanolicus. Int J Syst Bacteriol 43(1):41–51. https://doi.org/10.1099/00207713-43-1-41
Lee YE, Lowe SE, Zeikus JG (1993b) Gene cloning, sequencing, and biochemical characterization of endoxylanase from Thermoanaerobacterium saccharolyticum B6A-RI. Appl Environ Microbiol 59(9):3134–3137
Lemaire M, Ohayon H (1995) OlpB, a new outer layer protein of Clostridium thermocellum, and binding of Its S-layer-like domains to components of the cell envelope. J Bacteriol 177:2451–2459
Li X, Shi H, Ding HH, Zhang Y, Wang F (2014) Production, purification, and characterization of a cellulase-free thermostable endo-xylanase from Thermoanaerobacterium thermosaccharolyticum DSM 571. Appl Biochem Biotechnol 174(7):2392–2402. https://doi.org/10.1007/s12010-014-1135-4
Liu SY, Gherardini FC, Matuschek M, Bahl H, Wiegel J (1996) Cloning, sequencing, and expression of the gene encoding a large S-layer-associated endoxylanase from Thermoanaerobacterium sp strain JW/SL-YS 485 in Escherichia coli. J Bacteriol 178(6):1539–1547. https://doi.org/10.1128/jb.178.6.1539-1547.1996
Menon V, Rao M (2012) Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog Energ Combust 38(4):522–550. https://doi.org/10.1016/j.pecs.2012.02.002
Mirmohamadsadeghi S, Karimi K, Azarbaijani R, Yeganeh LP, Angelidaki I, Nizami AS, Bhat R, Dashora K, Vijay VK, Aghbashlo M, Gupta VK, Tabatabaei M (2021) Pretreatment of lignocelluloses for enhanced biogas production: a review on influencing mechanisms and the importance of microbial diversity. Renew Sust Energ Rev 135:ARTN110173. https://doi.org/10.1016/j.rser.2020.110173
Mullen CA, Boateng AA, Goldberg NM, Lima IM, Laird DA, Hicks KB (2010) Bio-oil and bio-char production from corn cobs and stover by fast pyrolysis. Biomass Bioenergy 34(1):67–74. https://doi.org/10.1016/j.biombioe.2009.09.012
O-Thong S, Prasertsan P, Karakashev D, Angelidaki I (2008) Thermophilic fermentative hydrogen production by the newly isolated Thermoanaerobacterium thermosaccharolyticum PSU-2. Int J Hydrogen Energ 33(4):1204–1214. https://doi.org/10.1016/j.ijhydene.2007.12.015
Qiu Z, Gao Q, Bao J (2018) Engineering Pediococcus acidilactici with xylose assimilation pathway for high titer cellulosic l-lactic acid fermentation. Bioresour Technol 249:9–15. https://doi.org/10.1016/j.biortech.2017.09.117
Qu CY, Chen LL, Fu HX, Wang JF (2020) Engineering Thermoanaerobacterium aotearoense SCUT27 with argR knockout for enhanced ethanol production from lignocellulosic hydrolysates. Bioresour Technol 310:ARTN123435. https://doi.org/10.1016/j.biortech.2020.123435
Ravindran R, Jaiswal AK (2016) A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresour Technol 199:92–102. https://doi.org/10.1016/j.biortech.2015.07.106
Salusjarvi L, Kaunisto S, Holmstrom S, Vehkomaki ML, Koivuranta K, Pitkanen JP, Ruohonen L (2013) Overexpression of NADH-dependent fumarate reductase improves d-xylose fermentation in recombinant Saccharomyces cerevisiae. J Ind Microbiol Biotechnol 40(12):1383–1392. https://doi.org/10.1007/s10295-013-1344-9
Saripan AF, Reungsang A (2013a) Biohydrogen production by Thermoanaerobacterium thermosaccharolyticum KKU-ED1: culture conditions optimization using mixed xylose/arabinose as substrate. Electron J Biotechnol 16(1). https://doi.org/10.2225/vol16-issue1-fulltext-1
Saripan AF, Reungsang A (2013b) Biohydrogen production by Thermoanaerobacterium thermosaccharolyticum KKU-ED1: culture conditions optimization using xylan as the substrate. Int J Hydrogen Energ 38(14):6167–6173. https://doi.org/10.1016/j.ijhydene.2012.12.130
Schonheit P, Schafer T (1995) Metabolism of hyperthermophiles. World J Microbiol Biotechnol 11(1):26–57. https://doi.org/10.1007/Bf00339135
Sedlak M, Ho NWY (2004) Characterization of the effectiveness of hexose transporters for transporting xylose during glucose and xylose co-fermentation by a recombinant Saccharomyces yeast. Yeast 21(8):671–684. https://doi.org/10.1002/yea.1060
Shao WL, Deblois S, Wiegel J (1995) A high-molecular-weight, cell-associated xylanase isolated from exponentially growing Thermoanaerobacterium Sp strain Jw/Sl-Ys485. Appl Environ Microbiol 61(3):937–940. https://doi.org/10.1128/Aem.61.3.937-940.1995
Shaw AJ, Jenney FE, Adams MWW, Lynd LR (2008a) End-product pathways in the xylose fermenting bacterium, Thermoanaerobacterium saccharolyticum. Enzyme Microb Tech 42(6):453–458. https://doi.org/10.1016/j.enzmictec.2009.01.005
Shaw AJ, Podkaminer KK, Desai SG, Bardsley JS, Rogers SR, Thorne PG, Hogsett DA, Lynd LR (2008b) Metabolic engineering of a thermophilic bacterium to produce ethanol at high yield. P Natl Acad Sci USA 105(37):13769–13774. https://doi.org/10.1073/pnas.0801266105
Shaw AJ, Covalla SF, Hogsett DA, Herring CD (2011) Marker removal system for Thermoanaerobacterium saccharolyticum and development of a markerless ethanologen. Appl Environ Microbiol 77(7):2534–2536. https://doi.org/10.1128/AEM.01731-10
Shaw AJ, Covalla SF, Miller BB, Firliet BT, Hogsett DA, Herring CD (2012) Urease expression in a Thermoanaerobacterium saccharolyticum ethanologen allows high titer ethanol production. Metab Eng 14(5):528–532. https://doi.org/10.1016/j.ymben.2012.06.004
Shen DK, Zhang LQ, Xue JT, Guan SP, Liu Q, Xiao R (2015) Thermal degradation of xylan-based hemicellulose under oxidative atmosphere. Carbohydr Polym 127:363–371. https://doi.org/10.1016/j.carbpol.2015.03.067
Singh N, Mathur AS, Gupta RP, Barrow CJ, Tuli DK, Puri M (2020) Enzyme systems of thermophilic anaerobic bacteria for lignocellulosic biomass conversion. Int J Biol Macromol 168:572–590. https://doi.org/10.1016/j.ijbiomac.2020.12.004
Sinha P, Pandey A (2011) An evaluative report and challenges for fermentative biohydrogen production. Int J Hydrogen Energ 36(13):7460–7478. https://doi.org/10.1016/j.ijhydene.2011.03.077
Sołowski G, Konkol I, Cenian A (2020) Production of hydrogen and methane from lignocellulose waste by fermentation A review of chemical pretreatment for enhancing the efficiency of the digestion process. J Clean Prod 267. https://doi.org/10.1016/j.jclepro.2020.121721
Tukacs-Hajos A, Pap B, Maroti G, Szendefy J, Szabo P, Retfalvi T (2014) Monitoring of thermophilic adaptation of mesophilic anaerobe fermentation of sugar beet pressed pulp. Bioresour Technol 166:288–294. https://doi.org/10.1016/j.biortech.2014.05.059
van Niel EWJ, Budde MAW, de Haas GG, van der Wal FJ, Claasen PAM, Stams AJM (2002) Distinctive properties of high hydrogen producing extreme thermophiles, Caldicellulosiruptor saccharolyticus and Thermotoga elfii. Int J Hydrogen Energ 27(11-12):1391–1398. https://doi.org/10.1016/S0360-3199(02)00115-5
Wang M, Qi Z, Li L, Niu K, Yi F (2016) Contributing factors in the improvement of cellulosic H2 production in Clostridium thermocellum/Thermoanaerobacterium co-cultures. Appl Microbiol Biotechnol:8607–8620. https://doi.org/10.1007/s00253-016-7776-1
Wu Y, Xia W, Cao M, Sun X-M (2019) Reach control problem for affine multi-agent systems on simplices. Automatica 107:264–271. https://doi.org/10.1016/j.automatica.2019.05.052
Yadav M, Paritosh K, Pareek N, Vivekanand V (2019) Coupled treatment of lignocellulosic agricultural residues for augmented biomethanation. J Clean Prod 213:75–88. https://doi.org/10.1016/j.jclepro.2018.12.142
Yang XF, Lai ZC, Lai CF, Zhu MZ, Li S, Wang JF, Wang XN (2013) Efficient production of L-lactic acid by an engineered Thermoanaerobacterium aotearoense with broad substrate specificity. Biotechnol Biofuels 6:Artn124. https://doi.org/10.1186/1754-6834-6-124
Zheng Y, Shi J, Tu M, Cheng Y-S (2017) Chapter one - principles and development of lignocellulosic biomass pretreatment for biofuels. In: Li Y, Ge X (eds) Advances in Bioenergy 2. Elsevier 1-68
Zhu MZ, Lu YP, Wang JF, Li S, Wang XN (2015) Carbon catabolite repression and the related genes of ccpA, ptsH and hprK in Thermoanaerobacterium aotearoense. PLoS One 10(11):ARTNe0142121. https://doi.org/10.1371/journal.pone.0142121
Funding
This work was supported by the National Key R&D Program of China (2018YFA0902200), National Natural Science Foundation of China (21978130, 22008113, 22078151 and 31961133017), Jiangsu Province Natural Science Foundation for Youths (BK20200683), and China Postdoctoral Science Foundation (No. 2020 M671465).
Author information
Authors and Affiliations
Contributions
Jiang M and Xin F provided ideas for the article. Jiang Y and Wu M wrote the manuscript. Xin F and Zhang W revised the article. Liu Y and Mou L contributed to the conception and reviewed the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, M., Jiang, Y., Liu, Y. et al. Microbial application of thermophilic Thermoanaerobacterium species in lignocellulosic biorefinery. Appl Microbiol Biotechnol 105, 5739–5749 (2021). https://doi.org/10.1007/s00253-021-11450-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11450-4