Abstract
The general public spends one-third of its time under artificial lighting, which lacks bands beneficial to human health, and long-term exposure will have a negative impact on bone health. Here, we report the effects of long-term, low-dose ultraviolet (UV) supplementation to white light-emitting diode (LED) light exposure on intestinal microorganisms and bone metabolism, as well as the correlations between the two. Normal and ovariectomized rats were irradiated with LED white light with or without supplementation with UV. The effects of UV supplementation on the intestinal flora and the relationship between the intestinal flora and bone were investigated by measuring the intestinal flora, bone metabolism markers, and bone histomorphology. UV supplementation affected the bone density and bone mass by changing the relative content of Firmicutes, Saccharibacteria, and Proteobacteria; however, the intestinal flora were not the only factors affecting bone. Ultraviolet supplementation changed the composition and function of the gut flora in the bone loss model. By increasing the synthesis of short-chain fatty acids and affecting immunomodulatory, intestinal flora directly or indirectly regulate the activity of osteoclasts and thus mediate UV-mediated improvements in bone metabolism. Our work shows that UV supplementation affects bone density by influencing the intestinal flora, introducing a novel strategy to develop healthier artificial light sources and prevent bone loss.
Key points
• We measured the bone metabolism markers and bone histomorphometry of rats.
• The diversity, composition, and function of intestinal flora were analyzed.
• The relationship between gut microbiota and host bone physiology was analyzed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In modern society, individuals spend most of their time working and living under artificial lighting environments; this is especially true for professional groups, such as astronauts and divers, who spend extended time periods working in environments without sunlight. Compared with sunlight, artificial lighting sources are often lacking infrared, ultraviolet, and other bands beneficial to human health (Jou et al. 2019). Working and living in an artificial lighting environment for a long duration has a negative impact on bone health in humans. It has been reported that submariners and night workers are more likely to suffer from bone mineral density decline and osteoporosis (Bukowska-Damska et al. 2019; Luria et al. 2010), while appropriate ultraviolet (UV) exposure can alleviate bone health issues (Cranney et al. 2007; Falkenbach et al. 1993).
UV irradiation accelerates the absorption of intestinal calcium, maintains bone mass, prevents osteoporosis, and reduces the incidence of bone fractures by promoting vitamin D synthesis (Mead 2008; Nikolikj-Dimitrova 2013). In addition, UV regulates bone metabolism through other distal effects on tissues. Recent studies have shown that alterations in the intestinal flora are also associated with the maintenance of bone mass and bone quality (Hernandez et al. 2016). Other studies have shown that manipulation of the microbiome or its metabolites may afford the ability to optimize bone growth and health (Yan et al. 2016). It has also been observed that gut microbiota regulate bone mass in mice by altering immune status in the bone and affecting osteoclast-mediated bone resorption (Sjögren et al. 2012). The studies mentioned above demonstrate that both UV and gut microbiota play important roles in bone health. However, it is unclear whether UV exposure modulates gut microbiota–associated bone metabolism.
Here, we examined the effects of long-term, low-dose UV supplementation to light-emitting diode (LED) white light on intestinal microorganisms and bone metabolism in normal and ovariectomized rats. We found that UV improved bone metabolism by affecting the intestinal microbial community. The results of our study not only provide the foundation for creating strategies and developing artificial lighting for a healthy artificial light environment but also reveal that intestinal flora play an important role in the UV prevention of bone loss.
Methods
Animals
Forty 180 ± 20 g specific-pathogen-free (SPF) 8-week-old female Sprague-Dawley (SD) rats (provided by Beijing Weitong Lihua Animal Experiment Technology Co., Ltd., Beijing, China) were used in this study. They were held in four plastic squirrel cages, with ten rats per group. SPF feed and drinking water were available ad libitum. The animals were maintained in controlled conditions (12-h light-dark cycle, temperature 20–25°C, air humidity 55–65%).
Experimental design
The SD rats were allowed to acclimate to the animal room for 1 week prior to the start of the experiment (supplementary Fig. S1). In order to observe the effects of UV supplementation on bone loss inhibition and osteoporosis prevention, ovariectomy was performed on 20 rats to establish a rat model of bone loss. The 40 rats were divided into four treatment groups: (1) normal (Normal UV-), (2) normal supplemented with ultraviolet (Normal UV+), (3) ovariectomized (Ovx UV-), and (4) ovariectomized supplemented with ultraviolet (Ovx UV+) as shown in supplementary Fig. S1a. The Normal UV- and Ovx UV- groups were reared under a white LED lighting environment. The Normal UV+ and Ovx UV+ groups were reared under white LED light supplemented with UV. Each group of rats was given light exposure from 10:00 to 22:00. The light intensity and UV light intensity of the active area of the rats were detected weekly by optical fiber spectrometry. The illumination intensity on each rat in the activity area of the cage was about 1000 lx, and the ultraviolet intensity was about 13 W/cm2 (supplementary Fig. S1b). The experiment lasted 12 weeks, and the rats were sacrificed at the end of the experiment.
Micro-CT analyses
Femurs were fixed in 10% phosphate-buffered-formalin and stored at −80°C (n=6/group). The mCT analyses were performed on the distal femur using a Skyscan 1076 scanner (Kontich, Belgium), X-ray tube potential = 18 kVp; X-ray intensity = 145 μA; and integration time = 200 ms. Calibrated three-dimensional images were reconstructed by NRecon (version 1.7.3.1) software. Femur trabecular bone morphology was analyzed using the CT Analyzer (version 1.17.7.2) software.
Dual-energy x-ray analysis
The rats were deeply anesthetized with a 1% pentobarbital sodium solution by intraperitoneal injection (n=6/group). The rats were scanned with a bone densitometer (Hologic Discovery A, San Diego, USA). The whole-body bone mass content (BMC) and bone mineral density (BMD) of each mouse were measured. Before measurements, a tissue calibration scan was performed with a Hologic phantom (HOLOGIC Discovery A Bone, San Diego, USA). All measurements were performed by the same operator, followed by the software analysis for small animals carried by Hologic Discovery A (San Diego, USA); the determined BMC and BMD were recorded.
Serum biochemical assays
Blood samples (n=6/group) were drawn from rat tail veins. Each blood sample was dispensed into a centrifuge tube and centrifuged at 845g for 20 min using a cryogenic centrifuge. Then, the separated plasma supernatant was collected and stored at −20°C until the time of measurement. Repeated freezing and thawing were avoided. This method was based on that described in a previous study (Abulmeaty 2017). 1,25-Dihydroxyvitamin D3 (1,25-(OH)2-D3), tartrate-resistant acid phosphatase (TRACP), and bone alkaline phosphatase (BALP) serum levels were measured using ELISA kits (Beijing Ruigebo Technology Development Co., Ltd., Beijing, China) according to the manufacturer’s instructions.
16S rRNA gene sequencing
Fresh stool samples were collected from the rats and immediately stored at −20°C (n=6/group). Genomic DNA was extracted from stool using the Qiagen QIAamp DNA stool Mini Kit (Hilden, Germany) according to the manufacturer’s instructions. The V3-V4 16S rRNA region (338F-806R, 338F: 5′-ACTCCTACGGGAGGCAGCA-3′; 806R: 5′-GGACTACHVGGGTWTCTAAT-3′) was amplified by thermal cycling consisting of an initial denaturation at 98°C for 2 min, followed by 30 cycles of denaturation at 98°C for 30 s, annealing at 50°C for 30 s, and elongation at 72°C for 1 min, and finally, holding at 72°C for 5 min. High-throughput pyrosequencing of the PCR products was performed on an Illumina MiSeq/HiSeq2500 platform (Biomarker Technologies Co, Ltd., Beijing, China).
Sequence data analysis
Reads were chimera-checked and clustered into 97% operational taxonomic units (OTUs) using the vsearch (Rognes et al. 2016) implementation of the UPARSE pipeline (Edgar 2013). A representative sequence (the most abundant) of each OTU was selected for searching against the SILVA 16S rRNA gene database (www.drive5.com/sintax/silva_16s_v123.fa.gz) using the syntax function (Edgar 2016) in vsearch version 2.8.1 with a confidence cut-off (P) value of 0.6. We then excluded the OTUs with < 10 reads from all the samples as their sequences could contain PCR or sequencing errors. The diversity indices of bacterial communities were assessed with the “vegan” package (Oksanen et al. 2007). Alpha and richness diversity indices were evaluated with the Chao1 and Shannon index. Community distance between samples was calculated using the Canberra distance, implemented in the vegan package. Distance-based redundancy analysis (DB-RDA) was performed with the capscale function in vegan. A statistically significant (p < 0.05) Spearman’s correlation coefficient with an absolute value greater than 0.6 was used as the basis for the selection of the core genera of the bacterial community in each group. All core genera in each group were visualized in a network, where genera and the correlation coefficients were set as nodes and edges, respectively, using the Gephi software (Bastian et al. 2009). The network features in each group were analyzed, including average node connectivity, average path length, diameter, and cumulative degree distribution. An open-source R package, Tax4Fun, was used to analyze the enrichment of functional genes of the microbiome of each group (Aßhauer et al. 2015). The output from QIIME (Caporaso et al. 2010) with a SILVA database extension (SILVA 123) was used for this analysis. Tax4Fun can survey the functional genes of bacterial communities based on the 16S rRNA sequencing data.
Statistical analysis
All statistical analyses were implemented in R version 3.6.1 (Team 2013). Observed OTU, Shannon, and Chao1 diversity measures were used to estimate alpha diversity, and the significance of differences in alpha diversity under different light treatments for normal rats or ovariectomized rats was evaluated by Student’s t-test. For beta diversity and partitioning of variance, Canberra distance matrices for bacteria communities were subjected to permutational analysis of variance (PERMANOVA) using the “adonis” test from the vegan package. The two-sided Student’s t-test analysis was performed to test the significance of the effect of UV supplementation on bone metabolism. Spearman’s correlation analysis between bone metabolism and bacterial taxa was performed in R. p < 0.05 was considered statistically significant.
Results
BMC, BMD, and the concentration of serum bone metabolism markers
In order to confirm the effects of UV supplementation on bone mineral content (BMC) and bone mineral density (BMD) in rats, we analyzed the BMC, BMD, and trabecular bone in the four treatment groups (Fig. 1, n=6/group). Although there was no significant difference in femur BMC (Fig. 1a, p > 0.05), UV supplementation significantly increased the BMC of normal and ovariectomized rats (p < 0.05, Fig. 1b). The BMD of normal and ovariectomized rats was significantly increased after the UV supplementation (p < 0.01).
Bone mass of the femur (a) and whole body (b) of rats. Bone mineral density of the femur (c) and whole body (d) of rats. Trabecular bone parameters were analyzed by mCT in the distal region of femurs from 12-week-old rats. e 1,25-Dihydroxyvitamin D3 (1,25-(OH)2-D3); f Bone alkaline phosphatase (BALP); g Tartrate-resistant acid phosphatase (TRACP). p < 0.05 indicate significant differences between conditioning treatments (two-sided Student’s t-tests)
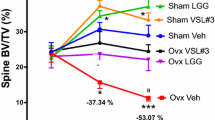
Through micro-CT scanning to obtain the cross-sectional image of the distal femur, we observed an increase in trabecular bone density after UV supplementation (supplementary Fig. S2e). Micro-CT analysis revealed that UV supplementation also increased trabecular separation (Tb.Sp; p = 0.08, supplementary Fig. S2d) in the distal femur of normal rats. Interestingly, Tb.Sp (supplementary Fig. S2d) and Tb.Th (trabecular thickness; p = 0.07, supplementary Fig. S2c) were decreased, but Tb.N (trabecular number; p = 0.09, supplementary Fig. S2b) was increased in the ovariectomized rats after the UV supplementation. The BV/TV (the trabecular bone volume fraction, supplementary Fig. S2a) of normal and ovariectomized rats was no significant change after the UV supplementation. These results show that long-term, low-energy UV supplementation has a positive effect on local BMD and BMC in both control and ovariectomized rats and can promote bone formation, especially in ovariectomized rats.
The results of serum bone metabolism marker assays are presented in Fig. 1. Concentrations of 1,25-(OH)2-D3 and BALP were significantly increased in both normal and ovariectomized rats after the UV supplementation (p < 0.05, Fig. 1e, f), suggesting that UV supplementation contributes to the increase in the concentration of bone formation markers in rats. The concentration of TRACP was decreased in normal (p = 0.458) and ovariectomized (p = 0.001) rats with bone loss (Fig. 1g), indicating that the UV supplementation decreased the bone absorption rate in rats.
Changes in microbial diversity after UV supplementation
Bacterial diversity within each sample was analyzed based on the Chao1 and the Shannon diversity index (Fig. 2, n = 6/group). For diversity and evenness estimates, we found a marked rise in the UV+ samples compared to that in the UV- samples in both ovariectomized and normal rats (p < 0.05; Fig. 2a, b). The four groups of rats harbored significantly different communities based on permutational multivariate analysis of variance on Canberra distances (p < 0.05; Fig. 2c). Thus, UV supplementation had a markedly significant effect on the diversity of gut bacterial communities.
a Chao1, b Shannon diversity, and c bacterial communities were constrained by four different surface types using distance-based redundancy analysis (DB-RDA). Significant differences among the types, based on Canberra taxonomic distances (p = 0.001 from permutational multivariate analysis of variation), were observed. p < 0.05 indicate significant differences between conditioning treatments (two-sided Student’s t-tests)
Correlation and network analysis
To dissect whether microbial communities were modulated by interactions between bacteria, we performed Spearman correlation analysis and observed both positive and negative microbial interactions. Although correlation analysis does not elucidate cause and effect, it reveals highly connected OTUs, regardless of their relative abundance. The network nodes were mostly Firmicutes, Bacteroidetes, and Proteobacteria (Fig. 3). Construction of correlation-based networks in each group resulted in four networks, consisting of 75, 81, 79, and 81 nodes connected by 213, 256, 225, and 592 edges, respectively (supplementary Table S1). Each network had a much higher number of strongly positive correlations than negative correlations. Furthermore, we found that the network of the Ovx UV+ group had a greater degree and was more modular but exhibited a lower average path length than the Ovx UV- network. However, network features in normal rats only exhibited a small change between UV+ and UV- treatments.
Taxonomic composition of the bacterial communities in rats
The bacterial community compositions in the normal (UV- and UV+) and ovariectomized (UV- and UV+) samples (n = 6/group) are shown in Fig. 4. In all samples, Firmicutes, Saccharibacteria, and Bacteroidetes were the three dominant phyla (Fig. 4a). The relative abundance of Firmicutes and Saccharomycetes showed an increase and a decrease, respectively, after the UV supplementation in both normal and ovariectomized rats, although these differences did not reach statistical significance (supplementary Fig. S3). The ratio of Firmicutes to Bacteroidetes showed a tendency to increase (supplementary Fig. S3e, p > 0.05). It is worth noting that Proteobacteria exhibited a significant decrease only in the ovariectomized rat group after UV supplementation (p = 0.025, supplementary Fig. S3d).
Taxonomic composition of the bacterial communities at the phylum and family levels. a Relative abundances of the 7 most abundant phyla. b Relative abundances of the 8 most abundance families. c Significantly different genera in ovariectomized rats with and without UV supplementation (p < 0.05). d Significantly different genera in normal rats with and without UV supplementation (p < 0.05)
At the family level, Ruminococcaceae, Lachnospiraceae, and Bacteroidales s24-7 group are the three most significant bacterial families (Fig. 4b). After the UV supplementation, the relative abundance of most Clostridiaceae_1, Desulfovibrionaceae significantly decreased in ovariectomized rats (p < 0.05, supplementary Table S2), but normal rats did not exhibit significant changes (supplementary Table S3). At the genus level, three genera showed significant increases, and ten genera showed significant decreases in relative abundance in Ovx rats after the UV supplementation (Fig. 4c). However, only one genus (Prevotella) in the normal rats significantly decreased (p < 0.05, Fig. 4d). The effect of UV supplementation on the ovariectomized rats was generally greater than that in normal rats.
Gut bacteria function prediction
To understand the effect of UV supplementation on the function of the flora, the 16s sequencing data were used to predict gene function. After the UV supplementation, the intestinal flora function of normal rats did not change significantly (supplementary Fig. S4), while the intestinal flora function of ovariectomized rats did exhibit a significant change (Fig. 5, supplementary Table S4). Genes related to hematopoietic cell lineage, antigen processing and presentation, fatty acid biosynthesis, biosynthesis of unsaturated fatty acids, DNA replication homologous recombination, mismatch repair, nucleotide excision repair, lipopolysaccharide biosynthesis, and glycosaminoglycan biosynthesis—chondroitin sulfate/dermatan sulfate were significantly increased after UV irradiation. Among the gene functions noted above, the abundance of genes related to immune diseases, the immune system, lipid metabolism, glycan biosynthesis, and metabolism and replication and repair were significantly higher in the Ovx UV+ samples than in the Ovx UV- samples. However, tyrosine metabolism, pyruvate metabolism, methane metabolism, the bacterial secretion system, and bacterial chemotaxis were significantly decreased (Fig. 5). Based on the prediction of bacterial gene function, it is apparent that supplementation with UV light exerts an important influence on the bacterial gene function in ovariectomized rats.
Correlation analysis of microbial communities and indicators of bone
To explore the relationship between gut microbiota and host bone physiology, we conducted a Spearman correlation analysis. The 18 bacterial families, based on their relative abundance, exhibited some differences between the different treatment groups (Fig. 6). The BMC, Femur BMC, BMD, Femur BMD, and Tb.Th correlated negatively with Ruminococcaceae, Bacteroidales_S24.7_group, Christensenellaceae, Streptococcaceae, Defluviitaleaceae, and positively with Lactobacillaceae. The TRACP and Tb.N show opposite trends in relation to the above-mentioned bacterial families. These results suggest that gut bacteria may increase BMC and BMD by inhibiting bone resorption.
Discussion
Working and living under artificial lighting for long durations does not permit sufficient sunlight exposure, resulting in an adverse effect on bone. White LED light often lacks the ultraviolet band, which is known to be important to human bone health (Jou et al. 2019), previous studies have shown that long-term exposure to low-dose ultraviolet irradiation promotes bone metabolism (Guo et al. 2018). Meanwhile, there has been accumulating evidence that the gut microbiome is a key regulator of bone health (Hsu and Pacifici 2018; Yan et al. 2016, 2018). With the long-term goal of creating a “healthy light” for maintaining bone health and to elucidate the potential role of gut flora in the UV regulation of bone metabolism, in this study, we examined the effects of UV supplementation to white LED light on the intestinal flora and bone metabolism in rats, including ovariectomized rats as a bone loss model.
The UV supplementation not only enhanced the BMD in normal rats but also effectively prevented the bone loss induced by the decrease in estrogen in the ovariectomized groups (Fig. 1). This result is consistent with the findings of previous studies (Ho-Pham et al. 2013; Micić et al. 2013). We also found that UV supplementation can significantly increase the concentration of 1,25(OH)2D3 and BALP in serum, indicating that UV promotes bone formation, which is also supported by a previous study (Morita et al. 2016). Interestingly, UV supplementation significantly increased the concentration of plasma TRACP, a major marker of bone resorption, in ovariectomized rats (p = 0.001), while there was no significant difference in normal rats (p = 0.458). These results indicate that UV exposure may affect bone absorption in the ovariectomized and normal rats, possibly via different mechanisms and effects on rats in different states. Additional experiments will be necessary to reveal the specific mechanisms and efficacy UV supplementation light on bone.
UV supplementation significantly decreased the diversity and richness of intestinal flora in ovariectomized rats but had no significant effect on the richness of intestinal flora in normal rats (Fig. 2). In addition, UV supplementation significantly increased the modularity of the gut bacterial network in ovariectomized rats (Fig. 3). However, there was no significant effect on the gut flora network of normal rats after UV supplementation, indicating that the intestinal flora in ovariectomized rats was more sensitive to UV exposure, compared to that of normal rats. These results show that the effects of UV supplementation on the intestinal flora of ovariectomized rats were more significant than those in normal rats.
At the phylum level, the relative abundance of Firmicutes increased, while that of Sacro bacteroides decreased after UV supplementation in the Ovx group (supplementary Fig. S3). Numerous studies have shown that Firmicutes are actively related to bone metabolism and bone mass increase, due to enhanced calcium absorption (Ai et al. 2016; Dhama et al. 2016; Kasselman et al. 2018; Wu et al. 2015). However, some members of Sacro bacteroides, such as Saccharibacteri (TM7), have exhibited an important relationship with oral pathogens and can affect the body’s immune function (Kuehbacher et al. 2008). Furthermore, UV supplementation significantly reduced the relative content of Proteobacteria in ovariectomized rats (supplementary Fig. S3d). Proteobacteria is positively associated with bone loss by causing inflammation in the intestines (Carvalho et al. 2012). The ratio of Firmicutes to Bacteroidetes gut microbiota is indicative of a dysregulation of various biological processes, such as maintenance of bone volume. As this ratio increases, intestinal permeability may be altered, leading to greater susceptibility to disease (David Yatsonsky et al. 2019). At the family level, the relative abundances of Desulfovibrionaceae and Clostridiaceae can cause intestinal inflammation (Babidge et al. 1998; Wagner et al. 1998); these were both significantly decreased only in the Ovx UV+ group. An animal study suggested that gut microbiota can regulate bone mass in mice by altering immune status in the bone and affecting osteoclast-mediated bone resorption (Sjögren et al. 2012). Thus, based on our results, UV supplementation may impart its effects on bone metabolism through mediating the intestinal flora.
To establish a role of intestinal flora in UV-improved bone metabolism, it is insufficient to only examine changes in the composition of the flora. Therefore, we further analyzed the mechanism of the effect of gut flora on bone metabolism in rats under UV supplementation conditions from a gene function perspective. Previous studies have demonstrated that functional genes of the bacterial community can be related to 16S rRNA marker genes, allowing the functional capacities of the gut microbiome to be surveyed using the 16S rRNA gene sequencing (Aßhauer et al. 2015). The UV irradiation did not significantly alter the microbiome function in normal rats (supplementary Fig. S4)., but strongly altered that of ovariectomized rats (Fig. 5). In particular, the relative content of fatty acid biosynthesis, antigen processing and presentation, glycosaminoglycan biosynthesis—chondroitin sulfate/dermatan sulfate genes in the intestinal flora of ovariectomized rats significantly increased. It has been shown that short-chain fatty acids, as an essential product of the fat synthesis pathway, indirectly regulate osteoclast activity by modulating the immune system, thus affecting the regulation of bone synthesis (Lucas et al. 2018). Bone metabolism is closely related to immune system activity, which can affect osteoclast activity through T cells (Grčević et al. 2000). In addition, chondroitin sulfate can promote chondroitin formation and reduce inflammation (McCarty et al. 2000; Ronca et al. 1998). Therefore, we speculate that the gut flora mediates UV-mediated improvements in bone metabolism in ovariectomized rats by affecting osteoclast activity by directly or indirectly affecting the immune system. The intestinal flora may also promote cartilage formation and reduce inflammation.
To further clarify the relationship between primary bacterial composition and indicators of bone, we performed correlation analysis between bacterial composition and indicators of bone (Fig. 6, supplementary Fig. S5). Most bacterial families are negatively correlated with BMC and BMD, especially the Bacteroidales_S24.7_group and Streptococcaceae. It has been shown that Streptococcaceae is related to osseous inflammation (Davidson et al. 2003). Lactobacillaceae was positively correlated with BMC and BMD, which is consistent with prior observations (Li et al. 2019). Lactobacillaceae also showed a negative correlation with the bone resorption marker, TRACP. These bacterial families are potential compositional targets involved in the UV improvement of bone metabolism.
In summary, our present study shows that UV supplementation to white LED exposure changes the structure and function of intestinal flora in a rat model of bone loss. By increasing the synthesis of short-chain fatty acids and affecting immunomodulatory, intestinal flora directly or indirectly regulate the activity of osteoclasts and thus mediates UV-mediated improvements in bone metabolism. However, the composition and function of the intestinal flora in normal rats did not apparently change after UV supplementation. Our findings open new avenues for the protection of bone health in individuals who work under artificial lighting for extended durations and provide a theoretical basis for the prevention and treatment of bone loss by combining ultraviolet radiation supplementation with intestinal probiotics. The detailed molecular mechanisms by which supplementation with UV affects bone metabolism through intestinal flora warrants further exploration.
References
Abulmeaty MMA (2017) Sunlight exposure vs. vitamin D supplementation on bone homeostasis of vitamin D deficient rats. Metab Clin Exp 11:1–9. https://doi.org/10.1016/j.yclnex.2016.10.003
Ai C, Ma N, Zhang Q, Wang G, Liu X, Tian F, Chen P, Chen W (2016) Immunomodulatory effects of different lactic acid bacteria on allergic response and its relationship with in vitro properties. PLoS One 11(10):e0164697. https://doi.org/10.1371/journal.pone.0164697
Aßhauer KP, Wemheuer B, Daniel R, Meinicke P (2015) Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 31(17):2882–2884. https://doi.org/10.1093/bioinformatics/btv287
Babidge W, Millard S, Roediger W (1998) Sulfides impair short chain fatty acid β-oxidation at acyl-CoA dehydrogenase level in colonocytes: implications for ulcerative colitis. Mol Cell Biochem 181(1-2):117–124. https://doi.org/10.1023/a:1006838231432
Bastian M, Heymann S, Jacomy M (2009) Gephi: an open source software for exploring and manipulating networks. Icwsm 8(2009):361–362. https://doi.org/10.13140/2.1.1341.1520
Bukowska-Damska A, Skowronska-Jozwiak E, Peplonska B (2019) Night shift work and osteoporosis: evidence and hypothesis. Chronobiol Int 36(2):171–180. https://doi.org/10.1080/07420528.2018.1528553
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336. https://doi.org/10.1038/nmeth.f.303
Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters WA, González A (2012) Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe 12(2):139–152. https://doi.org/10.1016/j.chom.2012.07.004
Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D (2007) Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (158):1
David Yatsonsky I, Pan K, Shendge VB, Liu J, Ebraheim NA (2019) Linkage of microbiota and osteoporosis: a mini literature review. World J Gastroenterol 10(3):123–127. https://doi.org/10.5312/wjo.v10.i3.123
Davidson D, Letts M, Khoshhal K (2003) Pelvic osteomyelitis in children: a comparison of decades from 1980–1989 with 1990–2001. J Pediatr Orthop 23(4):514–521. https://doi.org/10.1097/01241398-200307000-00019
Dhama K, Latheef KS, Munjal KA, Khandia R, Samad AH, Iqbal HMN, Joshi SK (2016) Probiotics in curing allergic and inflammatory conditions-research progress and futuristic vision. Recent Patents Inflamm Allergy Drug Discov 10(2):105–118. https://doi.org/10.2174/1872213X10666161226162229
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10):996–998. https://doi.org/10.1038/NMETH.2604
Edgar R (2016) SINTAX: a simple non-Bayesian taxonomy classifier for 16S and ITS sequences. BioRxiv 074161. https://doi.org/10.1101/074161
Falkenbach A, Unkelbach U, Boehm B, Regeniter A, Stein J, Seiffert U, Wendt T (1993) Bone metabolism before and after irradiation with ultraviolet light. Eur J Appl Physiol 66(1):55–59. https://doi.org/10.1007/BF00863400
Grčević D, Lee S-K, Marušić A, Lorenzo JA (2000) Depletion of CD4 and CD8 T lymphocytes in mice in vivo enhances 1, 25-dihydroxyvitamin D3-stimulated osteoclast-like cell formation in vitro by a mechanism that is dependent on prostaglandin synthesis. J Immunol 165(8):4231–4238. https://doi.org/10.4049/jimmunol.165.8.4231
Guo R, Du Y, Zhang S, Liu H, Fu Y (2018) The effects of ultraviolet supplementation to the artificial lighting on rats’ bone metabolism, bone mineral density, and skin. J Photochem Photobiol B-Biol 188:12–18. https://doi.org/10.1016/j.jphotobiol.2018.08.020
Hernandez CJ, Guss JD, Luna M, Goldring SR (2016) Links between the microbiome and bone. J Bone Miner Res 31(9):1638–1646. https://doi.org/10.1002/jbmr.2887
Ho-Pham LT, Nguyen ND, Nguyen TV (2013) Quantification of the relative contribution of estrogen to bone mineral density in men and women. BMC Musculoskelet Disord 14(1):366. https://doi.org/10.1186/1471-2474-14-366
Hsu E, Pacifici R (2018) From osteoimmunology to osteomicrobiology: how the microbiota and the immune system regulate bone. Calcif Tissue Int 102(5):512–521. https://doi.org/10.1007/s00223-017-0321-0
Jou J-H, Tai T-C, Liu S-H, He Z-K, Chen C-L, Chavhan SD, Chen Y-H, Chen C-C, Lee M-T, Lee J-R (2019) Pseudo-sunlight organic light-emitting diodes. Opt Laser Technol 112:494–499. https://doi.org/10.1016/j.optlastec.2018.11.046
Kasselman LJ, Vernice NA, DeLeon J, Reiss AB (2018) The gut microbiome and elevated cardiovascular risk in obesity and autoimmunity. Atherosclerosis 271:203–213. https://doi.org/10.1016/j.atherosclerosis.2018.02.036
Kuehbacher T, Rehman A, Lepage P, Hellmig S, Fölsch UR, Schreiber S, Ott SJ (2008) Intestinal TM7 bacterial phylogenies in active inflammatory bowel disease. J Med Microbiol 57(12):1569–1576. https://doi.org/10.1099/jmm.0.47719-0
Li C, Huang Q, Yang R, Dai Y, Zeng Y, Tao L, Li X, Zeng J, Wang Q (2019) Gut microbiota composition and bone mineral loss—epidemiologic evidence from individuals in Wuhan, China. Osteoporos Int 30(5):1003–1013. https://doi.org/10.1007/s00198-019-04855-5
Lucas S, Omata Y, Hofmann J, Böttcher M, Iljazovic A, Sarter K, Albrecht O, Schulz O, Krishnacoumar B, Krönke G (2018) Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun 9(1):1–10. https://doi.org/10.1038/s41467-017-02490-4
Luria T, Matsliah Y, Adir Y, Josephy N, Moran DS, Evans RK, Abramovich A, Eliakim A, Dan N (2010) Effects of a prolonged submersion on bone strength and metabolism in young healthy submariners. Calcif Tissue Int 86(1):8–13. https://doi.org/10.1007/s00223-009-9308-9
McCarty M, Russell A, Seed M (2000) Sulfated glycosaminoglycans and glucosamine may synergize in promoting synovial hyaluronic acid synthesis. Med Hypotheses 54(5):798–802. https://doi.org/10.1054/mehy.1999.0954
Mead MN (2008) Benefits of sunlight: a bright spot for human health. Environ Health Perspect 116(4):A160–A167. https://doi.org/10.1289/ehp.116-a160
Micić I, Jeon I-H, Park S-H, Hwa S-S, Chun J-M, Stojiljković P (2013) The effect of short-term low-energy ultraviolet B irradiation on bone mineral density and bone turnover markers in postmenopausal women with osteoporosis: a randomized single-blinded controlled clinical trial. Srp Ark Celok Lek 141(9-10):615–622. https://doi.org/10.2298/SARH1310615M
Morita D, Nishida Y, Higuchi Y, Seki T, Ikuta K, Asano H, Ishiguro N (2016) Short-range ultraviolet irradiation with LED device effectively increases serum levels of 25 (OH) D. J Photochem Photobiol B 164:256–263. https://doi.org/10.1016/j.jphotobiol.2016.09.036
Nikolikj-Dimitrova E (2013) The role of physical agents in treatment of osteoporosis. Maced J Med Sci 6(2):189–193. https://doi.org/10.3889/mjms.1857-5773.2013.0286
Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, Oksanen MJ, Suggests M (2007) The vegan package. Community ecology package 10:631–637 http://r-forge.r-project.org/projects/vegan/
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. https://doi.org/10.7717/peerj.2584
Ronca F, Palmieri L, Panicucci P, Ronca G (1998) Anti-inflammatory activity of chondroitin sulfate. Osteoarthr Cartil 6:14–21. https://doi.org/10.1016/S1063-4584(98)80006-X
Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C (2012) The gut microbiota regulates bone mass in mice. J Bone Miner Res 27(6):1357–1367. https://doi.org/10.1016/j.bone.2012.02.272
Team RC (2013) R: A language and environment for statistical computing. http://www.R-project.org
Wagner MA, Roger AJ, Flax JL, Brusseau GA, Stahl DA (1998) Phylogeny of dissimilatory sulfite reductases supports an early origin of sulfate respiration. J Bacteriol 180(11):2975–2982. https://doi.org/10.1128/JB.180.11.2975-2982.1998
Wu Z, Pan D, Guo Y, Sun Y, Zeng X (2015) Peptidoglycan diversity and anti-inflammatory capacity in Lactobacillus strains. Carbohydr Polym 128:130–137. https://doi.org/10.1016/j.carbpol.2015.04.026
Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF (2016) Gut microbiota induce IGF-1 and promote bone formation and growth. P Natl Acad Sci USA 113(47):E7554–E7563. https://doi.org/10.1073/pnas.1607235113
Yan J, Takakura A, Zandi-Nejad K, Charles JF (2018) Mechanisms of gut microbiota-mediated bone remodeling. Gut Microbes 9(1):84–92. https://doi.org/10.1080/19490976.2017.1371893
Acknowledgements
This work was financially supported by grants from the National Natural Science Foundation of China (81871520, 31870852) and the Foundation for the Excellent Youth Scholars of Shandong Educational Committee (72).
Availability of data and material
The data that support the findings of this study are openly available in the NCBI SRA repository, reference number PRJNA671564.
Funding
This work was financially supported by grants from the National Natural Science Foundation of China (81871520, 31870852) and the Foundation for the Excellent Youth Scholars of Shandong Educational Committee (72).
Author information
Authors and Affiliations
Contributions
JC and YF conceived and designed research. JC conducted experiments. JC, ZY, and CD analyzed data. YF and HL guided most experiments. JC and YF wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were performed with approval by the Science and Ethics Committee of the School of Beihang University (Approval ID: BM20180003).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 2330 kb)
Rights and permissions
About this article
Cite this article
Cui, J., Fu, Y., Yi, Z. et al. The beneficial effects of ultraviolet light supplementation on bone density are associated with the intestinal flora in rats. Appl Microbiol Biotechnol 105, 3705–3715 (2021). https://doi.org/10.1007/s00253-021-11282-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11282-2