Abstract
Root-associated microorganisms play an important role in plant nutrition and productivity. However, our understanding of how a plant-microbiome system responds to pre-planting soil management remains limited. Here, continuous labeling with 13CO2 gas combined with stable isotope probing (SIP) was applied to explore bacterial utilization of plant-derived carbon (C) in the tomato rhizosphere as affected by biochar amendment or reductive soil disinfestation (RSD). Our results showed that RSD treatment strongly shaped the soil bacterial community composition, while biochar soil amendment had little impact on the community in the rhizosphere of tomato. We observed that the bacterial community in the RSD treatment, which actively utilized plant-derived C, belonged to various phyla (i.e., Proteobacteria, Cyanobacteria, Verrucomicrobia, and Acidobacteria), while the genus Streptomyces (phylum Actinobacteria) was the main bacterial taxa that actively utilized plant-derived C in the biochar and control treatments. This study provides evidence that biochar application or RSD pre-planting soil management practices induced distinct bacterial utilization of plant-derived C, which may in turn regulate plant productivity in agricultural systems.
Key points
• Genus Streptomyces was the main bacterial group utilizing plant-derived carbon in both control and biochar treatments.
• Reductive soil disinfestation altered bacterial utilization of plant-derived carbon.
• Biochar did not alter the composition of the bacterial communities but had more labeled bacterial taxa utilizing plant-derived carbon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tomato is a staple vegetable crop grown and consumed globally (Olaniyi et al. 2010). Driven by the great demand from the market, intensive continuous mono-cropping and fertilization are common management practices in tomato producing. Nevertheless, these practices decrease soil quality through soil acidification and soil-borne pathogen accumulation (Blok et al. 2000; Huang et al. 2016). In order to alleviate the soil degradation induced by continuous mono-cropping, pre-planting chemical soil fumigation has been used extensive across the globe (Liu et al. 2016). However, chemical soil fumigation has environmental and human health hazards that make it unsuitable in sustainable agricultural systems (Butler et al. 2012). In recent years, much progress has been made in seeking environmentally friendly alternatives (Jaiswal et al. 2017). As alternatives to traditional pre-planting soil management regimes, biochar amendment and reductive soil disinfestation (RSD) are two popular soil treatments which have been increasingly used to improve soil quality (Messiha et al. 2007; Huang et al. 2015; Jaiswal et al. 2017; Kumar et al. 2018) due to their potential agronomic, environmental, and economic benefits.
Biochar is a C-rich product with a half-life ranging from hundreds to thousands of years (Zimmerman 2010). There is evidence that biochar soil amendment can improve soil quality (Glaser et al. 2002) and crop productivity (Graber et al. 2010). It has been shown that biochar application can increase soil pH, nutrient availability, and soil water-holding capacity (Lehmann et al. 2011; Abel et al. 2013; Whitman et al. 2016; Zheng et al. 2017). Additionally, it is known that biochar amendment is able to efficiently reduce soil pathogen density through pore adsorption (Gu et al. 2017) and alter microbial communities and their functions in soils (Kolton et al. 2017). Uzoma et al. (2011) found that the maize grain yield increased by 150% and 98% after the application of biochar at 15 and 20 t ha−1 in a degraded soil. Therefore, biochar is highly recommended as a soil amendment to remediate degraded soils (Kolton et al. 2011; Zheng et al. 2017).
RSD is another soil pre-planting treatment for improving soil quality. The RSD method, also called biological soil disinfestation, was simultaneously invented in the Netherlands (Blok et al. 2000) and Japan (Shinmura 2000). This method stimulates anaerobic processes by incubating organic wastes (e.g., corn stover, rice straw, or animal manures) in water-logged soils covered with a polyethylene mulch (Butler et al. 2012; Huang et al. 2015). RSD can improve the quality of degraded soils by increasing soil nutrients and suppressing pathogens. Huang et al. (2016) reported that disease incidences in a RSD treatment were about six times lower than the control during two seasons of cultivation in a field experiment. Increasingly, studies have indicated that the RSD treatment can be effective against a wide range of pathogens, including harmful nematodes (Goud et al. 2004). For example, the genera Bacillus and Pseudomonas have been shown as the dominant microbial populations in RSD-treated soils, which have capabilities to suppress pathogens via antibiotics, lytic enzymes, and volatile organic compound production (Khabbaz et al. 2015; Shen et al. 2015). However, despite the fact that increasing number of studies have demonstrated the influence of both biochar amendment and RSD treatment on general soil microbial communities, few studies have sought to explore the effects on those rhizosphere microbial communities that specifically utilize plant-derived C.
The rhizosphere is a unique interface, at which microbes and plants carry out complex and diverse molecular interactions (Gkarmiri et al. 2017; Liu et al. 2019). On average, rhizodeposition accounts for about 17% of the total photoassimilated C (Nguyen 2003). Previous studies have shown that root exudate composition depends on multiple factors, e.g., developmental phase, root traits, soil types, plant species, nutrient availability, and environmental conditions (Jones 1998; Aulakh et al. 2001; Badri and Vivanco 2009; Mendes et al. 2017). Root exudates consumed by microorganisms as a substrate play a vital role in affecting the composition and activity of the soil microbial community (Ahmed et al. 2018; Sarr et al. 2020). Studies have suggested that plant root exudates can recruit the rhizosphere microbial community to build a suitable habitat for plant growth (Bulgarelli et al. 2013). For example, root exudates released by tomato can stimulate beneficial Pseudomonad abundance in the rhizosphere (Lemanceau et al. 1995). Clearly, the complex interactions and feedbacks among plant roots, microbes, and the soil environmental conditions shape the microbial community composition in the rhizosphere (Chapelle et al. 2016), and this may further influence on nutrient cycling and plant productivity (Hannula et al. 2017). The differences between biochar amendment and RSD treatment on soil rhizosphere microbial community composition have not been explored adequately (Huang et al. 2015; Whitman et al. 2016). Our previous study, focusing on application of biochar in a faba bean-maize intercropping system found that biochar amendment can stimulate soil bacterial utilization of plant-derived C (Liao et al. 2019). However, whether different pre-planting soil managements (i.e., biochar and RSD) can alter bacterial communities utilizing plant-derived C in continuous mono-cropping systems is still an open question.
The aims of this study were (i) to investigate the influence of two environmentally friendly soil management practices (i.e., biochar addition and RSD) on bacterial communities in the tomato rhizosphere; (ii) to identify the impacts of biochar and RSD managements on bacteria that actively utilize plant-derived C in the rhizosphere; and (iii) to assess roles of active bacteria that may potentially regulate plant development in the rhizosphere. We hypothesized that the biochar and RSD treatments could enhance utilization of root exudates by soil bacteria in the rhizosphere of tomato. To verify this hypothesis, continuous 13CO2 labeling, stable isotope probing (SIP), and Illumina sequencing were performed to classify bacterial communities and their utilization of root exudates in the rhizosphere of tomato under different soil treatments.
Materials and methods
Soil sampling and biochar preparation
Soils used in this study were collected from a greenhouse in Changzhou (31°55′32″N, 119°51′39″E), Jiangsu Province, East China. The greenhouse soil is a clay loam texture, belonging to plinthosols according to the FAO/UNESCO classification. The greenhouse soil had received excessive fertilizer, was under continuous tomato cropping for 4 years, and therefore suffered severe bacterial wilt disease. The disease incidence was about 40% with approximately 106 CFU (colony forming units) of Ralstonia solanacearum per gram soil. Soil samples were collected from 0 to 20-cm depth. Plants and root residues were removed from the soil before passing it through a 2-mm sieve.
Biochar was made from wood chip solid waste. First, chips were air-dried and then charred in the lab in a furnace for 5 h at 500 °C. The biochar was then milled (< 2-mm sieve) for further use. For the RSD treatment, maize straw was air-dried for 4 weeks and finely chopped to less than 2 mm. The basic properties of soil, biochar, and maize straw are shown in Table 1.
Experimental procedure
A pot experiment was conducted with three treatments: biochar amendment, RSD, and a control (CK). To each pot (6.8-cm diameter, 14.8-cm height), a dry weight basis of 300 g soil was added. Both biochar and maize straws were added and well mixed with the soil just immediately before filling the pots. Then Milli-Q (Merck, Darmstadt, Germany) water was added to reach 40% water-holding capacity (Liao et al. 2019). For the biochar treatment, biochar was incorporated into the soil at 2% (w/w). For both the biochar and CK treatments, Milli-Q water was added to maintain the soil water content during soil incubation. For the RSD treatment, air-dried maize straw was incorporated into the soil at 2% (w/w), and the soil irrigated to 5 cm above the upper surface to ensure flood conditions. All the three treatments were put into a step-in incubator at 28 °C for 21 days. After 21 days of flood incubation, soils in the RSD treatment were air-dried to achieve approximately 40% water-holding capacity. In addition, 3 replicate pots of each treatment were randomly destructively sampled to investigate the soil bacterial community composition before planting.
Tomato (Lycopersicon esculentum Mill.) seeds (Hezuo 903, Changfeng Co., Ltd., Shanghai, China) were planted in November 2017. Before planting, soil was amended with NH4NO3 and KH2PO4 at the rate of 100 mg total N kg−1 soil, 50 mg P2O5 kg−1 soil, and 50 mg K2O kg−1 soil as base fertilizer. Three healthy seedlings were planted in each pot after the tomato plants had produced true leaves, and then, one healthy seedling per pot was kept after 7-day planting. The day and night periods and temperatures were set at 14 h and 10 h and at 25 °C and 18 °C, respectively.
The 13CO2 continuous labeling process followed the descriptions of Yao et al. (2012) and Wang et al. (2016) after 14-day tomato growth. Briefly, 99.8 atom % of 13CO2 gas (Sigma-Aldrich, Darmstadt, USA) at 350 ppm was applied to the labeled plant growth chamber. For the parallel unlabeled plant growth chamber, 12CO2 was used to reach the same CO2 concentration. Three pots for each treatment were put into both labeled and unlabeled plant growth chambers. Plants experienced similar temperature and light conditions in each growth chamber. Soil moisture was maintained by adding Milli-Q water every 3 days during the 35-day labeling. All soil in a given pot was considered as rhizosphere soil since root growth in each pot was extremely extensive. The collected soil was then separated into two parts, one was immediately freeze-dried for DNA extraction, and the other was stored at − 4 °C for soil property analysis. An overall conceptual diagram for the study design is shown in Fig. 1.
Soil property analysis
Soil pH (soil: water 1:2.5) was measured by a pH meter; total carbon and nitrogen contents were analyzed using an elemental analyzer (Vario MACRO cube, Hanau, Germany); soil NO3− and exchangeable NH4+ contents were extracted with 2 M KCl (1:10 w/v) for 30 min and then measured by a continuous flow analyzer (AutoAnalyzer3, Mequon, USA).
DNA extraction
Genomic DNA was extracted from 0.50 g of fresh soil samples using the Fast DNA SPIN Kit (MP Biomedicals, Santa Ana, USA). DNA content was determined by a NanoDrop™ 2000 (Thermo Scientific, Waltham, USA).
DNA-SIP
The DNA-SIP procedure was that by Liao et al. (2019). Briefly, for each sample, 3 μg DNA was added into a CsCl density fraction, then CsCl solutions were adjusted to 1.7102 g/ml with a refractometer (Reichert, Depew, USA). The solution was then centrifuged at 45,000 rpm (184,000g) at 20 °C for 44 h using a Vti 65.2 vertical rotor (Beckman Coulter, Brea, USA). Sixteen equal fractions of CsCl-DNA were then collected using a single-channel syringe pump. DNA samples were purified with PEG6000 and ethanol buffer (70%), then dissolved in 30 μL sterile water, and stored at − 20 °C for further use.
PCR amplification and Illumina sequencing
Six bulk DNA samples for each treatment (three 13CO2-labeled and three unlabeled soil samples) and three pre-planting soil DNA samples for each treatment were selected for sequencing.
qPCR results show that there were no clear differences in quantification of the16S rRNA gene between unlabeled and 13C-labeled samples (Supplemental Note 1; Fig. 2). Because the bacteria utilizing the plant-derived C accounted for a small proportion of the whole bacterial communities, and the qPCR technique may not be sensitive enough (Wang et al. 2019), the high-resolution SIP approach was used to identify those 13C-labeled bacteria that utilize plant rhizoexudates. Only buoyant densities ranging from 1.71 to 1.75 g/ml (7 fractions per sample) were analyzed. Bacterial 16S rRNA gene was amplified with barcoded primers 515f/907r (Hamady et al. 2008; Zhou et al. 2011). The PCR reaction was that used by Liao et al. (2019). Purified PCR samples were run on an Illumina HiSeq2500 platform.
Quantitative distribution of density-resolved bacterial 16S rRNA gene obtained from CK (a) and biochar (b) and RSD (c) 35-day labeling with either labeled (13CO2) or unlabeled (12CO2).The normalized data are the ratio of the copy number in each gradient fraction to the total quantities from each treatment
Sequenced data was analyzed by the QIIME 1.9.1 pipeline (Caporaso et al. 2010). Briefly, chimeras were checked and filtered using the USEARCH tool (Edgar 2010). Subsequently, high-quality sequences were clustered into operational taxonomic units (OTUs) using the UCLUST algorithm with 0.97 similarity. Representative sequence clusters were then annotated with the Greengenes database (release v13.8) (McDonald et al. 2012). The SSU rRNA sequences have been deposited at the BioProject database under NCBI accession number PRJNA563242.
Statistical analysis
The statistical approach for identifying 13C-labeled OTUs (high-resolution SIP) has been described previously (Pepe-Ranney et al. 2016; Angel et al. 2018; Youngblut et al. 2018; Koechli et al. 2019). In this study, the procedure for identifying 13C-labeled OTUs was that by Liao et al. (2019). Briefly, we defined > 1.71 g/ml as the heavy category fraction and discarded the OTUs with low sequence counts to perform the statistical analyses using the Benjamini-Hochberg method of P value corrections (Benjamini and Hochberg 1995) using the DESeq2 package (Love et al. 2014). Statistically enriched OTUs from 13C-labeled samples, compared to the unlabeled control, were expected to be the target 13CO2-labeled microbiome (Youngblut and Buckley 2014; Pepe-Ranney et al. 2016).
SPSS 16.0 software (SPSS Inc., Chicago, USA) was used to for standard statistical tests. The statistical significance was determined by the least significant difference (LSD) test at the P = 0.05 level. PCoA and NMDS ordinations based on Bray-Curtis similarities were analyzed using the “Phyloseq” package (McMurdie and Holmes 2013) and the “ggplot2” package (Wickham 2010) in R (version 3.4.1) (R Core Team 2013).
Results
Soil properties and plant biomass
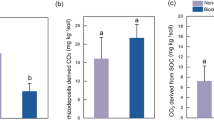
The biochar treatment significantly increases (P < 0.05) the tomato biomass (shoot + root) compared to RSD and CK, but no significant differences are found between the RSD and CK treatments (Table 2). Specifically, the RSD treatment had 34.2% lower biomass than the biochar treatment. Both biochar and RSD treatments significantly increase (P < 0.05) soil pH, total C and total N, and exchangeable NH4+ and C/N in the tomato rhizosphere compared with CK, while the NO3− concentration did not change among treatments (Table 2).
Changes in bacterial community composition as assessed by Illumina sequencing
Before planting, we measure the soil bacterial community composition among the different treatments (Supplemental Fig. S1). The RSD treatment significantly changed the bacterial community composition (Adonis test, P < 0.001) compared with CK, whereas no significant differences were observed between CK and biochar treatments. We found that bacterial species from phylum Firmicutes (e.g., Bacillales and Clostridiales) were significantly enriched in the RSD treatment. After planting, the RSD treatment maintains a distinct bacterial community composition compared with the control and biochar treatments (Adonis test, P < 0.001; Supplemental Fig. S2). Proteobacteria and Actinobacteria, which accounted for more than 50% of sequences, are the two major phyla occurring in the tomato rhizosphere among treatments (Fig. 3). In contrast to our hypotheses, the bacterial community composition in the biochar and CK treatments is similar, and only two taxa, Gemmatimonadetes and Planctomycetes, are significantly (P < 0.05) altered in their relative abundances (Supplemental Fig. S3). The RSD treatment significantly decreases (P < 0.05) the relative abundance of Actinobacteria while increases the relative abundance of Firmicutes compared with CK (Supplemental Fig. S3).
13C incorporation into DNA in the heavy density fractions
The CsCl fraction density plays an important role in composition of bacterial communities, in both the unlabeled and the 13C-labeled samples (Fig. 4). This phenomenon was mainly attributed to the genome G + C concentrations of different bacteria in the CsCl buoyant density. We found that bacterial communities in the 13C-labeled samples differed from unlabeled samples in the heavy gradient fraction, and significant differences were observed within the biochar treatment (Adonis test, P < 0.001; Fig. 4).
Ordination of rhizosphere bacterial communities using the heavy (> 1.71 g ml−1) fractions of different soil amendments and Bray-Curtis similarities based on their OTU contents. The size of a point indicates its fraction density, while the distance between points represents the similarity in bacterial community composition
13C-labeled rhizosphere bacterial communities
A total of 126 fractions (density > 1.71 g/ml) are chosen for 16S rRNA gene sequencing (Fig. 5). In total, there were 6923, 7651, and 8303 OTUs that passed the sparsity threshold in the CK, biochar, and RSD treatments, respectively. The OTUs that were significantly enriched in the heavy fraction relative to the unlabeled control were considered to have 13C-labeled DNA. Among these, 91, 201, and 72 OTUs, which accounted for 1.3%, 2.6%, and 0.87% of the total detected OTUs in the CK, biochar, and RSD treatments, respectively, are enriched significantly in the 13C-labeled samples (Fig. 5).
Log2-fold changes in the relative abundances of OTUs (13C-labeled vs. 12C-labeled) in the heavy (> 1.71 g ml−1) fractions for three soil amendments. All the OTUs passed a 0.35 sparsity threshold for the heavy fractions. Each circle represents a single OTU (at 97% similarity level). The dashed and dotted lines denote the 2- and 10-fold changes, respectively (increases and decreases). Colored circles denote percentage fold-changes that had an adjusted P value below a false discovery rate of 10%
Various identified phyla (e.g., Proteobacteria, Cyanobacteria, Verrucomicrobia, and Acidobacteria) were more prominent among the 13C-labeled OTUs in the RSD treatment. In contrast, Actinobacteria and Proteobacteria are identified as the major labeled taxa in both the CK and biochar treatments (Fig. 5). A Venn diagram shows that there were 33 labeled OTUs shared by the CK and biochar treatments, while there is only one labeled OTU shared by the CK and RSD treatments, and only two OTUs between the biochar and RSD treatments (Supplemental Fig. S4). Among the labeled OTUs, there are numerous shared 13C-labeled OTUs between the CK and biochar treatments, while the RSD treatment had few shared 13C-labeled OTUs with either biochar or CK treatment (Supplemental Fig. S5). After the absolute counts of OTUs were transformed into their relative abundances, we found relatively more Actinobacteria utilizing root exudates in the biochar treatment, while the phylum Proteobacteria that actively utilized root exudates was identified in the RSD treatment (Supplemental Fig. S6).
At the genus level, DNA-SIP reveals that Streptomyces was the dominant genus that utilized root exudates in both the CK and biochar treatment, which had 25 and 93 labeled OTUs, respectively (Fig. 6). In addition, two Streptomyces OTUs (OTU.113924 and OTU.599) are among the 10 most 13CO2-labeled OTUs among the treatments (Supplemental Fig. S8). The relative abundances of Streptomyces (phylum Actinobacteria) are 15.9% and 12.0% in the rhizosphere of the CK and biochar treatments, respectively, which were significantly higher (P < 0.05) than that of the RSD treatment (Supplemental Fig. S7). In contrast, DNA-SIP reveals that the13CO2-labeled OTUs in the RSD treatment were mainly those of the phylum Proteobacteria (e.g., Anaeromyxobacter, Bosea, Dechloromonas, and Geobacter) (Fig. 6).
Discussion
Our results showed that the RSD treatment harbored a specific bacterial community either at pre-planting or after harvesting of the tomato plants. This phenomenon is likely due to the RSD treatment strongly influencing on soil pH, total C, total N, and the C/N ratio due to the flood incubation and amendment with maize straw. These changes likely shifted the soil bacterial community composition in the tomato rhizosphere and selected a distinct enriched bacterial community compared with the control and biochar treatments. Specifically, Clostridium and Coprococcus (phylum Firmicutes) were the two major genera contributing to shifts in the rhizosphere microbial community composition. The Clostridium genus is known to be involved in the anaerobic degradation of rice straw (Huang et al. 2016). Weber et al. (2001) reported that the abundance of Clostridium spp. substantially increased in rice straw, colonizing and decomposing the straw within the first 2 weeks after flooding. Members of the genus Coprococcus can produce organic acids (Holdeman and Moore 1974), such as propionic, butyric, and acetic acids, which can decrease the abundance of pathogens (Huang et al. 2015).
We observed that the biochar application gave very similar bacterial communities to the control. Streptomyces accounted for about 10% of the sequences in the biochar and control samples. Members of the genus Streptomyces are ubiquitous in soils, where they play a significant role in global soil C cycling (Schrempf 2001). Streptomyces is the most abundant genus (> 600 species) producing antibiotics (Labeda et al. 2012). In addition, they have the capacity to produce a wide range of volatile organic compounds and increase soil nutrient levels, which directly and indirectly stimulate plant growth (Citron et al. 2015; Cordovez et al. 2015). Therefore, Streptomyces is recognized as a plant growth-promoting bacterial genus (Dias et al. 2017) and has been proven to stimulate tomato growth (El-Tarabily 2008). Interestingly, we observed that the biochar treatment had the highest tomato biomass, while bacterial community composition was still similar to the control. The higher tomato biomass might be attributed to higher soil nutrients released by the biochar into the rhizosphere in comparison to the RSD and control treatments during the 49-day tomato growth (Enders et al. 2012). In general, our results suggest that the RSD treatment harbors distinct bacterial communities in the rhizosphere compared with the biochar treatment.
The 13CO2-labeled plant-derived C released by tomato can participate in a series of microbial processes (Hernandez et al. 2015; Ge et al. 2019). A relatively short sampling time should be considered to avoid secondary metabolism of labeled C source between soil microbes. However, a short sampling time point that ensures high enough 13C label in the soil is hard to obtain in practice during DNA-SIP studies (Yao et al. 2015; Liao et al. 2019). Despite the limitations, DNA-SIP coupled with continuous labeling is still the best technique for detecting those microbial communities that actively utilize root exudates (Haichar et al. 2008). DNA-SIP results revealed that different numbers of OTUs were detected under the three treatments. Only a low percentage (< 3%) of total OTUs was labeled, thus being involved in the use of root exudates and confirming previous findings (Ai et al. 2015; Gschwendtner et al. 2016).
Similar to what was observed for the total rhizosphere bacterial community, phylogenetic affiliation revealed that the RSD treatment had a distinct bacterial community utilizing plant-derived C compared with the biochar and control treatments. Studies have documented that soil type (Lian et al. 2017), plant host (Haichar et al. 2008), and N fertilization (Gschwendtner et al. 2016) have considerable effects on soil microbial utilization of root exudates. The biochar treatment had the highest numbers of 13C-labeled OTUs among treatments. Streptomyces (phylum Actinobacteria) was the dominant genus consuming the 13C root exudate in both control and biochar treatments. Biochar may have facilitated microbial activity due to root exudate absorption (Gu et al. 2017) and to its porous properties (Pietikäinen et al. 2000). In contrast, the phylum Proteobacteria (e.g., Anaeromyxobacter, Bosea, Dechloromonas, and Geobacter) was the main labeled bacterial taxa in the rhizosphere of the RSD treatment, favored by the incorporation of maize straw into the soil and the legacy of flood incubation (Huang et al. 2015). Generally, compared with the control and biochar treatments, the microbial communities of the RSD-treated soil had diverse abilities to decompose organic materials (Huang et al. 2019). More importantly, a relatively high diversity of various bacterial phyla (e.g., Proteobacteria, Cyanobacteria, Verrucomicrobia, and Acidobacteria) using plant-derived C in the rhizosphere soil might have altered more diversity and functions in the RSD treatment than in the biochar and the control treatments.
In general, the RSD had a stronger influence on rhizosphere bacterial communities and their utilization of root exudates relative to the biochar amendment. Biochar did not alter the composition of the bacterial communities but had more labeled bacterial taxa utilizing plant-derived C in tomato rhizosphere soil. Our results provide experimental evidence that bacterial utilization of root exudates is strongly altered by soil management regimes. Further studies should be conducted to investigate the interactions among root exudate compounds on rhizosphere microbial community activity and function by using RNA-SIP or metagenomic techniques.
Data availability
All datasets generated for this study are included in the article/Supplementary Material.
References
Abel S, Peters A, Trinks S, Schonsky H, Facklam M, Wessolek G (2013) Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 202:183–191
Ahmed MA, Banfield CC, Sanaullah M, Gunina A, Dipopold MA (2018) Utlilisation of mucilage C by microbial communities under drought. Biol Fertil Soils 54:89–94
Ai C, Liang G, Sun J, Wang X, He P, Zhou W, He X (2015) Reduced dependence of rhizosphere microbiome on plant-derived carbon in 32-year long-term inorganic and organic fertilized soils. Soil Biol Biochem 80:70–78
Angel R, Panholzl C, Gabriel R, Herbold C, Wanek W, Richter A, Eichorst SA, Woebken D (2018) Application of stable-isotope labelling techniques for the detection of active diazotrophs. Environ Microbiol 20:44–61
Aulakh MS, Wassmann R, Bueno C, Kreuzwieser J, Rennenberg H (2001) Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol 3:139–148
Badri D, Vivanco J (2009) Regulation and function of root exudate. Plant Cell Environ 6:666–681
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B (Methodol) 57:289–300
Blok WJ, Lamers JG, Termorshuizen AJ, Bollen GJ (2000) Control of soilbrone plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 90:253–259
Bulgarelli D, Schlaeppi K, Spaepen S, van Themaat E, Schulze-Lefert P (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838
Butler DM, Rosskopf EN, Kokalis-Burelle N, Albano JP, Muramoto J, Shennan C (2012) Exploring warm-season cover crops as carbon sources for anaerobic soil disinfestation (ASD). Plant Soil 355:149–165
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Chapelle E, Mendes R, Bakker PA, Raaijmakers JM (2016) Fungal invasion of the rhizosphere microbiome. ISME J 10:265–268
Citron CA, Barra L, Wink J, Dickschat JS (2015) Volatiles from nineteen recently genome sequenced actinomycetes. Org Biomol Chem 13:2673–2683
Cordovez V, Carrion VJ, Etalo DW, Mumm R, Zhu H, Van Wezel GP, Raaijmakers JM (2015) Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Front Microbiol 6:1081
Dias MP, Bastos MS, Xavier VB, Cassel E, Astarita LV, Santarém ER (2017) Plant growth and resistance promoted by Streptomyces spp. in tomato. Plant Physiol Biochem 118:479–493
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
El-Tarabily KA (2008) Promotion of tomato (Lycopersicon esculentum mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing streptomycete actinomycetes. Plant Soil 308:161–174
Enders A, Hanley K, Whitman T, Joseph S, Lehmann J (2012) Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour Technol 144:644–653
Ge T, Luo Y, He XH (2019) Quantitative and mechanistic insights into the key process in the rhizodeposited carbon stabilization, transformation and utlization of carbon, nitrogen, and phosphorus in paddy soil. Plant Soil 445:1–5
Gkarmiri K, Mahmood S, Ekblad A, Alström S, Högberg N, Finlay R (2017) Identifying the active microbiome associated with roots and rhizosphere soil of oilseed rape. Appl Environ Microbiol 83:e01938–e01917
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal – a review. Biol Fertil Soils 35:219–230
Goud J-KC, Termorshuizen AJ, Blok WJ, van Bruggen AHC (2004) Long-term effect of biological soil disinfestation on Verticillium wilt. Plant Dis 88:688–694
Graber ER, Harel YM, Kolton M, Cytryn E, Silber A, David DR, Tsechansky L, Borenshtein M, Elad Y (2010) Biochar impcat on development and productivity of pepper and tomato growth in fertligated soilless media. Plant Soil 337:481–496
Gschwendtner S, Engel M, Lueders T, Buegger F, Schloter M (2016) Nitrogen fertilization affects bacteria utilizing plant-derived carbon in the rhizosphere of beech seedlings. Plant Soil 407:203–215
Gu Y, Hou Y, Huang D, Hao Z, Wang X, Wei Z, Jousset A, Tan S, Xu D, Shen Q, Xu Y, Friman V (2017) Application of biochar reduces Ralstonia solanacearum infection via effecs on pathogen chemotaxis, swarming motility, and root exudate adsorption. Plant Soil 415:269–281
Haichar FZ, Marol C, Berge O, Rangel-Castro JI, Prosser JI, Balesdent J, Heulin T, Achouak W (2008) Plant host habitat and root exudates shape soil bacterial community structure. ISME J 2:1221–1230
Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R (2008) Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods 5:235–237
Hannula SE, Morrien E, de Hollander M, van der Putten WH, van Veen JA, de Boer W (2017) Shifts in rhizosphere fungal community during secondary succession following abandonment from agriculture. ISME J 11:2294–2304
Hernandez M, Dumont MG, Yuan Q, Conrad R (2015) Different bacterial populations associated with the roots and rhizosphere of rice incorporate plant-derived carbon. Appl Environ Microbiol 81:2244–2253
Holdeman LV, Moore W (1974) New genus, Coprococcus, twelve new species, and emended descriptions of four previously described species of bacteria from human feces. Int J Syst Evol Microbiol 24:260–277
Huang X, Liu L, Wen T, Zhu R, Zhang J, Cai Z (2015) Illumina MiSeq investigations on the changes of microbial community in the Fusarium oxysporum f.sp. cubense infected soil during and after reductive soil disinfestation. Microbiol Res 181:33–42
Huang X, Liu L, Wen T, Zhang J, Wang F, Cai Z (2016) Changes in the soil microbial community after reductive soil disinfestation and cucumber seedling cultivation. Appl Microbiol Biotechnol 100:5581–5593
Huang X, Zhao J, Zhou X, Zhang J, Cai ZC (2019) Differential respones of soil bacterial community and functional diversity to reductive soil disinfestation and chemical soil disinfestation. Geoderma 348:124–134
Jaiswal AK, Elad Y, Paudel I, Graber ER, Cytryn E, Frenkel O (2017) Linking the belowground microbial composition, diversity and activity to soilborne disease suppression and growth promotion of tomato amended with biochar. Sci Rep 7:44382
Jones DL (1998) Organic acids in rhizosphere - a critical review. Plant Soil 205:25–44
Khabbaz SE, Zhang L, Cáceres LA, Sumarah M, Wang A, Abbasi PA (2015) Characterisation of antagonistic Bacillus and Pseudomonas strains for biocontrol potential and suppression of damping-off and root rot disease. Ann Appl Biol 166:456–471
Koechli C, Campbell AN, Pepe-Ranney C, Buckley DH (2019) Assessing fungal contributions to cellulose degradation in soil by using high-throughput stable istope probing. Soil Biol Biochem 130:150–158
Kolton M, Harel YM, Pasternak Z, Graber ER, Elad Y, Cytryn E (2011) Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl Environ Microbiol 77:4924–4930
Kolton M, Graber ER, Tsehansky L, Elad Y, Cytryn E (2017) Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol 213:1393–1404
Kumar A, Elad Y, Tsechansky L, Abrol V, Lew B, Offenbach R, Graber ER (2018) Biochar potential in intensive cultivation of Capsicum annuum L. (sweet pepper): crop yield and plant protection. J Sci Food Agric 98:495–503
Labeda D, Goodfellow M, Brown R, Ward A, Lanoot B, Vanncanneyt M, Swings J, Kim S.-B, Liu Z, Chun J (2012) Phylogenetic study of the species within the family Streptomycetaceae. Antonie Van Leeuwenhoek 101: 73–104
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota–a review. Soil Biol Biochem 43:1812–1836
Lemanceau P, Corberand T, Gardan L, Latour X, Laguerre Boeufgras J, Alabouvette C (1995) Effect of two plant species, flax (Linum usitatissinum L.) and tomato (Lycopersicon esculentum Mill.), on the diversity of soilborne populations of fluorescent pseudomonads. Appl Environ Microbiol 61:1004–1012
Lian T-X, Wang G-H, Yu Z-H, Li Y-S, Liu X-B, Zhang S-Q, Herbert SJ, Jin J (2017) Bacterial communities incorporating plant-derived carbon in the soybean rhizosphere in Mollisols that differ in soil organic carbon content. Appl Soil Ecol 119:375–383
Liao HK, Li YY, Yao HY (2019) Biochar amendment stimulates untlization of plant-derived carbon by soil bacterial in an intercropping system. Front Microbiol 10:1361
Liu L, Sun C, Liu X, He X, Liu M, Wu H, Tang C, Jin C, Zhang Y (2016) Effect of calcium cyanamide, ammonium bicarbonate and lime mixture, and ammonia water on survival of Ralstonia solanacearum and microbial community. Sci Rep 6:19037
Liu Y, Ge T, Zhu Z, Liu S, Luo Y, Li Y, Wang P, Gavrichkova O, Xu X, Wang J, Wu J, Guggenberger G, Kuzyakov Y (2019) Carbon input and allocation by rice into paddy soils: a review. Soil Biol Biochem 133:97–107
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550
McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P (2012) An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6:610–618
McMurdie PJ, Holmes S (2013) Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8:e61217
Mendes LW, Raaijmakers JM, de Hollander M, Mendes R, Tsai SM (2017) Influence of resistance breeding in common bean on rhizosphere microbiome composition and function. ISME J 12:212–224
Messiha NAS, van Diepeningen AD, Wenneker M, van Beuningen AR, Janse JD, Coenen TGC, Termorshuizen AJ, van Bruggen AHC, Blok WJ (2007) Biological soil disinfestation (BSD), a new control method for potato brown rot, caused by Ralstonia solanacearum race 3 biovar 2. Eur J Plant Pathol 117:403–415
Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23:375–396
Olaniyi JO, Akanbi WB, Adejumo TA, Akande OG (2010) Growth, fruit yield and nutritional quality of tomato varieties. Afr J Food Sci 4:398–402
Pepe-Ranney C, Koechli C, Potrafka R, Andam C, Eggleston E, Garcia-Pichel F, Buckley DH (2016) Non-cyanobacterial diazotrophs mediate dinitrogen fixation in biological soil crusts during early crust formation. ISME J 10:287–298
Pietikäinen J, Kiikkilä O, Fritze H (2000) Charcoal as a habitat for microbes and its effects on the microbial community of the underlying humus. Oikos 89:231–242
R Core Team (2013) Development Core. R: a language and environment for statistical computing. Vienna, Austria
Sarr PS, Ando Y, Nakamura S, Deshpande S, Subbarao GV (2020) Sorgoleone release from sorghum roots shapes the composition of nitrifying populations, total bacteria, and archaea and determines the level of nitrification. Biol Fertil Soils 56:145–166
Schrempf H (2001) Recognition and degradation of chitin by Streptomycetes. Antonie Van Leeuwenhoek 79:285–289
Shen Z, Ruan Y, Xue C, Zhong S, Li R, Shen Q (2015) Soils naturally suppresive to banana Fusarium wilt disease harbor unique bacterial communities. Plant Soil 393:21–33
Shinmura A (2000) Causal agent and control of root rot of welsh onion. PSJ Soil-borne Dis Workshop Rep 20:133–143
Uzoma KC, Inoue M, Andry H, Fujimaki H, Zahoor A, Nishihara E (2011) Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag 27:205–212
Wang J, Chapman SJ, Yao H (2016) Incorporation of 13C-labelled rice rhizodeposition into soil microbial communities under different fertilizer applications. Appl Soil Ecol 101:11–19
Wang J, Chapman SJ, Ye Q, Yao H (2019) Limited effect of planting transgenic rice on the soil microbiome studied by continous 13CO2 labeling combined with high-throughput sequencing. Appl Microbiol Biotechnol 103:4217–4227
Weber S, Stubner S, Conrad R (2001) Bacterial populations colonizing and degrading rice straw in anoxic paddy soil. Appl Environ Microbiol 67:1318–1327
Whitman T, Pepe-Ranney C, Enders A, Koechli C, Campbell A, Buckley DH, Lehmann J (2016) Dynamics of microbial community composition and soil organic carbon mineralization in soil following addition of pyrogenic and fresh organic matter. ISME J 10:2918–2930
Wickham H (2010) ggplot2: elegant graphics for data analysis. J Stat Softw 35:65–88
Yao H, Thornton B, Paterson E (2012) Incorporation of 13C-labelled rice rhizodeposition carbon into soil microbial communities under different water status. Soil Biol Biochem 53:72–77
Yao H, Chapman SJ, Thornton B, Paterson E (2015) 13C PLFAs: a key to open the soil microbial black box? Plant Soil 392:3–15
Youngblut ND, Buckley DH (2014) Intra-genomic variation in G+C content and its implications for DNA stable isotope probing. Environ Microbiol Rep 6:767–775
Youngblut ND, Barnett S, Buckley DH (2018) A modeling toolkit to predict accurary and aid design of DNA-SIP experiments. Front Microbiol 9:570
Zheng H, Wang X, Chen L, Wang Z, Xia Y, Zhang Y, Wang H, Luo X, Xing B (2017) Enhanced growth of halophyte plants in biochar-amended coastal soil: roles of nutrient availability and rhizosphere microbial modulation. Plant Cell Environ 41:517–532
Zhou J, Wu L, Deng Y, Zhi X, Jiang YH, Tu Q, Xie J, Van Nostrand JD, He Z, Yang Y (2011) Reproducibility and quantitation of amplicon sequencing-based detection. ISME J 5:1303–1313
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44:1295–1301
Funding
This work was supported by the National Natural Science Foundation of China (41525002, 41471206, 41601249, and 41877051), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB15020301), the Talents of Guizhou Science and Technology Cooperation Platform ([2017]5726-52), and the Ningbo Municipal Science and Technology Bureau (2015C10031).
Author information
Authors and Affiliations
Contributions
HL and HY conceived and designed the research. HL and YL conducted the experiments. HF contributed new reagents or analytical tools. HL and HY wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 657 kb)
Rights and permissions
About this article
Cite this article
Liao, H., Fan, H., Li, Y. et al. Influence of reductive soil disinfestation or biochar amendment on bacterial communities and their utilization of plant-derived carbon in the rhizosphere of tomato. Appl Microbiol Biotechnol 105, 815–825 (2021). https://doi.org/10.1007/s00253-020-11036-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-11036-6