Abstract
Plant virus-based expression systems are an alternative expression platform for the production of clinically and industrially useful recombinant proteins. Nonetheless, due to a lack of viral vector with the commercial potentials, it is urgent to design and develop new, versatile, and efficient plant virus vectors. The genome of Tomato bushy stunt virus (TBSV) offers an attractive alternative to being modified as a vector for producing heterologous proteins in plants. Here, we developed a set of novel fusion and non-fusion TBSV-CP replacement vectors, which provide more flexible and efficient tools for expressing proteins of interest in plants. An alternative tobacco plant, Nicotiana excelsiana, was used in this study as a host for newly constructed TBSV vectors because the unwanted necrotic effects were reported on the commonly used Nicotiana benthamiana host associated with expression of TBSV-encoded P19 protein. The data showed that TBSV vectors caused a symptomless infection and overexpressed reporter gene in N. excelsiana leaves, demonstrating that N. excelsiana is an ideal host plant for TBSV-mediated heterologous gene expression. Moreover, a TBSV non-fusion vector, dAUG, shows the similar accumulation level of reporter proteins to that of TMV- and PVX-based vectors in side-by-side comparison and provides more flexible aspects than the previously developed TBSV vectors. Collectively, our newly developed TBSV expression system adds a new member to the family of plant viral expression vectors and meanwhile offers a flexible and highly effective approach for producing proteins of interest in plants.
Key points
• The TBSV-based transient expression system has been significantly improved.
• The necrotic effects caused by viral P19 protein were avoided by the usage of N. excelsiana as a host plant.
• The expression level of the non-fusion vector was similar to the most effective virus vectors reported so far.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant virus-based vectors have been regarded as a potential tool for the production of proteins of interest with a limited cost (Streatfield 2007). An outstanding advantage of the viral vector system is that it can express the heterologous proteins at exceedingly high levels within a short time frame as a result of viral replication (Hefferon 2017). Plant viruses were used to produce heterologous proteins in plants in the early 1980s (Giritch et al. 2017). The first plant viral vector was developed from Cauliflower mosaic virus (CaMV), in which its genome is a double-stranded (ds) DNA, to express a bacterial gene in plants (Brisson et al. 1984). Shortly afterward, the plant RNA viruses got attention in the field of virus-mediated expression systems. The relative abundance of available species and the ease of reverse genetic manipulation make RNA viruses a promising candidate to deliver the foreign gene into plants. Brome mosaic virus (BMV) is the first plant RNA virus that was engineered to be a virus-based gene vector in 1986 (French et al. 1986). Since then, various plant viruses from genera of RNA viruses were created into expression vectors, including the tobamoviruses (Takamatsu et al. 1987), potexviruses (Chapman et al. 1992), potyviruses (Dolja et al. 1992), comoviruses (Gopinath et al. 2000), bromoviruses (Ding et al. 2006), tombusviruses (Scholthof 1999), benyviruses (Schmidlin et al. 2005), and comoviruses (Zhao et al. 2000). However, the well-established systems based on viral RNA genomes are still limited. The viral vectors with the commercial potentials are confined to a few kinds of plant RNA viruses, such as Tobacco mosaic virus (TMV) (Lindbo 2007; Marillonnet et al. 2004; Marillonnet et al. 2005), Cowpea mosaic virus (CPMV) (Sainsbury and Lomonossoff 2008), and Potato virus X (PVX) (Komarova et al. 2006; Mardanova et al. 2017). Moreover, the accumulation levels of different recombinant proteins are totally different for a designated viral vector (Lindbo 2007), even when the most efficient and widely used TMV based-vector was employed. Furthermore, the expression of structural complex heterologous proteins, such as antibodies, requires the simultaneous expression of two or more subunits in the same cells. Using separate vectors possessing the same virus backbone is unable to efficiently express different polypeptides within single cells due to superinfection exclusion (Sainsbury et al. 2008). One of the approaches to overcome this limitation requires the co-inoculation of non-competitive plant viral vectors (Giritch et al. 2006; Mendoza et al. 2017). Hence, it is urgent for now to develop new and efficient plant virus-based systems.
Tomato bushy stunt virus (TBSV) belongs to the genus Tombusvirus within the family Tombusviridae (Yamamura and Scholthof 2005). The genome of TBSV is composed of a positive-sense single-stranded RNA (+ssRNA) of approximately 4.8 kb, which encodes five major open reading frames (ORFs) (Hearne et al. 1990). Translation of those ORFs adopts a cap- and poly(A) tail-independent strategy, which involves an RNA-RNA interaction between untranslated regions (UTRs) of the TBSV RNAs (Fabian and White 2004). The 5′-proximal ORFs (encoded P33 and P92) are translated by the genomic RNA (gRNA), and the products are crucial for replication of viral RNA or synthesis of subgenomic messenger RNAs (sg mRNAs) during infections (Oster et al. 1998), while the 3′-proximally encoded ORFs (P41, P22, and P19) are expressed via two sg mRNAs (Miller and Koev 2000). The capsid protein (CP) is translated from the internal p41 ORF on the larger 2.1-kb sg mRNA1 and is required for encapsidation of virus gRNA (Desvoyes and Scholthof 2002). Two nested ORFs for translation of the P22 and P19 are expressed via the smaller 0.9-kb sg mRNA2 (Hearne et al. 1990). The P22 protein is functional for cell-to-cell movement of TBSV (Chu et al. 1999), whereas P19 is an RNA-silencing suppressor (RSS) (Silhavy et al. 2002) and an elicitor of the hypersensitive response (HR) on particular Nicotiana species (Scholthof et al. 1995). Transcription of two sg mRNAs also relies on long-distance RNA-RNA interactions (Jiwan and White 2011). The cis-acting RNA elements, such as activator sequences (AS) and its partner receptor sequences (RS), distal element (DE) and proximal element (CE), involved in the long-distance interactions (Nicholson and White 2014). The partial sequences of DE and CE are located in the coding region of the CP gene (Lin and White 2004).
TBSV is a good candidate for modification as a viral expression vector because it possesses several potential advantages over the other plant RNA viruses. The size of about 4.8-kb genome is relatively smaller among the known plant RNA viruses that make it much easier for genetic manipulation. And TBSV is not an economically important pathogen and is absent of a known biological vector (Yamamura and Scholthof 2005) that reduced the risk of biological contamination. Another attractive aspect of TBSV is the P19 protein, which enhances gene expression levels as a powerful suppressor of gene silencing, whatever acting in cis or in trans. Moreover, numerous advances have been achieved in the molecular biology of TBSV in the past decade (White and Nagy 2004) that make it possible to generate TBSV to be a vector for the expression of the heterologous proteins. Indeed, it has been reported that the TBSV genome can be developed to be a gene expression vector. The early-developed TBSV vectors used pUC-derived cloning vector as backbone under control of T7 or CaMV 35 promoter and were delivered into host cells by mechanical inoculation with plasmid DNA or with in vitro transcripted infectious viral RNA (Scholthof 1999; Zhang et al. 2000). Only a few leaf cells can be inoculated by the method of directly rubbing nucleic acid onto host plants (Peyret and Lomonossoff 2015). Whereafter, a next-generation TBSV vector was developed, which can be inoculated through agroinfiltration on various plant species, such as Nicotiana benthamiana, cowpea, tomato, and lettuce (Shamekova et al. 2014; Mendoza et al. 2017; Zhumabek et al. 2018). However, the expression level from this agroinfection-compatible TBSV vector was lower than what is obtained with a TMV vector (Shamekova et al. 2014). And the infiltrated leaves of N. benthamiana started to be necrotic after 7 days due to the side effects of P19 (Mendoza et al. 2017). This unwanted side effect associated with the expression of P19 might interfere with the optimum expression of TBSV-derived expression vectors. Although this TBSV vector did not induce necrosis in inoculated leaves of the other tested plant species, the expression levels in those plants were much lower than in N. benthamiana (Mendoza et al. 2017). Therefore, the efficiency of the present TBSV-based vector should be improved. And alternative host plant, which can support high efficiently TBSV-mediated expression without the side effect of P19, should be investigated. In addition, the foreign proteins produced by present TBSV vectors were fused in-frame to the N-termini of CP, which needed an additionally laborious process to remove if required. And the gene fusion-expression approach also restricted the scope of the application of the TBSV vector, such as delivering the target proteins to the different subcellular organelles. Hence, it is necessary to generate a flexible and user-convenient TBSV-based vector to meet multiple purposes.
In this study, we engineered a TBSV-based fusion expression vector carrying the GFP reporter gene for investigating the possibility and potentiality of Nicotiana excelsiana as an alternative host of the TBSV vector. Their capabilities of infecting and expressing GFP in N. excelsiana or the toxigenic effect of P19 on inoculated N. excelsiana plants were analyzed by visual inspections and quantitative immunoblot assay. Then a flexible and user-friendly TBSV non-fusion vector was generated and assessed on N. excelsiana leaves. And the efficiency of TBSV vectors was further compared with the other two widely used plant viral vectors, TMV (TRBO-G) and PVX (PVXdt) vectors.
Materials and methods
Plant materials and growth conditions
The seeds of N. excelsiana were planted in 5-inch (in diameter) plastic pots filled with the sterilized soils. After germination, the young seedlings were maintained under conditions at 22–25 °C with 65% relative humidity and 16 h of light per day. All plants were well-watered when required and fed with standard Hoagland’s culture solution once a week.
Construction of TBSV-based vectors
All TBSV viral cDNA constructs were created with in vitro DNA synthesis and conventional restriction enzyme-mediated cloning.
A fragment, which successively contained ApaI restriction enzymes site (GGGCCC), 35S promoter of CaMV with double enhancers, the full-length cDNA sequence of TBSV cherry (GenBank accession number NC_001554), a hammerhead ribozyme cDNA sequence, CaMV transcription terminator, and PstI restriction enzymes site (CTGCAG), was synthesized in vitro (GenScript, Nanjing, China) and then was cloned into the mini binary vector pCB301 (Xiang et al. 1999) between the ApaI and PstI sites to generate pCB301-TBSV.
For the construction of the pTBSV-WY2.1 vector, an in vitro synthesized sequence, which included the TBSV cDNA region from nt 2480 to nt 2718 (including 5′ end NheI restriction enzymes site), FspI restriction enzymes site (TGCGCA), GFP gene sequence (GenBank accession number U57607) with an in-frame TAA stop codon, and AgeI restriction enzymes site (ACCGGT), was cloned into pCB301-TBSV vector between the NheI and AgeI sites. To engineer the pTBSV-WY2.1/delP19 vector that required P19 to be functionally inactive, single nucleotide mutation (C to T) was introduced in the coding region of P19 protein at the 157 position from the P19 start codon. This mutation was designed to change the glutamine codon to a stop codon, without altering the primary sequence of the overlapping P22. Then the TBSV cDNA region (including the single nucleotide mutation) between the NcoI and SalI sites was synthesized in vitro and replaced the NcoI-SalI-digested fragment of pTBSV-WY2.1.
To create TBSV vectors for non-fusion expression of heterologous proteins, the three in-frame AUGs at 5′ end of the TBSV CP gene were mutated or deleted as shown in Fig. 3a. The corresponding designed sequences were synthesized in vitro respectively and substituted the NheI-AgeI-digested fragment of pTBSV-WY2.1 as described above.
For the construction of sg1-P9, sg1-P16, sg1-P24, and Pl-P9 vector, fragments, which successively contained PacI restriction enzymes site (TTAATTAA), the corresponding designed sequences (Fig. 4a), the full length of GFP gene sequence, and AgeI restriction enzymes site (ACCGGT), were synthesized in vitro respectively and then cloned into dAUG vector between the PacI and AgeI sites one by one to generate the four vectors as mentioned above.
Agroinfiltration of N. excelsiana leaves
The recombinant plasmids were transformed into competent Agrobacterium tumefaciens cells of strain GV3101 according to procedures of the freeze-thaw method, and then transformed cells were plated on LB agar plate with appropriate antibiotics for plasmid selection. A PCR-based method was used for identifying the positive recombinant colony. Selected A. tumefaciens colony was transferred into 4 mL of LB liquid medium containing appropriate antibiotics and then incubated (28 °C and 250 rpm shaking) for 24 h. After adding another 100 mL of LB medium (containing 200 μM acetosyringone), the cultured cells were kept growing overnight under the same condition. Cells harvested by centrifugation at 4000 g were suspended and adjusted to an OD600 of 1.0 by using MES buffer (pH 5.6, 10 mM MgCl2, and 200 μM acetosyringone). The cell suspensions were put into the dark chamber and allowed to stand at room temperature for 2 h just before infiltration. After treatment for 2-h standing, the suspensions were infiltrated into N. excelsiana leaves via a needleless syringe. The infiltrated leaves were sampled on the date, and the harvested leaves were stored in a − 80 °C refrigerator until use.
GFP imaging
GFP signals were observed in infiltrated leaves under irradiation by a handheld UV lamp. And all of the pictures of images were taken in the darkened room by the use of a digital camera.
Protein extraction
Leaf tissue samples were frozen in liquid nitrogen and ground to a fine powder. 0.1 g of leaf powder was then mixed with 400 μL of extraction buffer (50 mM Tris-HCl, 150 mM NaCl, 10 mM EDTA, pH 8.0). The tissue homogenates were centrifuged at 12,000 g for a total of 15 min at 4 °C. The supernatant containing total soluble proteins was transferred to a new tube and stored at 4 °C until use.
SDS-PAGE and western blot analysis
Protein samples were heated at 95 °C for about 5 min and then loaded on a 12% polyacrylamide gel for protein separation. After electrophoresis (1 h at 180 V), the gel was stained overnight in the solution of Coomassie brilliant blue G-250 with gently shaking. For western blot assays, a semi-dry electrophoresis transfer (Bio-Rad, Shanghai, China) was used to transfer the proteins form SDS gel to a 0.45 μm nitrocellulose (NC) membrane (Sigma-Aldrich, Shanghai, China). Following an overnight-block with PBS made 5% non-fat dry milk, strips from the blot were developed by rabbit polyclonal anti-GFP antibody (Abcam, Shanghai, China) with 1:1000 dilutions, next by 1:5000 goat anti-rabbit HRP conjugated secondary antibody (Sigma-Aldrich, Shanghai, China). Specific protein bands were visualized after the treatment of the strips with ECL solution (GE Healthcare, Shanghai, China).
GFP quantification assay
Enzyme-linked immunosorbent assay (ELISA) was applied to measure the accumulation of recombinant GFP in the inoculated leaves. ELISA plate filled with 100 μL of protein samples per well was incubated overnight at 4 °C for antigen coating. Following washing procedures (three times and 5 min for each), each well of plate was added 100 μL of 1:10,000 rabbit polyclonal anti-GFP antibody, and then the plate was put into an incubator at 37 °C for 2 h. Next, 100 μL of 1:5000 secondary antibody (HRP conjugated goat anti-rabbit antibody) was added into the carefully washed plate. After a 2-h incubation and a washing step, each plate well was refilled with 100 μL of tetramethylbenzidine and incubated for 15–30 min. After that, phosphoric acid (1.0 M) was pipetted into wells to stop the reaction. A microplate reader (Bio-Rad, Shanghai, China) was employed to read optical density (OD) value at a wavelength of 450 nm. Meanwhile, E. coli-produced GFP (BioVision, San Francisco, USA) was used to generate an ELISA standard curve. Then the standard curve was utilized for calculating the GFP accumulation in the collected leaf samples. The observed values from three independent experiments were performed with Student’s t test, and a value of p < 0.05 was considered significant.
RNA extraction and northern blot analysis
Total RNAs of N. excelsiana were extracted from 0.1 g of fresh tissue using the RNAprep Pure Plant Kit (TIANGEN, Beijing, China) according to instructions. Equal aliquots of samples were separated in 1.2% agarose gel. The gels were stained to ensure that the samples were loaded evenly. RNAs were then transferred to NC membrane and followed by northern blot analysis using PCR digoxigenin (DIG)-labeled probe, which was complementary to the 3′ terminus of the viral genome. The viral RNAs were showed up by DIG Luminescent Detection Kit (Roche, Shanghai, China) according to instructions. The blot images were captured with a digital camera, and the relative levels of viral RNAs were determined by densitometry using the Quantity One analysis software (Bio-Rad, Hercules, CA).
Results
Description of TBSV vectors for fusion expression of heterologous proteins
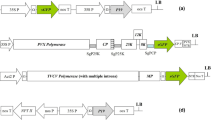
The infectious clone of pCB301-TBSV was initially developed and then was further modified to be functional as agroinfection-compatible expression vectors. Since the product of the CP gene is not necessary for efficient infection of tobacco plants by wild-type TBSV (Scholthof et al. 1993; Russo et al. 1994), GFP reporter gene was chosen to replace the middle part of the CP gene (nt 2718 to 3750) to take advantage of high production capacity of the viral CP. As mentioned before, the CP is produced through TBSV subgenomic mRNA1 (sgRNA1). The flanking sequences of the CP coding regions were proved to be essential for itself for translation and to be crucial for the efficient transcription of subgenomic mRNA2 (sgRNA2), which is the template for the p22 and p19 gene translation. Therefore, the 31 nts of 5′-UTR (including 5′-GACCAAG sequence required for sgRNA1translation), the initial 63 nts (containing the intact DE cis-element), and the last 69 nts (having the complete CE cis-element) of the CP coding sequence were maintained to preserve the RNA structures, which are critical for CP translation and sgRNA2 production. Thus, a TBSV-derived vector-denominated pTBSV-WY2.1 (Fig. 1a), in which GFP was fused in-frame with 21 extra amino acids of the N-terminal of CP, was generated based on the infectious clone of pCB301-TBSV.
Schematic representation of the construction of Tomato bushy stunt virus (TBSV)-based expression vectors and RNA-silencing suppressor expression vector. TBSV coding regions are represented as boxes with approximate molecular masses (in kDa) prefixed with “p.” The coat protein (CP) region from nt 97 to nt 1129 of subgenomic mRNAs1 was replaced by the GFP (green). The vectors contain the native initiation codon of the CP gene and allow expression of GFP fused in-frame with the N-terminal 27 amino acids of CP. (a) pTBSV-WY2.1, a TBSV-based vector with wild-type P19. P35S, enhanced Cauliflower mosaic virus (CaMV) 35S promoter; p33 and p92, the gene coding replication proteins, which involved in genome replication and subgenomic mRNAs transcription; sg1 and sg2, the transcription initiation sites for subgenomic mRNAs 1 and 2 indicated by right-angled arrows; gfp, enhanced green fluorescent protein gene; p19, silencing suppressor gene; p22, cell-to-cell movement protein (MP) gene; Rz, ribozyme; T35S, CaMV polyA signal sequence/terminator; NdeI, FspI, and AgeI, restriction enzymes sites. (b) pTBSV-WY2.1/delP19, a TBSV-based vector with inactivated p19 gene. (c) pCBNox P19, a silencing suppressor expression vector. TE, tCUP translational enhancer; p19, the gene of silencing suppressor P19 from TBSV. Note that boxes are not drawn to scale, and the backbone regions of vectors are not shown on the maps
TBSV-encoded P19 is a multifunctional pathogenicity protein (Scholthof 2006). Besides its function as an RNA-silencing suppressor (Chiba et al. 2006), P19 is also vital for persistent infections of TBSV because viral RNAs will be eliminated from infected leaves in its absence within 2 weeks (Scholthof 2006). However, the systemic necrosis was observed for certain Nicotiana species along with TBSV expressing P19 (Yamamura and Scholthof 2005). N. excelsiana was used as host plants in this study, but no evidence was yet available that the P19 protein would not induce the toxigenic effect on it. Thus, another modified vector with an inactive p19 gene, named pTBSV-WY2.1/delP19 (Fig. 1b), was created based on pTBSV-WY2.1.
Examining the capability of N. excelsiana as a host plant for TBSV-based vector
Fully expanded leaves of N. excelsiana were infiltrated with A. tumefaciens cultures carrying an empty vector (negative control, Mock), pTBSV-WY2.1 (P19 acting in cis, cis P19), pTBSV-WY2.1/delP19 (absence of P19, del P19), or a mixture of pTBSV-WY2.1/delP19 and pCBNox P19 (P19 acting in trans, trans P19). Agroinfiltration zones for each inoculum are shown in Fig. 2a. The infiltrated N. excelsiana leaves were observed under UV illumination for GFP expression at 4–8 days post-infiltration (dpi). The green signals were easily visualized in each infiltrated zone with TBSV-based vectors even with the room lights turned on, whereas zones infiltrated with the empty vector were not able to observe any fluorescence (Fig. 2b), indicating successful infection of these viral constructs in this tobacco species. Afterward, expressed GFP was confirmed by both SDS-PAGE and western blot assay (Fig. 2c, upper and middle panel). In addition, the intensity of GFP fluorescence was greatly enhanced overtime throughout the infiltrated area with P19 expression, whatever it acted in cis or in trans (Fig. 2b). In contrast, the fluorescence signals were decreased dramatically within the infiltrated area in the absence of P19. SDS-PAGE and western blot assay revealed the same temporal variations of GFP protein as that of fluorescence intensity (Fig. 2c, upper and middle panel). Furthermore, P19 protein was detected using immunoblotting in leaf samples infiltrated with inoculum carrying pTBSV-WY2.1 (cis P19) or pCBNox P19 (trans P19) but not in leaves inoculated with the empty vector (Mock) or pTBSV-WY2.1/delP19 (del P19) (Fig. 2c, bottom panel). Collectively, these results declared that the presence of P19 could enhance and prolong the TBSV-mediated heterologous expression. The expression of GFP was then quantified using GFP ELISA. The accumulation of GFP reached 3.0–5.0 mg/g fresh leaf at 8 dpi in the presence of P19 (Fig. 2d), indicating that the TBSV vector can overexpress heterologous proteins in N. excelsiana plants.
The effect of P19 on the expression of recombinant GFP proteins by TBSV-based vectors in N. excelsiana. (a) Schematic representation of the agroinfiltrated zones in N. excelsiana leaf. MOCK, infiltrated by pCB301 empty vector; del P19, infiltrated by pTBSV-WY2.1/delP19 vector; trans P19, co-infiltrated by pTBSV-WY2.1/delP19 and pCBNox P19 vector; cis P19, infiltrated by pTBSV-WY2.1 vector. (b) GFP expression pattern (under UV light) of agroinfiltrated N. excelsiana leaves at 4, 6, and 8 days post-inoculation (dpi), respectively. (c) SDS-PAGE analysis and immunodetection of proteins isolated from the agroinfiltrated zones of N. excelsiana leaves. The gel was stained with Coomassie brilliant lue (upper panel), and the corresponding antibodies were used to detect GFP (middle panel) and TBSV P19 (lower panel) in agroinfiltrated N. excelsiana leaves by western blots. M, molecular weight marker (kDa); del, tra, and cis, total soluble protein extracts from the corresponding agroinfiltrated zones of N. excelsiana leaves in (a). (d) Time course for the accumulation of recombinant GFP protein in N. excelsiana leaves by ELISA. (e) Zoomed-in view of GFP expression in TBSV-infected N. excelsiana leaves with cis- or trans-P19 observed under UV light at 10 dpi. White arrows indicate GFP fluorescent spots that were derived by recombinant TBSV cell-to-cell movement. (f) The photograph of inoculated N. excelsiana plants showing GFP fluorescence under UV light at 14 dpi
GFP fluorescence was observed in uninoculated areas around the border of the inoculated zone on the leaves since 6 dpi (Fig. 2b), indicating the ability of cell-to-cell movement of TBSV vectors in N. excelsiana leaves. Fluorescence signals generated by the viral cell-to-cell movement were faint with the P19-defected vector, while they were bright with the P19 functioned vector s (Fig. 2e). Those observations indicated that the viral vector, accompanied by a cis-acting silencing suppressor P19, may be more efficient and persistent than the other trans-acting one. Moreover, the GFP signal was never observed in the upper leaves (not infiltrated) until 14 dpi (Fig. 2f), showing the absence of virus systemic movement during the timespan of our experiments. This local response was expected because the CP is required for effective long-distance movement of TBSV (Scholthof et al. 1993). Interestedly, no symptoms were observed in the infiltrated zones (Fig. 2b) or whole plant of N. excelsiana (Fig. 2f) until 14 dpi, even in the presence of P19, demonstrating that P19 did not induce the hypersensitive resistance response (HR) on this tobacco species.
Since the TBSV vector can successfully infect and overexpress heterologous proteins in the N. excelsiana plant without the necrotic effects of the P19 protein, we concluded that N. excelsiana is more suitable for TBSV-mediated expression systems than another popularly used Nicotiana species, N. benthamiana, and offers a promising alternative for virus-based expression system.
Engineering of TBSV as a non-fusion expression vector
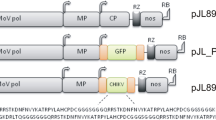
Considering the limitation of the first-generation TBSV vectors, non-fusion expression vectors were created based on pTBSV-WY2.1by mutation or deletion of three in-frame AUGs at 5′ end of the CP fusion sequence and by addition of PacI restriction enzyme site following a start codon (AUG), where GFP translation is expected to initiate (Fig. 3a). In brief, the third in-frame AUG was mutated to ACG in all expression cassettes, while the first and the second in-frame AUG was mutated to AGG or UAG or was deleted to generate m1AUG, m2AUG, and dAUG vector, respectively (Fig. 3a). Following transformation into A. tumefaciens, the constructs were agroinfiltrated into N. excelsiana leaves (Fig. 3b), and the expression levels of GFP were assessed.
Development of “non-fusion” TBSV expression vectors. (a) The nucleic acid sequences of 5′ end of the expression cassettes. The AUGs and mutated AUGs are indicated in red, and the GFP sequence is shown as green. (b) Schematic representation of the agroinfiltrated zones in N. excelsiana leaf. (c) GFP expression pattern (under UV light) of agroinfiltrated N. excelsiana leaves at 4, 6, and 8 days post-inoculation (dpi), respectively. (d) SDS-PAGE analysis and immunodetection of recombinant GFP proteins isolated from the agroinfiltrated zones of N. excelsiana leaves at 6 dpi. The gel was stained with Coomassie brilliant blue (upper panel), and the GFP antibodies were used to detect GFP (lower panel) in agroinfiltrated N. excelsiana leaves by western blots. M, molecular weight marker (kDa); H, non-inoculated plant leaves; WY2.1, m1AUG, m2AUG, and dAUG, total soluble protein extracts from the corresponding agroinfiltrated zones of N. excelsiana leaves in (b); S, GFP reference standard. (e) GFP accumulation in infiltrated leaves at 6 dpi measured by ELISA. (f) Northern blots analysis of TBSV RNA accumulation in WY2.1- or dAUG-infected leaf areas at 6 dpi. The relative values below the lanes correspond to means (± standard deviations, SD) from three independent experiments and were normalized to the accumulation level of the corresponding viral RNAs of the WY2.1, set at 100. EtBr-stained rRNAs (lower panel) are shown as loading controls
Observation of leaf under UV illumination at 4–8 dpi showed that the fluorescence of the inoculated zones of non-fusion vectors (m1AUG, m2AUG, and dAUG) was less than that of fusion vector pTBSV-WY2.1 (WY2.1), indicating a decrease in GFP expression along with non-fusion vectors (Fig. 3c). By contrast, the deletion construct dAUG, in which the first two in-frame AUGs were removed, appeared to be more efficient than the mutated constructs m1AUG and m2AUG, in which the first two in-frame AUGs were mutated to AGG or UAG (Fig. 3c). These findings were confirmed by analysis of protein samples at 6 dpi using SDS-PAGE and western blot assay (Fig. 3d). The relative level of GFP expression was determined by ELISA assay, and approaching 3.0 mg/g GFP of fresh-weight tissue was obtained at 6 dpi when using non-fusion vector dAUG (Fig. 3e). Although the non-fusion vector dAUG was not as efficient as the fusion vector pTBSV-WY2.1 in terms of GFP accumulation, it indeed provided a more flexible tool to produce heterologous non-fusion proteins.
To determine whether the variation in expression between fusion and non-fusion vectors is due to the modification of the DE element, northern blot assay was applied to detect the viral RNAs in the infiltrated leaf of the pTBSV-WY2.1 and dAUG constructs. The levels of viral RNAs (g RNA, sg mRNA1, and sg mRNA2) at 6 dpi did not vary significantly between the two kinds of constructs (Fig. 3f). The result indicates that the expression variation is not due to the change of viral RNAs accumulation, but rather to the different efficiency of protein translation.
One exploration of inefficient GFP expression of the non-fusion vectors might be the artificially generated start codon located downstream of the PacI restriction enzyme sequence (TTAATTAA), which is not an optimal context in theory for initiation of translation. This led us to construct the other vectors, in an attempt to improve the GFP expression (Fig. 4a). The sg1-P9, sg1-P16, and sg1-P24 vector carry the original sequences just before the CP start codon with different lengths, which were regarded as favorable context sequence for yielding high levels of CP. The Pl-P9 vector contains the optimum context sequence (AAAACAACA) of translation initiation codon in dicots (Rangan et al. 2008). All selected sequences were inserted between PacI restriction enzyme sequence and GFP start codon (Fig. 4a). GFP fluorescence was visualized in the zones infiltrated with inoculum carrying various vectors (Fig. 4b and c), but it did not reveal a significant difference between the newly generated constructs. The result was confirmed by SDS-PAGE detection, immunoblot analysis, as well as ELISA assay (Fig. 4d and e). Those data suggested that the position of CP initiation codon instead of its context sequence may be more important for efficient protein expression.
The evaluation of the context sequence of the GFP translation initiation codon on transient expression of GFP in N. excelsiana leaves. (a) The nucleic acid sequences around the GFP start codon of the various expression cassettes. The PacI restriction enzyme site is indicated in underline and the GFP start codon and GFP sequence are shown as green. (b) Schematic representation of the agroinfiltrated zones in N. excelsiana leaf. (c) GFP expression pattern (under UV light) of agroinfiltrated N. excelsiana leaves at 4, 6, and 8 days post-inoculation (dpi), respectively. (d) SDS-PAGE analysis and immunodetection of recombinant GFP proteins isolated from the agroinfiltrated zones of N. excelsiana leaves at 6 dpi. The gel was stained with Coomassie brilliant blue (upper panel), and the GFP antibodies were used to detect GFP (lower panel) in agroinfiltrated N. excelsiana leaves by western blots. M, molecular weight marker (kDa); H, non-inoculated plant leaves; WY2.1, dAUG, sg1-P9, sg1-P16, sg1-P24, and Pl-P9, total soluble protein extracts from the corresponding agroinfiltrated zones of N. excelsiana leaves in (b). (e) GFP accumulation in infiltrated leaves at 6 dpi measured by ELISA.
Comparing the efficiency of TBSV vectors with other viral vectors
The expression levels of GFP were further compared with TMV (TRBO-G) (Lindbo 2007) and PVX (PVXdt) vectors (Komarova et al. 2006). Both TMV and PVX vectors resulted in strong GFP signals with co-infiltration of P19 RNA-silencing inhibitor, while the fluorescence signal in two TBSV vectors was similar to that of TMV and PVX vectors (Fig. 5a and b). The TMV and TBSV vectors have shown cell-to-cell movement ability on the inoculated zones but not for the PVX vector (Fig. 5b). The observation can be expected because the triple gene block and coat protein gene in the PVX vector were substituted by the GFP gene. Furthermore, the soluble proteins were extracted from infiltrated leaves, and expressed GFP was detected by SDS-PAGE and western blots analysis (Fig. 5c). The GFP expression of dAUG vector was equivalent to that of TMV and PVX vectors; pTBSV-WY2.1 expression was higher than that of two viral vectors.
Comparing the efficiency of TBSV-based expression vectors with TMV- and PVX-based vector for transient expression of recombinant GFP in N. excelsiana leaves. (a) Schematic representation of the agroinfiltrated zones in N. excelsiana leaf. (b) GFP expression pattern (under UV light) of agroinfiltrated N. excelsiana leaves at 4, 6, and 8 days post-inoculation (dpi), respectively. (c) SDS-PAGE analysis and immunodetection of recombinant GFP proteins isolated from the agroinfiltrated zones of N. excelsiana leaves at 4 dpi and 8 dpi. The gel was stained with Coomassie brilliant blue (upper panel), and the GFP antibodies were used to detect GFP (lower panel) in agroinfiltrated N. excelsiana leaves by western blots. M, molecular weight marker (kDa); H, non-inoculated plant leaves; TMV, PVX, WY2.1, and dAUG, total soluble protein extracts from the corresponding agroinfiltrated zones of N. excelsiana leaves in (a). (d) GFP accumulation in infiltrated leaves at 4 dpi and 8 dpi measured by ELISA
To further support the above results, GFP from various vectors was quantified by ELISA in comparison with GFP standard curves. The amount of GFP produced per gram fresh leaf was determined. GFP was expressed by pTBSV-WY2.1 at levels of approaching 5.0 mg/g fresh leaf at 8 dpi, while dAUG was about 3.0 mg/g fresh leaf, similar to that of TRBO-G and PVXdt (Fig. 5d).
Discussion
The host plant is one of the critical factors for the successful establishment of a transient expression system. N. benthamiana is currently utilized as a fundamental host for virus-mediated protein production owing to its high susceptibility to A. tumefaciens and most of the plant viruses (Goodin et al. 2008). However, the apical necrosis and subsequently systemic lethal collapse were observed upon the TBSV-infected N. benthamiana plants (Scholthof et al. 1995). The P19 protein was proved to be the major component, which was responsible for eliciting the systemically lethal collapse, although other factors may involve in. Inactivation of P19 led to an amelioration of virus-induced symptoms in N. benthamiana, but this also reduces the rate of systemic invasion, and viral RNAs will be eliminated from infected leaves within 2 weeks. A mutation version of the P19 protein (P19/R43W) largely reduces the necrotic symptom during TBSV infection (Chu et al. 1999) while maintaining its function as an RNA-silencing suppressor (Saxena et al. 2011). However, the ability of this harmless suppressor to enhance transient expression appeared to be about half as much when the wt-P19 was used (Saxena et al. 2011). Moreover, some other characteristics of N. benthamiana, such as relatively low biomass and not growing well in the outdoor field, hamper its application in the plant molecular pharming (Smith et al. 2009). Therefore, the change of the host provides a promising way to improve the TBSV-based expression system. Theoretically, an ideal host for the TBSV vectors should own the aspects of high-level expression, easy growth in designated growth conditions, and reduction of harmful effects of P19. But none of the previously tested plant species can yet meet the requirements as an ideal host for the TBSV vector. N. excelsiana, which is a hybrid species of N. benthamiana and Nicotiana excelsior, produces more biomass in the field than N. benthamiana (Shamloul et al. 2014). N. excelsiana supported the large-scale production of heterologous proteins, and the expression levels of GFP with TMV-based vectors were comparable with that of N. benthamiana (Shamloul et al. 2014). To examine the capability of N. excelsiana as host of TBSV-based vector and figure out whether or not the necrotic effects of P19 will happen, we constructed two CP replacement TBSV vectors for fusion expression of reporter protein (Fig. 1). The p19 gene is active in one construct and is inactive in another one by a single nucleotide mutation, in which its function can be restored by in trans co-expression of P19. Successful infection of TBSV vectors was established in N. excelsiana (Fig. 2b). And no necrosis was observed in the infiltrated zones (Fig. 2b) or the whole plant of N. excelsiana (Fig. 2f) until 14 dpi, even in the presence of P19. TBSV vectors also can overexpress heterologous proteins in N. excelsiana leaves (Fig. 2d). Our current results demonstrate that N. excelsiana is an ideal host for TBSV-mediated protein production because of well-supporting for infiltration, high-level production, and none of P19-induced HR. Additionally, we also observe that the presence of P19 can enhance and prolong the TBSV-mediated heterologous expression (Fig. 2b and e), and the better result was obtained from the one with cis-acting P19.
The yield and quality of the final product in terms of functionality are two major principles that should be considered for a successful expression system. The final location of target proteins within the host plant cells has influential effects for the extent of expression level, integrity, stability, proper folding, and posttranslational processing (Di Fiore et al. 2002). Thus, targeting strategies have been applied to improve the expression of a target protein and to control posttranslational modifications in plant bioreactors (Egelkrout et al. 2012). An appropriate signal sequence can direct the target protein to a particular cellular compartment (Emanuelsson et al. 2007). Generally, the commonly used signal sequence is located at the C- or N-terminus of its guided proteins, especially at N-terminus (Arnau et al. 2006). The previously reported TBSV vectors have no such ability to guide the target proteins to subcellular compartments because the heterologous gene was fused in-frame to the N-termini of the CP gene. In addition, the removal of the CP fusion from recombinant proteins is usually necessary because it may interfere with the functional properties of the recombinant proteins (Terpe 2003). The fusion part was typically removed by the usage of proteases, which recognize and cleave the specific peptide bond in the fusion proteins (Frey and Görlich 2014). However, most of them leave extra undesirable residues on the target protein after cleavage (Lichty et al. 2005). The other disadvantages of proteases-mediated cleavage are low efficiency, need for conditions optimization, the expense of proteases, and difficulty for recovering the intact proteins (Arnau et al. 2006). This additional process also increases the cost of the final product. We attempted to overcome this limitation of the TBSV vector in this study. The results demonstrate that the TBSV vectors can be constructed to be a non-fusion expression vector. This non-fusion virus vector allowed high-level expression of the reporter gene, and the expression level was equivalent to that of the other two highly effective virus vectors. Although the non-fusion vector was not as efficient as the fusion vector, it broadens the range of application of TBSV vectors.
In a side-by-side comparison, it is interesting that the expression capacity of the TBSV fusion vector described here is more than that of the TMV vector, TRBO. This is in sharp contrast to the previously reported TBSV vector, by which the level of GFP expression was lower than what is obtained with the same TMV vector (Mendoza et al. 2017). Two TBSV vectors share the same T-DNA backbone, and both are based on the TBSV cherry strain. Furthermore, they are both coat protein gene replacement vector carrying GFP reporter gene, and all are delivered to plant by A. tumefaciens strain GV3101. One difference between these two vectors is the site used to insert a foreign gene. The 5′ end of GFP in the older TBSV vector and our fusion vector is fused with 17 (Mendoza et al. 2017) and 21 remnant amino acids of the CP N-terminal end, respectively. Theoretically, this subtle difference may not lead to the alteration of the GFP expression level because the DE element has not been destroyed. In the older TBSV vector, the 3′ end of GFP is located at ~ 150 bp upstream of the start codon of the p22 gene (Shamekova et al. 2014), while it is located at 100 bp upstream in our fusion TBSV vector. The non-translated CP gene sequences, which are located downstream of GFP, were regarded to be essential for the efficiency of the TBSV vector because it provided the opportunity for CP restoration (Shamekova et al. 2014). Our results also demonstrated that the length of those redundant sequences keeping in the TBSV vector did have a significantly negative effect on GFP expression, and the shorter one showed the higher level of GFP expression (data not shown). Thus, we speculated that the extra ~ 50 bp CP RNA nucleotides might be responsible for the expression reduction of the older TBSV vector. Another difference is the host plant that used to evaluate the efficiency of viral vectors. Our vector seems to be more efficient than the older TBSV vector probably because N. excelsiana is more suitable than N. benthamiana as host for TBSV-mediated recombinant protein production.
The restrictions on insert size are one of the disadvantages of plant virus RNA-based vectors reported so far. People may be concerned that for the TBSV-based vector, the expression of larger proteins may be limited because of its small genome size (~ 4.8 kb). Although we did not define the insert size constraints of the TBSV-derived vector in this study, other studies have shown that it can express proteins as large as 1,4-β-endoglucanase E1 (~ 1.6 kb) (Makenova et al. 2015). Therefore, the TBSV-based vector is probably not inferior to those existing expression vectors in terms of gene-carrying capacity.
In this study, by reasonable redesign of the TBSV genome and the employment of an alternative host N. excelsiana, we have significantly improved the TBSV-based transient expression system. The newly developed TBSV expression systems do not have the unwanted necrotic effects caused by viral P19 protein. Expression levels are similar to that of the other two highly effective virus vectors. Additionally, the non-fusion viral vector provides more flexible aspects than the previously developed TBSV vectors. Our TBSV RNA-based system added an effectively new member to the family of plant viral expression vectors and offered an alternative and flexible approach for producing proteins of interest in plants.
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Arnau J, Lauritzen C, Petersen GE, Pedersen J (2006) Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr Purif 48:1–13. https://doi.org/10.1016/j.pep.2005.12.002
Brisson N, Paszkowski J, Penswick JR, Gronenborn B, Potrykus I, Hohn T (1984) Expression of a bacterial gene in plants by using a viral vector. Nature 310:511–514. https://doi.org/10.1038/310511a0
Chapman S, Kavanagh T, Baulcombe D (1992) Potato Virus-X as a vector for gene-expression in plants. Plant J 2:549–557. https://doi.org/10.1046/j.1365-313X.1992.t01-24-00999.x
Chiba M, Reed JC, Prokhnevsky AI, Chapman EJ, Mawassi M, Koonin EV, Carrington JC, Dolja VV (2006) Diverse suppressors of RNA silencing enhance agroinfection by a viral replicon. Virology 346:7–14. https://doi.org/10.1016/j.virol.2005.09.068
Chu M, Park JW, Scholthof HB (1999) Separate regions on the tomato bushy stunt virus p22 protein mediate cell-to-cell movement versus elicitation of effective resistance responses. Mol Plant-Microbe Interact 12:285–292. https://doi.org/10.1094/MPMI.1999.12.4.285
Desvoyes B, Scholthof HB (2002) Host-dependent recombination of a Tomato bushy stunt virus coat protein mutant yields truncated capsid subunits that form virus-like complexes which benefit systemic spread. Virology 304:434–442. https://doi.org/10.1006/viro.2002.1714
Di Fiore S, Li Q, Leech MJ, Schuster F, Emans N, Fischer R, Schillberg S (2002) Targeting tryptophan decarboxylase to selected subcellular compartments of tobacco plants affects enzyme stability and in vivo function and leads to a lesion-mimic phenotype. Plant Physiol 129:1160–1169. https://doi.org/10.1104/pp.010889
Ding XS, Schneider WL, Chaluvadi SR, Mian MR, Nelson RS (2006) Characterization of a Brome mosaic virus strain and its use as a vector for gene silencing in monocotyledonous hosts. Mol Plant-Microbe Interact 19:1229–1239. https://doi.org/10.1094/MPMI-19-1229
Dolja VV, McBride HJ, Carrington JC (1992) Tagging of plant potyvirus replication and movement by insertion of beta-glucuronidase into the viral polyprotein. Proc Natl Acad Sci U S A 89:10208–10212. https://doi.org/10.1073/pnas.89.21.10208
Egelkrout E, Rajan V, Howard JA (2012) Overproduction of recombinant proteins in plants. Plant Sci 184:83–101. https://doi.org/10.1016/j.plantsci.2011.12.005
Emanuelsson O, Brunak S, Von Heijne G, Nielsen H (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc 2:953–971. https://doi.org/10.1038/nprot.2007.131
Fabian MR, White KA (2004) 5′-3′ RNA-RNA interaction facilitates cap-and poly (A) tail-independent translation of tomato bushy stunt virus mRNA A potential common mechanism for tombusviridae. J Biol Chem 279(28):28862–28872. https://doi.org/10.1074/jbc.M401272200
French R, Janda M, Ahlquist P (1986) Bacterial gene inserted in an engineered RNA virus: efficient expression in monocotyledonous plant cells. Science 231:1294–1297. https://doi.org/10.1126/science.231.4743.1294
Frey S, Görlich D (2014) A new set of highly efficient, tag-cleaving proteases for purifying recombinant proteins. J Chromatogr A 1337:95–105. https://doi.org/10.1016/j.chroma.2014.02.029
Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J, Klimyuk V, Gleba Y (2006) Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci U S A 103:14701–14706. https://doi.org/10.1073/pnas.0606631103
Giritch A, Klimyuk V, Gleba Y (2017) 125 years of virology and ascent of biotechnologies based on viral expression. Cytol Genet 51:87–102. https://doi.org/10.3103/S0095452717020037
Goodin MM, Zaitlin D, Naidu RA, Lommel SA (2008) Nicotiana benthamiana: its history and future as a model for plant-pathogen interactions. Mol Plant-Microbe Interact 21:1015–1026. https://doi.org/10.1094/MPMI-21-8-1015
Gopinath K, Wellink J, Porta C, Taylor KM, Lomonossoff GP, van Kammen A (2000) Engineering cowpea mosaic virus RNA-2 into a vector to express heterologous proteins in plants. Virology 267:159–173. https://doi.org/10.1006/viro.1999.0126
Hearne PQ, Knorr DA, Hillman BI, Morris TJ (1990) The complete genome structure and synthesis of infectious RNA from clones of tomato bushy stunt virus. Virology 177:141–151. https://doi.org/10.1016/0042-6822(90)90468-7
Hefferon K (2017) Plant virus expression vectors: a powerhouse for global health. Biomedicines 5:44. https://doi.org/10.3390/biomedicines5030044
Jiwan SD, White KA (2011) Subgenomic mRNA transcription in Tombusviridae. RNA Biol 8:287–294. https://doi.org/10.4161/rna.8.2.15195
Komarova TV, Skulachev MV, Zvereva AS, Schwartz AM, Dorokhov YL, Atabekov JG (2006) New viral vector for efficient production of target proteins in plants. Biochem Mosc 71:846–850. https://doi.org/10.1134/S0006297906080049
Lichty JJ, Malecki JL, Agnew HD, Michelson-Horowitz DJ, Tan S (2005) Comparison of affinity tags for protein purification. Protein Expr Purif 41:98–105. https://doi.org/10.1016/j.pep.2005.01.019
Lin HX, White KA (2004) A complex network of RNA-RNA interactions controls subgenomic mRNA transcription in a tombusvirus. EMBO J 23:3365–3374. https://doi.org/10.1038/sj.emboj.7600336
Lindbo JA (2007) TRBO: a high-efficiency tobacco mosaic virus RNA-based overexpression vector. Plant Physiol 145:1232–1240. https://doi.org/10.1104/pp.107.106377
Makenova AT, Scholthof HB, Ramankulov EM, Manabayeva SA (2015) Transient expression of Acidothermus cellulolyticus endoglucanase E1 by a Tomato bushy stunt virus-based plant expression vector. J Biotechnol 208:S29–S30. https://doi.org/10.1016/j.jbiotec.2015.06.080
Mardanova ES, Blokhina EA, Tsybalova LM, Peyret H, Lomonossoff GP, Ravin NV (2017) Efficient transient expression of recombinant proteins in plants by the novel pEff vector based on the genome of potato virus X. Front Plant Sci 8:247. https://doi.org/10.3389/fpls.2017.00247
Marillonnet S, Giritch A, Gils M, Kandzia R, Klimyuk V, Gleba Y (2004) In planta engineering of viral RNA replicons: efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc Natl Acad Sci U S A 101:6852–6857. https://doi.org/10.1073/pnas.0400149101
Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, Gleba Y (2005) Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat Biotechnol 23:718–723. https://doi.org/10.1038/nbt1094
Mendoza MR, Payne AN, Castillo S, Crocker M, Shaw BD, Scholthof HB (2017) Expression of separate proteins in the same plant leaves and cells using two independent virus-based gene vectors. Front Plant Sci 8:1808. https://doi.org/10.3389/fpls.2017.01808
Miller WA, Koev G (2000) Synthesis of subgenomic RNAs by positive-strand RNA viruses. Virology 273:1–8. https://doi.org/10.1006/viro.2000.0421
Nicholson BL, White KA (2014) Functional long-range RNA-RNA interactions in positive-strand RNA viruses. Nat Rev Microbiol 12:493–504. https://doi.org/10.1038/nrmicro3288
Oster SK, Wu B, White KA (1998) Uncoupled expression of p33 and p92 permits amplification of tomato bushy stunt virus RNAs. J Virol 72:5845–5851. https://doi.org/10.1128/JVI.72.7.5845-5851.1998
Peyret H, Lomonossoff GP (2015) When plant virology met Agrobacterium: the rise of the deconstructed clones. Plant Biotechnol J 13:1121–1135. https://doi.org/10.1111/pbi.12412
Rangan L, Vogel C, Srivastava A (2008) Analysis of context sequence surrounding translation initiation site from complete genome of model plants. Mol Biotechnol 39:207–213. https://doi.org/10.1007/s12033-008-9036-9
Russo M, Burgyan J, Martelli GP (1994) Molecular biology of Tombusviridae. Adv Virus Res 44:381–428. https://doi.org/10.1016/S0065-3527(08)60334-6
Sainsbury F, Lomonossoff GP (2008) Extremely high-level and rapid transient protein production in plants without the use of viral replication. Plant Physiol 148:1212–1218. https://doi.org/10.1104/pp.108.126284
Sainsbury F, Lavoie PO, D’Aoust MA, Vezina LP, Lomonossoff GP (2008) Expression of multiple proteins using full-length and deleted versions of Cowpea mosaic virus RNA-2. Plant Biotechnol J 6:82–92. https://doi.org/10.1111/j.1467-7652.2007.00303.x
Saxena P, Hsieh YC, Alvarado VY, Sainsbury F, Saunders K, Lomonossoff GP, Scholthof HB (2011) Improved foreign gene expression in plants using a virus-encoded suppressor of RNA silencing modified to be developmentally harmless. Plant Biotechnol J 9:703–712. https://doi.org/10.1111/j.1467-7652.2010.00574.x
Schmidlin L, Link D, Mutterer J, Guilley H, Gilmer D (2005) Use of a Beet necrotic yellow vein virus RNA-5-derived replicon as a new tool for gene expression. J Gen Virol 86:463–467. https://doi.org/10.1099/vir.0.80720-0
Scholthof HB (1999) Rapid delivery of foreign genes into plants by direct rub-inoculation with intact plasmid DNA of a tomato bushy stunt virus gene vector. J Virol 73(9):7823–7829. https://doi.org/10.1128/JVI.73.9.7823-7829.1999
Scholthof HB (2006) The Tombusvirus-encoded P19: from irrelevance to elegance. Nat Rev Microbiol 4:405–411. https://doi.org/10.1038/nrmicro1395
Scholthof HB, Morris TJ, Jackson AO (1993) The capsid protein gene of tomato bushy stunt virus is dispensable for systemic movement and can be replaced for localized expression of foreign genes. Mol Plant-Microbe Interact 6:309–309. https://doi.org/10.1094/MPMI-6-309
Scholthof HB, Scholthof KB, Jackson AO (1995) Identification of tomato bushy stunt virus host-specific symptom determinants by expression of individual genes from a potato virus X vector. Plant Cell 7:1157–1172. https://doi.org/10.1105/tpc.7.8.1157
Shamekova M, Mendoza MR, Hsieh YC, Lindbo J, Omarov RT, Scholthof HB (2014) Tombusvirus-based vector systems to permit over-expression of genes or that serve as sensors of antiviral RNA silencing in plants. Virology 452:159–165. https://doi.org/10.1016/j.virol.2013.12.031
Shamloul M, Trusa J, Mett V, Yusibov V (2014) Optimization and utilization of Agrobacterium-mediated transient protein production in Nicotiana. J Vis Exp 19:86. https://doi.org/10.3791/51204
Silhavy D, Molnár A, Lucioli A, Szittya G, Hornyik C, Tavazza M, Burgyán J (2002) A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J 21:3070–3080. https://doi.org/10.1093/emboj/cdf312
Smith ML, Fitzmaurice WP, Turpen TH, Palmer KE (2009) Display of peptides on the surface of tobacco mosaic virus particles. In: Karasev AV (ed) Plant-produced microbial vaccines. Springer, Berlin, pp 13–31. https://doi.org/10.1007/978-3-540-70868-1_2
Streatfield SJ (2007) Approaches to achieve high-level heterologous protein production in plants. Plant Biotechnol J 5:2–15. https://doi.org/10.1111/j.1467-7652.2006.00216.x
Takamatsu N, Ishikawa M, Meshi T, Okada Y (1987) Expression of bacterial chloramphenicol acetyltransferase gene in tobacco plants mediated by TMV-RNA. EMBO J 6:307–311. https://doi.org/10.1002/j.1460-2075.1987.tb04755.x
Terpe K (2003) Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 60:523–533. https://doi.org/10.1007/s00253-002-1158-6
White KA, Nagy PD (2004) Advances in the molecular biology of tombusviruses: gene expression, genome replication, and recombination. Prog Nucleic Acid Res 78:187–226. https://doi.org/10.1016/S0079-6603(04)78005-8
Xiang C, Han P, Lutziger I, Wang K, Oliver DJ (1999) A mini binary vector series for plant transformation. Plant Mol Biol 40:711–717 https://springerlink.bibliotecabuap.elogim.com/article/10.1023/A:1006201910593
Yamamura Y, Scholthof HB (2005) Tomato bushy stunt virus: a resilient model system to study virus-plant interactions. Mol Plant Pathol 6:491–502. https://doi.org/10.1111/j.1364-3703.2005.00301.x
Zhang G, Leung C, Murdin L, Rovinski B, White KA (2000) In planta expression of HIV-1 p24 protein using and RNA plant virus-based expression vector. Mol Biotechnol 14:99–107. https://doi.org/10.1385/MB:14:2:99
Zhao Y, Hammond J, Tousignant ME, Hammond RW (2000) Development and evaluation of a complementation-dependent gene delivery system based on cucumber mosaic virus. Arch Virol 145:2285–2295. https://doi.org/10.1007/s007050070021
Zhumabek AT, Abeuova LS, Mukhametzhanov NS, Scholthof HB, Ramankulov YM, Manabayeva SA (2018) Transient expression of a bovine leukemia virus envelope glycoprotein in plants by a recombinant TBSV vector. J Virol Methods 255:1–7. https://doi.org/10.1016/j.jviromet.2018.01.016
Acknowledgments
We would like to thank Dr. John A. Lindbo (Ohio State University, USA) for kindly providing the Tobacco mosaic virus (TMV) RNA-based expression vector (TRBO) and Dr. Joseph G. Atabekov (Lomonosov Moscow State University, Russia) for giving the Potato virus X (PVX)-based expression vector (PVXdt).
Funding
This study was supported by the National Natural Science Foundation of China (Project Nos. 31660037 and 31760039).
Author information
Authors and Affiliations
Contributions
SW conceived and designed the research. XZ conducted the experiments. XD analyzed the data. ZL and SW wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, X., Ding, X., Li, Z. et al. Development of Tomato bushy stunt virus-based vectors for fusion and non-fusion expression of heterologous proteins in an alternative host Nicotiana excelsiana. Appl Microbiol Biotechnol 104, 8413–8425 (2020). https://doi.org/10.1007/s00253-020-10837-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10837-z