Abstract
Bacillus subtilis naturally produces large amounts of 2,3-butanediol (2,3-BD) as a main by-product during poly-γ-glutamic acid (γ-PGA) production. 2,3-BD is a promising platform chemical in various industries, and co-production of the two chemicals has great economic benefits. Co-production of γ-PGA and 2,3-BD by a newly isolated B. subtilis CS13 was investigated here. The fermentation medium and culture parameters of the process were optimized using statistical methods. It was observed that sucrose, l-glutamic acid, ammonium citrate, and MgSO4·7H2O were favorable for γ-PGA and 2,3-BD co-production at culture pH of 6.5 and 37 °C. An optimal medium composed of 119.8 g/L sucrose, 48.8 g/L l-glutamic acid, 21.1 g/L ammonium citrate, and 3.2 g/L MgSO4·7H2O was obtained by response surface methodology (RSM). The results show that the titers of γ-PGA and 2,3-BD reached 27.8 ± 0.9 g/L at 24 h and 57.1 ± 1.3 g/L at 84 h with the optimized medium, respectively. γ-PGA and 2,3-BD production by B. subtilis CS13 was significantly enhanced in fed-batch fermentations. γ-PGA (36.5 ± 1.1 g/L, productivity of 1.22 ± 0.04 g/L/h) and 2,3-BD concentrations (119.6 ± 2.8 g/L, productivity of 2.49 ± 0.66 g/L/h) were obtained in the optimized medium with feeding sucrose. The co-production of 2,3-BD and γ-PGA provides a new perspective for industrial production of γ-PGA and 2,3-BD.
Key points • A strategy for co-production of γ-PGA and 2,3-BD was developed. • The culture parameters for the co-production of γ-PGA and 2,3-BD were studied. • RSM was used to optimize the medium for γ-PGA and 2,3-BD co-production. • 36.5 g/L γ-PGA and 119.6 g/L 2,3-BD were obtained from the optimum medium in fed-batch fermentation. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Production of bulk chemicals and biopolymers by microbial fermentation has aroused widespread interest in industry due to its safety and environmental friendliness. Natural microorganism metabolism is a complex process and generally accumulates various by-products along with the target products. Therefore, screening microorganisms with high yield target products has been an inevitable research direction for researchers. Moreover, bioengineering technologies have been developed to minimize the concentration of by-products or maximize the concentration of the target products (Guo et al. 2014). However, most researches for microbial production of bulk chemicals or polymers are just limited in laboratory scale due to the high cost of fermentation process and subsequent purification steps (Lee et al. 2011).
Under certain circumstances, two or more metabolites can accumulate simultaneously in organisms, and co-production strategies have been developed in a variety of microorganisms to make the processes more efficient, such as co-production of 3-hydroxypropionic acid and 1,3-propanediol (Huang et al. 2013), simultaneous production of butanol and acetoin (Liu et al. 2015), and simultaneous production of surfactin and 2,3-butanediol (2,3-BD) (Andrade et al. 2016). The co-production strategies realized the redox balance in cultivation and improved the mass yields and product values.
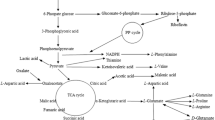
Bacillus subtilis is a poly-γ-glutamic acid (γ-PGA) producer (Zeng et al. 2014; Zhang et al. 2012). γ-PGA is a biopolymer consisting of l- and d-glutamic acid units and has applications in medicine, food, agriculture, cosmetics, and wastewater treatment industries due to its excellent properties such as water solubility, super absorption, biodegradability, and non-toxicity to humans and the environment (Bajaj and Singhal 2011; Luo et al. 2016). γ-PGA production by B. subtilis is an l-glutamic acid–dependent process. Meanwhile, B. subtilis consists of a 2,3-BD synthesis pathway. Pyruvate is converted to acetoin with the catalysis of acetolactate synthase (ALS) and acetolactate decarboxylase (ALD), and the latter is reduced to 2,3-butanediol (2,3-BD) by 2,3-BD dehydrogenase (BDH) (Fig. 1). 2,3-BD is an important chemical and has applications in food, pharmaceutical, cosmetics, fine chemical, and other industries. In addition, 2,3-BD can be used as a fuel due to its high heat energy (Zeng and Sabra 2011). In recent years, microbial route of 2,3-BD production has been reported in a series of bacteria species, such as Klebsiella spp. (Guragain and Vadlani 2017), Enterobacter spp. (Zhang et al. 2014), Paenibacillus spp. (Gao et al. 2010), and Bacillus spp. (Białkowska et al. 2016; Andrade et al. 2016; Yang et al. 2013). B. subtilis is a GRAS (generally regarded as safe) bacteria and is considered to be a promising microbe for the production of 2,3-BD (Feng et al. 2017; Fu et al. 2014).
The metabolic pathways of 2,3-BD and γ-PGA co-production in B. subtilis CS13. The coding genes of enzymes around pyruvate branch for 2,3-BD and γ-PGA production: lactate dehydrogenase (ldh); acetolactate synthase (alsS); acetolactate decarboxylase (alsD); d-butanediol dehydrogenase (bdhA); butanediol dehydrogenase (budC); pyruvate dehydrogenase (pdhABCD); glutamate dehydrogenase (gdh); glutamate racemase (glr); γ-PGA synthetase (pgsBCAE)
The production of 2,3-BD from acetoin requires oxidation of NADH to NAD+. Glutamate dehydrogenase (GDH) is the key enzyme of glutamic acid metabolism and mainly uses NADPH as a coenzyme (Meng et al. 2016). The decrease of NADPH will increase the NADH accumulation, which was beneficial for 2,3-BD production (Fig. 1). Fermentative production of γ-PGA is an aerobic process, while 2,3-BD synthesis is inhibited at aerobic conditions (Luo et al. 2016; Fu et al. 2016). However, the γ-PGA broth has high viscosity, which decreases the dissolved oxygen and leads to the metabolic flux from carbon sources mainly into 2,3-BD synthesis pathway (Fu et al. 2014). Therefore, simultaneous production of 2,3-BD and γ-PGA was proposed and investigated for the first time. Temperature and pH affect the activities of related enzymes, which in turn affect γ-PGA and 2,3-BD production. Besides, carbon sources, nitrogen sources, and inorganic salts have a significant effect on the γ-PGA or 2,3-BD production individually by various Bacillus species (Bajaj and Singhal 2011; Zeng et al. 2014; Yang et al. 2012; Yang et al. 2013). Thus, medium components can be manipulated by statistical methods to improve the yield of γ-PGA and 2,3-BD. Statistical methods are more rapid, reliable, and reduce the total number of experiments.
In this paper, a new B. subtilis strain was isolated and identified, which can co-produce 2,3-BD and γ-PGA. Detailed research on the effects of the medium components and cultivation conditions on the 2,3-BD and γ-PGA co-production was carried out. The Plackett–Burman design (PBD) was used to identify significant factors affecting the 2,3-BD and γ-PGA co-production. An optimal initial media, which could maximize the co-production of 2,3-BD and γ-PGA, was obtained by the response surface methodology (RSM). As far as we know, this is the first report of 2,3-BD and γ-PGA co-production. The present study provides a new perspective on theoretical research and industrial applications.
Materials and methods
Isolation and identification of the γ-PGA-producing strain
B. subtilis strains were isolated from Chung-kook-jang sauce, which is a traditional Korean food, purchased at a local market (Jeongeup-si, South Korea). Samples of 10 g were diluted in 90 mL of distilled water and boiled for 5 min. The suspension was diluted 10−1 to 10−6, and an aliquot (200 μL) of each suspension was spread on basal agar medium plates, containing 10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl, 20 g/L glucose, 20 g/L l-glutamic acid, and 15 g/L agar. After incubation at 37 °C for 48 h, high-viscosity and high-mucosity colonies were isolated as potential strains and purified on new agar plates. Pure colonies were inoculated into 15-mL tubes containing 3 mL of liquid basal medium and cultured for 12 h at 37 °C with agitation at 200 rpm, and 1% (v/v) of the samples was transferred into 50 mL of fermentation medium (pH 6.5) composed of 30 g/L glucose, 30 g/L l-glutamic acid, 5 g/L NH4Cl, 1 g/L KH2PO4, 0.5 g/L MgSO4·7H2O, 0.04 g/L FeCl3·6H2O, 0.15 g/L CaCl2·2H2O, 0.12 g/L MnCl2·4H2O, and 0.5 g/L NaCl in 250-mL baffled flasks. The cells were incubated at 37 °C and shaken at 200 rpm for 24 h. The concentrations of γ-PGA in the broth were measured, and the strains with the highest γ-PGA yield were chosen for further study.
The 16S rDNA gene of the isolated strain was amplified, sequenced, and manually edited with the BioEdit software as previously described (Wang et al. 2020). The sequence was used for comparison with reported type strains from the EzBioCloud database (https://www.ezbiocloud.net/). The phylogenetic tree was constructed using the neighbor-joining method in MEGA 7.0.
Culture conditions for co-production of γ-PGA and 2,3-BD
The isolated strain was first cultured in 50-mL tubes containing 15 mL basal medium at 37 °C and 200 rpm for 12 h. Then, the optical density of the seed cultures was adjusted to an OD600 of 5.0 ± 0.1, and 1% (v/v) was inoculated into 250-mL baffled flasks containing 50 mL of fresh fermentation medium and cultured for 24–36 h according to the experiment conditions. All experiments were performed independently in triplicates.
The effects of temperature were studied at 30, 34, 37, 42, and 45 °C with the agitation of 200 rpm and pH 6.5. The effects of pH on the 2,3-BD and γ-PGA co-production were carried out at an initial pH of 5.5, 6.0, 6.5, 7.0, 7.5, and 8.0. Incubation was conducted at the determined optimum temperature. The optimum temperature and initial pH were fixed for all subsequent experiments.
The effect of different carbon sources on the 2,3-BD and γ-PGA co-production was investigated by individually replacing glucose with sucrose, fructose, glycerol, maltose, and lactose at 30 g/L and keeping the others at the same levels. The optimal carbon source was selected for further study.
Various nitrogen sources including ammonium chloride, ammonium citrate, ammonium sulfate, sodium nitrate, potassium nitrate, yeast extract, peptone, and corn steep liquor were added to the fermentation medium at 5 g/L individually to investigate their effects on the 2,3-BD and γ-PGA co-production. The nitrogen source supporting the maximum production of 2,3-BD and γ-PGA was used for all subsequent experiments.
Screening of significant nutrients by PBD
The PBD as an efficient technique was used for screening of the main factors that significantly influenced the 2,3-BD and γ-PGA co-production. In the present study, the optimum temperature and initial pH were used. Eight variables including sucrose, l-glutamic acid, ammonium citrate, KH2PO4, MgSO4·7H2O, FeCl3·6H2O, MnCl2·4H2O, and CaCl2·2H2O were used to generate the experiment design by the Design-Expert 8.0.6 software (Stat-Ease Inc., Minneapolis, MN, USA). Each variable at two values (+ 1 for high and − 1 for low) were chosen and shown in Table 1. The true values of each variable and its analysis of variance (ANOVA) are shown in Table 2.
Optimization of the medium composition by response surface methodology
A face-centered central composite design (FCCD) was used for the RSM to optimize the levels of significant variables in the optimization of the 2,3-BD and γ-PGA production. The chosen variables were sucrose, l-glutamic acid, ammonium citrate, and MgSO4·7H2O identified by PBD (Table 2), while 1 g/L KH2PO4, 0.04 g/L FeCl3·6H2O, 0.15 g/L CaCl2·2H2O, 0.12 g/L MnCl2·4H2O, and 0.5 g/L NaCl in the medium were fixed. The effect of each variable on the 2,3-BD and γ-PGA production was studied at three coded levels (− 1, 0, + 1), and a total of 30 experiments including six replicates at the central point were conducted. The coded and real values are shown in Table 3. The 2,3-BD and γ-PGA concentrations were measured in triplicates and as the average of the maximum as the response after 84 h. The data obtained from the RSM for the 2,3-BD and γ-PGA co-production were subjected to ANOVA using the Design-Expert 8.0.6 software. The experimental results of the RSM were fitted to the second-order polynomial equation:
where Y is the predicted response; β0 is the intercept term; βi is the linear coefficient; βij is the quadratic coefficient; βii is the squared term, and Xi, Xj are the coded independent variables.
Fed-batch fermentation
Fed-batch fermentation was carried out in a 3-L fermentor (FMT-ST-D03; Bio System Engineering & Machine Company; Korea) with 0.9 L optimized fermentation medium at 37 °C, and 100 mL seed culture as previously described was inoculated. The aeration and the agitation speed were maintained at 3 L/min and 600 rpm, respectively; the pH was automatically controlled at 6.5 ± 0.1 by adding NH4OH or 2 M HCl. Bottles of sucrose solution (80%, w/v) were added into the fermentor when the residue sugar concentration was about 20–30 g/L.
Analytical methods
The biomass was determined according to the standard calibration curve between the OD600 and dry cell weight by measuring the OD600 of the broth (Wang et al. 2020).
γ-PGA was purified by the ethanol precipitation method as previously described (Wang et al. 2020). The concentration of γ-PGA was determined by gel permeation chromatography system using an Agilent 1100 high-performance liquid chromatography (HPLC) system equipped with a PL aquagel-OH MIXED-H column (300 × 7.5 mm, 8 μm; Agilent Technologies, Inc., UK) and refractive index detector. The mobile phase was HPLC-grade water at a flow rate of 1.0 mL/min. The γ-PGA yield was determined by the standard curve between the area peak and purified γ-PGA (Bioleaders Corporation, Daejeon, South Korea).
The concentrations of 2,3-BD, acetoin, glucose, and fructose in the broth were measured by HPLC with an Aminex HPX-87H column and sucrose with the Aminex HPX-87P column (300 × 7.8 mm; Bio-Rad, USA) at 65 °C. The mobile phase consisted of 5 mM H2SO4 and HPLC-grade water, respectively, and the flow rate was 0.6 mL/min. l-Glutamic acid was measured using a Chirex® 3126 (D)-phenicillamine column (250 × 4.6 mm; Phenomenex Inc., Torrance, CA, USA) and a UV detector (254 nm) as described before (Wang et al. 2020).
Results
Isolation and identification of the γ-PGA-producing strain
Based on the characteristics of the colonies which were highly mucoid, 15 strains (CS1–CS15) were picked up from the agar plates. These strains were transferred into the fermentation medium, and eight strains showed a γ-PGA production ability. Of these strains, strain CS13 produced the highest concentration of γ-PGA (9.6 ± 0.3 g/L) (Table S1). In addition, high concentration of 2,3-BD (14.2 ± 0.5 g/L) was found during an analysis of residual sugars in the fermentation broth (Fig. S1). Hence, the idea of the co-production γ-PGA and 2,3-BD arose due to strain CS13.
The 16S rDNA gene sequence of CS13 showed the similarity to B. subtilis subsp. subtilis NCIB 3610 (99.93%), B. subtilis subsp. stercoris D7XPN1 (99.92%), B. tequilensis KCTC 13622 (99.85%), and B. subtilis subsp. inaquosorum KCTC 13429 (99.85%) when blasting the sequence on EzBioCloud. A phylogenetic tree was constructed based on the 16S rDNA sequence and is shown in Fig. 2. CS13 formed a cluster with B. subtilis subsp. stercoris D7XPN1 and was classified as the species B. subtilis. The 16S rDNA gene sequence of B. subtilis CS13 was deposited in GenBank with a gene ID of MG722817. B. subtilis CS13 was deposited at the Korean Collection for Type Cultures with the accession number KCTC 14094 BP.
Effect of temperature and pH for co-production of 2,3-BD and γ-PGA
As shown in Fig. 3a, the biomass decreased from 5.2 ± 0.2 g/L to 3.2 ± 0.1 g/L with increasing temperature from 30 to 45 °C. The maximum concentration of γ-PGA reached 10.3 ± 0.3 g/L at 45 °C, and the concentration of 2,3-BD was 12.6 ± 0.4 g/L; meanwhile, the highest concentration of 2,3-BD (14.5 ± 0.5 g/L) was obtained at 37 °C with 9.8 ± 0.3 g/L of γ-PGA. The high temperature (45 °C) is favorable for γ-PGA production, but not conducive for 2,3-BD synthesis. Although the biomass was increased with the increase of pH from 5.5 to 8.0, the production of γ-PGA and 2,3-BD was significantly changed (Fig. 3b). B. subtilis CS13 showed the highest γ-PGA (9.7 ± 0.3 g/L) and 2,3-BD (14.4 ± 0.5 g/L) production at pH 6.5, followed by 6.0 and 7.0. Considering the production of 2,3-BD, the temperature of 37 °C and pH 6.5 were chosen as the optimum temperature and pH.
Effect of carbon sources and nitrogen sources for co-production of 2,3-BD and γ-PGA
All the tested carbon sources could promote cell growth, but the yield of γ-PGA and 2,3-BD were different (Fig. 3c). In the case of sucrose and glucose, a high yield of 2,3-BD (14.5 ± 0.5 and 14.3 ± 0.5 g/L) was attained, and the yield of γ-PGA was 9.8 ± 0.3 and 9.7 ± 0.3 g/L, respectively. Lactose was good for cell growth but did not affect the γ-PGA and 2,3-BD co-production. Glycerol was the best carbon source for the γ-PGA production, and 12.2 ± 0.4 g/L of γ-PGA accumulated; but interestingly, glycerol is unfavorable for the synthesis of 2,3-BD, and only 6.9 ± 0.2 g/L of 2,3-BD was produced. Thus, considering the yield of the 2,3-BD, sucrose as a cheap carbon source was chosen for the γ-PGA and 2,3-BD co-production.
Figure 3d shows the effects of different nitrogen sources on the γ-PGA and 2,3-BD co-production of B. subtilis CS13. All the nitrogen sources could be used by B. subtilis CS13 to synthesize 2,3-BD. In general, the strain preferred to utilize organic nitrogen sources showing improved cell growth and the inorganic nitrogen sources showed an enhanced γ-PGA production. Among the nitrogen sources tested, ammonium citrate yielded the highest γ-PGA (11.7 ± 0.4 g/L) and 2,3-BD (16.3 ± 0.5 g/L) production.
Screening of significant nutrients by PBD for co-production
In the present study, B. subtilis CS13 produced the highest yield of γ-PGA (22.4 ± 0.6 g/L) and 2,3-BD (26.2 ± 0.6 g/L) in combinations 6 and 9 (Table 1), and the ANOVA is shown in Table 2. Three variables, namely, sucrose, l-glutamic acid, and ammonium citrate, influenced the γ-PGA fermentation process significantly (P < 0.05) and showed a positive coefficient (Table 2), suggesting that the levels for these variables can be increased to improve the γ-PGA production. In the process of 2,3-BD fermentation, sucrose, l-glutamic acid, ammonium citrate, MgSO4·7H2O, FeCl3·6H2O, and MnCl2·4H2O showed a significant effect. The coefficients of l-glutamic acid, FeCl3·6H2O, and MnCl2·4H2O were negative, and lower concentrations of these chemicals are suggested for future experiments (Table 3). The coefficient of determination (R2), which equaled 0.9941 and 0.9998 for γ-PGA and 2,3-BD, indicates that 99.41% and 99.98% of the variability in the response could be explained by the model. The high values of the adjusted determination coefficient (R2adj = 0.9787; 0.9993) imply a high significance of the model.
Optimization of the medium composition by RSM
Sucrose, l-glutamic acid, ammonium citrate, and MgSO4·7H2O were used for further optimization with a FCCD to maximize the γ-PGA and 2,3-BD production. The averages of the maximum values of γ-PGA and 2,3-BD were used as the responses after an 84-h fermentation with 30 experiments in triplicate, and the experimental design is shown in Table 4.
Response surface of the γ-PGA yield
The regression models in the form of ANOVA are given in Table 5. The “Model P value” (< 0.0001) implies the models are significant. The values of the determination coefficient (R2 = 0.9918) indicate a 99.18% variability in the γ-PGA yield. The high values of the adjusted determination coefficient (R2adj = 0.9842) advocate a good term fit for the models. The “Lack of Fit P value” (0.8992) indicates that the “Lack of Fit” was insignificant relative to the pure error. The “P value” (< 0.005) showed the significant influence of the coefficients. Among the model terms, sucrose (X1), l-glutamic acid (X2), ammonium citrate (X3), the interaction term of sucrose and l-glutamic acid (X1X2), sucrose and ammonium citrate (X1X3), l-glutamic acid and ammonium citrate (X2X3), squared term of sucrose (X12), l-glutamic acid (X22), ammonium citrate (X32), and MgSO4·7H2O (X52) had a significant influence on the γ-PGA production (Table 5). The effect of the MgSO4·7H2O and its interaction between the other variables on the γ-PGA yield were not significant. The variables such as sucrose with a positive linear coefficient (2.03) indicate that the production of γ-PGA increased with the increasing concentration of sucrose. Whereas the negative squared coefficient of sucrose (− 1.77) suggests the existence of a maximum as a function of the sucrose concentration, and beyond this point, sucrose had an inhibitory effect (Table 5). According to ANOVA, the following polynomial quadratic equations were obtained in coded level:
The surface response plots for the optimization of the fermentation conditions of γ-PGA were generated by holding two constants at the central point while keeping another two variables within the experiment range (Fig. 4). Figure 4a–c shows there were significant interactions of sucrose concentration with other parameters on the γ-PGA yield. The γ-PGA concentration (21–27 g/L) increased significantly when the sucrose concentration increased from 40 to 100 g/L. However, if the sucrose concentration is above 100 g/L, it will decrease the γ-PGA yield, which may be caused by the substrate limitation. A similar effect for ammonium citrate was observed; the ammonium citrate increased to the optimum point increased the γ-PGA production to the maximum level, and a further increase in the ammonium citrate decreased the γ-PGA production (Fig. 4b, d, f). Figure 4a, d, and e shows that a high γ-PGA yield could be achieved if the concentration of l-glutamic acid is from 30 to 50 g/L. The increase of the l-glutamic acid concentration could decrease the γ-PGA yield because l-glutamic acid at too high of a concentration could not be used by the strain and substrate limitation occurs. The γ-PGA yield did not change as the MgSO4·7H2O concentration increased due to its insignificant effect (Fig. 4c, e, f).
Response surface and contour plots for γ-PGA production by B. subtilis CS13. (a) Effect of l-glutamic acid and sucrose, (b) effect of ammonium citrate and sucrose, (c) effect of MgSO4·7H2O and sucrose, (d) effect of ammonium citrate and l-glutamic acid, (e) effect of MgSO4·7H2O and l-glutamic acid, (f) effect of MgSO4·7H2O and ammonium citrate
Ignoring the effect of MgSO4·7H2O, the maximum value of γ-PGA was predicted by the “Numerical Optimization” tool of the Design-Expert 8.0.6 software. The predicted maximum γ-PGA yield was 27.8 g/L, which was obtained with a sucrose of 100.5 g/L, l-glutamic acid of 50.5 g/L, and ammonium citrate of 21.1 g/L.
Response surface of the 2,3-BD yield
The 2,3-BD concentration varied from 13.9 to 56.8 g/L for the 30 experiments shown in Table 4. The model was highly significant as the “Model P value” (< 0.0001). The determination coefficient (R2 = 0.9984) and adjusted determination coefficient (R2adj = 0.9969) suggest good fits were achieved by the model (Table 6). The variables with obvious effect were sucrose (X1), l-glutamic acid (X2), ammonium citrate (X3), MgSO4·7H2O (X5), interaction term of ammonium citrate and MgSO4·7H2O (X3X5), squared term of sucrose (X12), ammonium citrate (X32), and MgSO4·7H2O (X52) (Table 6). The polynomial quadratic equation in the coded level was given by:
Figure 5a, b, and c deposited the interaction effect of l-glutamic acid, ammonium citrate, MgSO4·7H2O, and sucrose on 2,3-BD production, respectively. The 2,3-BD concentration increased significantly when the sucrose concentration increased from 40 to 120 g/L, different from the other variables, and a high concentration of sucrose did not inhibit the 2,3-BD production within the experiment range. An increase in ammonium citrate and MgSO4·7H2O increased the 2,3-BD production gradually, and at a higher ammonium citrate and MgSO4·7H2O concentration, the trend was reversed but with a less significant tendency (Fig. 5d). l-Glutamic acid inhibited the production of 2,3-BD due to the negative linear coefficient (Table 6) and thus decreased the 2,3-BD concentration with an increase in l-glutamic acid from 30 to 70 g/L (Fig. 5e, f). The predicted highest value of 2,3-BD was 57.6 g/L, which was obtained at a sucrose of 120 g/L, l-glutamic acid of 30 g/L, ammonium citrate of 24.5 g/L, and MgSO4·7H2O of 3.6 g/L.
Response surface and contour plots for 2,3-BD production by B. subtilis CS13. (a) Effect of l-glutamic acid and sucrose, (b) effect of ammonium citrate and sucrose, (c) effect of MgSO4·7H2O and sucrose, (d) effect of ammonium citrate and l-glutamic acid, (e) effect of MgSO4·7H2O and l-glutamic acid, (f) effect of MgSO4·7H2O and ammonium citrate
Optimization and validation of the medium for the γ-PGA and 2,3-BD co-production
The optimum medium composition was calculated by a numerical iteration procedure using the Design-Expert 8.0.6 software. The optimum conditions for the maximum γ-PGA and 2,3-BD co-production was found to be 119.8 g/L sucrose, 48.9 g/L l-glutamic acid, 21.1 g/L ammonium citrate, and 3.2 g/L MgSO4·7H2O. In this condition, the γ-PGA and 2,3-BD yields predicted by Design-Expert 8.0.6 software were 27.5 and 56.8 g/L, respectively. At the optimum level, the highest yields of γ-PGA and 2,3-BD were 27.8 ± 0.9 and 57.1 ± 1.3 g/L (Fig. 6), which were close to the predicted values.
Fed-batch fermentation
To further verify the potential application of γ-PGA and 2,3-BD co-production strategy, fed-batch culture was performed primarily. Figure 7a illustrates the changes of residual sugars, γ-PGA, 2,3-BD, biomass, and l-glutamic acid in the fed-batch process. The maximum biomass reached 12.2 ± 0.4 g/L at 60 h. The maximum γ-PGA concentration of 36.5 ± 1.1 g/L was obtained at 30 h with a productivity of 1.22 ± 0.04 g/L/h and the conversion rate of l-glutamic acid to γ-PGA up to 1.04 gγ-PGA/gl-glutamic acid. The highest concentration of 2,3-BD reached 119.6 ± 2.8 g/L at 48 h with a 2,3-BD yield (0.48 g2,3-BD/gsucrose) and productivity of 2.49 ± 0.06 g/L/h. Moreover, B. subtilis CS13 produced a mixture of meso-2,3-BD and D-2,3-BD in the proportion of 8.7:1.3 at 48 h (Fig. 7b). The production of acetoin begins after 24 h, and the final titer of acetoin was 13.8 ± 0.6 g/L (Fig. 7b).
Discussion
2,3-BD and γ-PGA production is temperature dependent because of the dependence of the enzyme activity. The high biomass at lower temperatures might be caused by active energy metabolism (Zeng et al. 2014). Perego et al. found that butanediol production increased to the highest value when the temperature was increased to 37 °C and decreased over 37 °C (Perego et al. 2003). This phenomenon was consistent with our results. Interestingly, the highest γ-PGA production was obtained at 45 °C. According to the reported literature, the high temperature (45 °C) increased the activity of isocitrate dehydrogenase (ICDH) and glutamate dehydrogenase (GDH), which led to an enhanced γ-PGA production (Zeng et al. 2014). Moreover, the high temperature can reduce the molecular weight of γ-PGA, decrease the viscosity, and improve mass transfer.
pH influences bacterial metabolism and the formation of products. Zhu et al. found that 2,3-BD was a major by-product at pH 6.5 and 7.3 during γ-PGA fermentation. In contrast, the synthesis of 2,3-BD was limited, and acetoin had a high concentration at pH 5.7 (Zhu et al. 2013). However, for the B. subtilis CS13 in this study, a low concentration of acetoin (< 0.5 g/L) was detected, and the low pH only decreased the yield of 2,3-BD, which may be caused by the high viscosity of broth promoting metabolic flux from acetoin to 2,3-BD.
Most of the γ-PGA producers prefer glucose and glycerol as carbon sources. In our results (Fig. 3c), glycerol obviously increased the yield of γ-PGA but reduced the yield of 2,3-BD compared with sucrose or glucose. Sucrose is mainly derived from sugar cane and sugar beet, and is a cheap carbon source for biochemical production through microbial fermentation (Jiang et al. 2014; Zhang et al. 2017). Additionally, sucrose was a popular carbon source for production of 2,3-BD (Feng et al. 2017). Nitrogen sources promote cell growth and γ-PGA synthesis but are strain dependent, which may be caused by differences in metabolism. Previous reports have found that l-glutamic acid and citric acid as precursors successfully improve γ-PGA production (Ashiuchi 2010); moreover, B. subtilis CS13 belongs to this group. Ammonium citrate improved the production of γ-PGA and 2,3-BD (Fig. 3d), suggesting that citrate enhanced the production of α-ketoglutarate as the precursor for glutamate and γ-PGA in the TCA metabolism (Ashiuchi 2010). NH4+ enhanced glutamate metabolism conversion more of NADPH to NADP+, therefore, it increased the NADH generation and promoted the synthesis of 2,3-BD (Fig. 1). l-α-Acetolactate synthase is a key enzyme from pyruvate to acetoin and 2,3-BD, and the activity of the enzyme is dependent on Mg2+ (Poulsen and Stougaard 1989). Therefore, MgSO4·7H2O showed a positive and significant effect on 2,3-BD synthesis (Tables 3 and 6).
This design successfully utilizes the metabolic pathways and fermentation conditions of γ-PGA and 2,3-BD. Sucrose was rapidly hydrolyzed to glucose and fructose before 12 h due to the sucrose utilization systems (Reid and Abratt 2005). All of the sugars were exhausted at 84 h, and 57.1 ± 1.3 g/L 2,3-BD was obtained with a productivity of 0.68 g/L/h, and the conversion rate of sucrose to 2,3-BD was 0.48 g2,3-BD/gsucrose (Fig. 6a). As shown in Fig. 6b, the maximum γ-PGA concentration reached 27.8 ± 0.9 g/L at 24 h of fermentation, and then showed a slow decline, suggesting the γ-PGA depolymerase was activated (Yao et al. 2009). Furthermore, compared with the batch culture in flasks, fed-batch operation achieved a high γ-PGA (36.5 ± 1.1 g/L) and 2,3-BD (119.6 ± 2.8 g/L) production. Meanwhile, the productivity of γ-PGA and 2,3-BD improved from 1.16 ± 0.04 g/L/h to 1.22 ± 0.04 g/L/h and 0.68 ± 0.02 g/L/h to 2.49 ± 0.06 g/L/h, respectively. The fermentor overcomes the substrate shortages and provides comfortable fermentation conditions (dissolved oxygen and pH) which are crucial to γ-PGA and 2,3-BD production. Our results proved that co-production of γ-PGA and 2,3-BD has potential application in industry, and further research will focus on the fermentation conditions on a large-scale fermentor.
Many B. subtilis species have the ability of produce γ-PGA and 2,3-BD individually. Until now, the highest concentration of 2,3-BD (103.7 g/L) was produced by B. subtilis 168 with a productivity of 0.46 g/L/h (Table 7). B. subtilis NX-2, B. subtilis MJ80, and B. subtilis ZJU7 have good advantages in the final titers of γ-PGA, but the productivities were lower than B. subtilis CS13 (Table 8). Compared with these previously reported systems, the co-production by B. subtilis CS13 has the highest final 2,3-BD level and highest productivities of γ-PGA and 2,3-BD. This might be caused by the co-production of γ-PGA and 2,3-BD in the optimized medium which balanced the production and consumption of cofactors perfectly (Fig. 1). Actually, 2,3-BD is the major by-product during γ-PGA production by Bacillus species. Previous researches have focused on reducing the production of 2,3-BD by regulating metabolic flux during γ-PGA production, enhancing NADPH generation (Cai et al. 2017), supplementing nitrate (Li et al. 2014), and controlling the pH in the γ-PGA fermentation process (Zhu et al. 2013). Compared with previous studies, we believe that the co-production of 2,3-BD with a value-added γ-PGA is a more novel direction.
This research not only provides a new strategy to obtain γ-PGA and 2,3-BD simultaneously but also demonstrates the potential for industrial scale. The design utilizes the properties of the products, reduces the production cost, and simplifies the steps of industrial separation and purification. In the fermentation downstream processing, 2,3-BD is difficult to separate from the fermentation broth due to its high boiling point (180–184 °C) and high affinity for water (Jiang et al. 2009). The steam stripping and vacuum distillation methods consume a large amount of energy, preventing its application in the industrial separation of 2,3-BD (Shao and Kumar 2011). A novel aqueous two-phase extraction showed a good advantage because of the low energy, but it requires a large amount of ethanol (Jiang et al. 2009; Li et al. 2010). To separate γ-PGA, large amounts of ethanol usually need to be added to precipitate the γ-PGA after removing the biomass by centrifugation (Luo et al. 2016). 2,3-BD dissolved in ethanol could be separated from water by aqueous two-phase extraction (Jiang et al. 2009; Li et al. 2010). First, trichloroacetic acid (TCA) solution is added to the fermentation broth to separate the cells and proteins by centrifugation; second, γ-PGA is obtained through alcohol precipitation and drying and then, the alcohol and 2,3-BD mixture are obtained by aqueous two-phase extraction. The mixture could be used as a fuel or by distillation separation. The co-production of 2,3-BD with a value-added γ-PGA improves the economics of the fermentation process and facilitates its application.
References
Andrade CJ, Andrade LM, Bution ML, Dolder MAH, Fábio F, Barros C, Pastore GM (2016) Optimizing alternative substrate for simultaneous production of surfactin and 2,3-butanediol by Bacillus subtilis LB5a. Biocatal Agric Biotechnol 6:209–218. https://doi.org/10.1016/j.bcab.2016.04.004
Ashiuchi M (2010) Occurrence and biosynthetic mechanism of poly-gamma-glutamic acid. In: Hamano Y (ed) Amino-acid homopolymers occurring in nature. Microbiol Monogr, 15, pp 77–93
Bajaj IB, Singhal RS (2011) Poly (glutamic acid)—an emerging biopolymer of commercial interest. Bioresour Technol 102:5551–5561. https://doi.org/10.1016/j.biortech.2011.02.047
Białkowska AM, Marzena JK, Gromek E, Krysiak J, Sikora B, Kalinowska H, Kubik C, Schütt F, Turkiewicz M (2016) Effects of genetic modifications and fermentation conditions on 2,3-butanediol production by alkaliphilic Bacillus subtilis. Appl Microbiol Biotechnol 100:2663–2676. https://doi.org/10.1007/s00253-015-7164-2
Cai DB, He PH, Lu XC, Zhu CJ, Zhu J, Zhan YY, Wang Q, Wen ZY, Chen SW (2017) A novel approach to improve poly-γ-glutamic acid production by NADPH regeneration in Bacillus licheniformis WX-02. Sci Rep 7:43404. https://doi.org/10.1038/srep43404
Chen J, Shi F, Zhang B, Zhu F, Cao WF, Xu ZN, Xu GH, Cen PL (2010) Effects of cultivation conditions on the production of γ-PGA with Bacillus subtilis ZJU-7. Appl Biochem Biotechnol 160:370–377. https://doi.org/10.1007/s12010-008-8307-z
de Cesaro A, da Silva SB, Ayub MAZ (2014) Effects of metabolic pathway precursors and polydimethylsiloxane (PDMS) on poly-(gamma)-glutamic acid production by Bacillus subtilis BL53. J Ind Microbiol Biotechnol 41:1375–1382. https://doi.org/10.1007/s10295-014-1477-5
Feng J, Gu YY, Yan PF, Song CJ, Wang Y (2017) Recruiting energy-conserving sucrose utilization pathways for enhanced 2,3-butanediol production in Bacillus subtilis. ACS Sustain Chem Eng 5:11221–11225. https://doi.org/10.1021/acssuschemeng.7b03636
Fu J, Wang ZW, Chen T, Liu WX, Shi T, Wang GL, Tang YJ, Zhao XM (2014) NADH plays the vital role for chiral pure D-(−)-2,3-butanediol production in Bacillus subtilis under limited oxygen conditions. Biotechnol Bioeng 111:2126–2131. https://doi.org/10.1002/bit.25265
Fu J, Huo GX, Feng LL, Mao YF, Wang ZW, Ma HW, Chen T, Zhao XM (2016) Metabolic engineering of Bacillus subtilis for chiral pure meso-2,3-butanediol production. Biotechnol Biofuels 9:90. https://doi.org/10.1186/s13068-016-0502-5
Gao G, Xu H, Li QJ, Feng XH, Li S (2010) Optimization of medium for one-step fermentation of inulin extract from Jerusalem artichoke tubers using Paenibacillus polymyxa ZJ-9 to produce R,R-2,3-butanediol. Bioresour Technol 101:7076–7082. https://doi.org/10.1016/j.biortech.2010.03.143
Guo XW, Cao CH, Wang YZ, Li CQ, Wu MY, Chen YF, Zhang CY, Pei HD, Xiao DG (2014) Effect of the inactivation of lactate dehydrogenase, ethanol dehydrogenase, and phosphotransacetylase on 2,3-butanediol production in Klebsiella pneumoniae strain. Biotechnol Biofuels 7:44. https://doi.org/10.1186/1754-6834-7-44
Guragain YN, Vadlani PV (2017) 2,3-Butanediol production using Klebsiella oxytoca ATCC 8724: evaluation of biomass derived sugars and fed-batch fermentation process. Process Biochem 58:25–34. https://doi.org/10.1016/j.procbio.2017.05.001
Huang YN, Li ZM, Shimizu K, Ye Q (2013) Co-production of 3-hydroxypropionic acid and 1,3-propanediol by Klebseilla pneumoniae expressing aldH under microaerobic conditions. Bioresour Technol 128:505–512. https://doi.org/10.1016/j.biortech.2012.10.143
Jiang B, Li ZG, Dai JY, Zhang DJ, Xiu ZL (2009) Aqueous two-phase extraction of 2,3-butanediol from fermentation broths using an ethanol/phosphate system. Process Biochem 44:112–117. https://doi.org/10.1016/j.procbio.2008.09.019
Jiang M, Dai WY, Xi YL, Wu MK, Kong XP, Ma JF, Zhang M, Chen KQ, Wei P (2014) Succinic acid production from sucrose by Actinobacillus succinogenes NJ113. Bioresour Technol 153:327–332. https://doi.org/10.1016/j.biortech.2013.11.062
Ju WT, Song YS, Jung WJ, Park RD (2014) Enhanced production of poly-γ-glutamic acid by a newly-isolated Bacillus subtilis. Biotechnol Lett 36:2319–2324. https://doi.org/10.1007/s10529-014-1613-3
Lee JW, Kim HU, Choi S, Yi JH, Lee SY (2011) Microbial production of building block chemicals and polymers. Curr Opin Biotechnol 22:758–767. https://doi.org/10.1016/j.copbio.2011.02.011
Lee NR, Lee SM, Cho KS, Jeong SY, Hwang DY, Kim DS, Hong CO, Son HJ (2014) Improved production of poly-γ-glutamic acid by Bacillus subtilis D7 isolated from Doenjang, a Korean traditional fermented food, and its antioxidant activity. Appl Biochem Biotechnol 173:918–932. https://doi.org/10.1007/s12010-014-0908-0
Li ZG, Teng H, Xiu ZL (2010) Aqueous two-phase extraction of 2,3-butanediol from fermentation broths using an ethanol/ammonium sulfate system. Process Biochem 45:731–737. https://doi.org/10.1016/j.procbio.2010.01.011
Li X, Gou XY, Long D, Ji ZX, Hu LF, Xu DH, Liu J, Chen SW (2014) Physiological and metabolic analysis of nitrate reduction on poly-gamma-glutamic acid synthesis in Bacillus licheniformis WX-02. Arch Microbiol 196:791–799. https://doi.org/10.1007/s00203-014-1014-y
Liu D, Chen Y, Ding FY, Guo T, Xie JJ, Zhuang W, Niu HQ, Shi XC, Zhu CJ, Ying HJ (2015) Simultaneous production of butanol and acetoin by metabolically engineered Clostridium acetobutylicum. Metab Eng 27:107–114. https://doi.org/10.1016/j.ymben.2014.11.002
Luo ZT, Guo Y, Liu JD, Qiu H, Zhao MM, Zou W, Li SB (2016) Microbial synthesis of poly-γ-glutamic acid: current progress, challenges, and future perspectives. Biotechnol Biofuels 9:134. https://doi.org/10.1186/s13068-016-0537-7
Meng YH, Dong GR, Zhang C, Ren YY, Qu YL, Chen WF (2016) Calcium regulates glutamate dehydrogenase and poly-γ-glutamic acid synthesis in Bacillus natto. Biotechnol Lett 38:673–679. https://doi.org/10.1007/s10529-015-2023-x
Perego P, Converti A, Borghi MD (2003) Effects of temperature, inoculum size and starch hydrolyzate concentration on butanediol production by Bacillus licheniformis. Bioresour Technol 89:125–131. https://doi.org/10.1016/S0960-8524(03)00063-4
Poulsen C, Stougaard P (1989) Purification and properties of Saccharomyces cerevisiae acetolactate synthase from recombinant Escherichia coli. Eur J Biochem 185:433–439. https://doi.org/10.1111/j.1432-1033.1989.tb15133.x
Reid SJ, Abratt VR (2005) Sucrose utilisation in bacteria: genetic organisation and regulation. Appl Microbiol Biotechnol 67:312–321. https://doi.org/10.1007/s00253-004-1885-y
Shao PH, Kumar A (2011) Process energy efficiency in pervaporative and vacuum membrane distillation separation of 2,3-butanediol. Can J Chem Eng 89:1255–1265. https://doi.org/10.1002/cjce.20468
Tanimura K, Takashima S, Matsumoto T, Tanaka T, Kondo A (2016) 2,3-Butanediol production from cellobiose using exogenous beta-glucosidase-expressing Bacillus subtilis. Appl Microbiol Biotechnol 100:5781–5789. https://doi.org/10.1007/s00253-016-7326-x
Wang DX, Hwang JS, Kim DH, Lee SB, Kim DH, Joe MH (2020) A newly isolated Bacillus siamensis SB1001 for mass production of poly-γ-glutamic acid. Process Biochem 92:164–143. https://doi.org/10.1016/j.procbio.2019.11.034
Wu Q, Xu H, Ying HJ, Ouyang PK (2010) Kinetic analysis and pH shift control strategy for poly(γ-glutamic acid) production with Bacillus subtilis CGMCC 0833. Biochem Eng J 50:24–28. https://doi.org/10.1016/j.bej.2010.02.012
Yang TW, Zhang X, Rao ZM, Gu SH, Xia HF, Xu ZH (2012) Optimization and scale-up of 2,3-butanediol production by Bacillus amyloliquefaciens B10−127. World J Microbiol Biotechnol 28:1563–1574. https://doi.org/10.1007/s11274-011-0960-7
Yang TW, Rao ZM, Zhang X, Xu MJ, Xu ZH, Yang ST (2013) Effects of corn steep liquor on production of 2,3-butanediol and acetoin by Bacillus subtilis. Process Biochem 48:1610–1617. https://doi.org/10.1016/j.procbio.2013.07.027
Yao J, Jing J, Xu H, Liang JF, Wu Q, Feng XH, Ouyang PK (2009) Investigation on enzymatic degradation of γ-polyglutamic acid from Bacillus subtilis NX-2. J Mol Catal B-Enzym 56:158–164. https://doi.org/10.1016/j.molcatb.2007.12.027
Yao J, Xu H, Shi NN, Cao X, Feng XH, Li S, Ouyang PK (2010) Analysis of carbon metabolism and improvement of γ-polyglutamic acid production from Bacillus subtilis NX-2. Appl Biochem Biotechnol 160:2332–2341. https://doi.org/10.1007/s12010-009-8798-2
Zeng AP, Sabra W (2011) Microbial production of diols as platform chemicals: recent progresses. Curr Opin Biotechnol 22:749–757. https://doi.org/10.1016/j.copbio.2011.05.005
Zeng W, Chen GG, Wang QL, Zheng SF, Shu L, Liang ZQ (2014) Metabolic studies of temperature control strategy on poly(γ-glutamic acid) production in a thermophilic strain Bacillus subtilis GXA-28. Bioresour Technol 155:104–110. https://doi.org/10.1016/j.biortech.2013.12.086
Zhang D, Feng XH, Zhou Z, Zhang Y, Xu H (2012) Economical production of poly(γ-glutamic acid) using untreated cane molasses and monosodium glutamate waste liquor by Bacillus subtilis NX-2. Bioresour Technol 114:583–588. https://doi.org/10.1016/j.biortech.2012.02.114
Zhang CY, Peng XP, Li W, Guo XW, Xiao DG (2014) Optimization of 2,3-butanediol production by Enterobacter cloacae in simultaneous saccharification and fermentation of corncob residue. Biotechnol Appl Biochem 61:501–509. https://doi.org/10.1002/bab.1198
Zhang JZ, Yu L, Lin M, Yan QJ, Yang ST (2017) N-Butanol production from sucrose and sugarcane juice by engineered Clostridium tyrobutyricum overexpressing sucrose catabolism genes and adhE2. Bioresour Technol 233:51–57. https://doi.org/10.1016/j.biortech.2017.02.079
Zhu F, Cai J, Wu XT, Huang J, Huang L, Zhu JZ, Zheng Q, Cen PL, Xu ZN (2013) The main byproducts and metabolic flux profiling of γ-PGA-producing strain B. subtilis ZJU-7 under different pH values. J Biotechnol 164:67–74. https://doi.org/10.1016/j.jbiotec.2012.12.009
Acknowledgments
This project was supported by the Nuclear R&D Program of the Ministry of Science and ICT (MSIT), Republic of Korea.
Author information
Authors and Affiliations
Contributions
D.X.W. performed the experiments. D.X.W. and M.-H.J. designed the experiments, analyzed the data, and drafted the manuscript. H.M.K., S.B.L., D.-H.K., and M.-H.J. designed and guided the study, and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 109 kb)
Rights and permissions
About this article
Cite this article
Wang, D., Kim, H., Lee, S. et al. Simultaneous production of poly-γ-glutamic acid and 2,3-butanediol by a newly isolated Bacillus subtilis CS13. Appl Microbiol Biotechnol 104, 7005–7021 (2020). https://doi.org/10.1007/s00253-020-10755-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10755-0