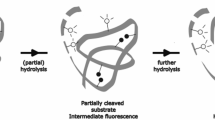
Abstract
Proteins, an important fraction of the organic matter in wastewater, typically enter a treatment facility as high molecular weight components. These components are degraded by extracellular protein hydrolytic enzymes, denoted as proteases. Adequate protein hydrolysis monitoring is crucial, since protein hydrolysis is often a rate-limiting step in wastewater treatment. However, current monitoring tools lack a high sample throughput and reliable quantification. Here, we present an improved assay for high-throughput protein hydrolysis rate measurements in wastewater treatment applications. A BODIPY FL casein model substrate was implemented in a microplate format for continuous fluorescent quantification. Case studies on a conventional and a high-rate aerobic municipal wastewater treatment plant and a lab-scale, two-stage, anaerobic reactor provided proof-of-concept. The assay presented in this study can help to obtain monitoring-based process insights, which will in turn allow improving biological performance of wastewater treatment installations in the future.
Key points
• Protein hydrolysis is a crucial step in biological wastewater treatment.
• Quantification of the protein hydrolysis rate enables in-depth process knowledge.
• BODIPY FL casein is a suitable model substrate for a protein hydrolysis assay.
• High sample throughput was obtained with fluorescent hydrolysis quantification.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological conversion of organic matter is the cornerstone of all aerobic and anaerobic wastewater treatment facilities. This conversion process relies on the coordinated action of a mixed microbial community to metabolize the protein, carbohydrate, and lipid fractions in the organic matter. This organic matter typically enters the treatment systems as high molecular weight (MW) components (MW > 10,000 Da) or particulates in the micro- and millimeter range, while only molecules in the nanometer range (MW < 1000) can be taken up directly through the microbial cell membrane (Cadoret et al. 2002; Hashimoto et al. 2005). Therefore, the microbial community actively excretes substrate-specific, hydrolytic enzymes to break down the organics in the extracellular environment. This hydrolysis of particulates is often cited as the overall rate-limiting step for the conversion of organic matter in wastewater treatment (Burgess 2008). A significant fraction of the particulates consists of proteins, up to 30% of the total incoming chemical oxygen demand (COD) (Raunkjær et al. 1994), which is hydrolyzed by protein hydrolytic enzymes, denoted as proteases.
Adequate protein hydrolysis monitoring is crucial in any system aiming at understanding and improving protein hydrolysis. Hydrolytic activity assays should mimic the full-scale situation as closely as possible with careful selection of model substrates. Casein has been widely acknowledged as a suitable protease model substrate because it has (i) a relatively open, globular secondary structure with limited α-helices and β-sheets (Haugland and Zhou 1998), (ii) a diverse amino acid composition (Holt 2016), and (iii) a high solubility in water including amphiphilic behavior with other hydrophobic molecules (Fox and Brodkorb 2008).
The most common colorimetric assay applied in wastewater treatment research is the azocasein assay, originally described by Charney and Tomarelli (1947). However, this assay has two main limitations. First, the chromogenic azocasein substrate and colored products have to be separated by precipitation with trichloroacetic acid. Precipitated azocasein substrate is then removed by centrifugation, to obtain a clear supernatant for spectrophotometric determination of product formation. This additional separation step inherently limits the sampling frequency and reduces the sample throughput, as the method is labor-intensive. Second, spectrophotometric methods have been recently criticized as they are highly susceptible to interference by components in the complex sludge matrix (Felz et al. 2019; Le et al. 2016) and have a low sensitivity (Nybroe 1992). Fluorogenic casein substrates have been proposed as a robust and sensitive alternative for azocasein. The labeling degree with the fluorophore in these substrates is such that fluorescence is quenched intramolecular, but is released upon digestion of casein by proteases. Two examples of commercially available fluorogenic casein substrates are fluorescein isothiocyanate (FITC)-casein (Twining 1984) and 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene (BODIPY FL) casein (Jones et al. 1997). The BODIPY FL spectral characteristics are similar to those of fluorescein, but the fluorescent properties are stable over a wider pH range (Schade et al. 1996). Neither the use of FITC-casein nor BODIPY FL casein has found application in wastewater treatment research so far with one exception. Xia et al. (2007) combined BODIPY FL casein staining with fluorescence in situ hybridization (FISH) on samples from full-scale aerobic wastewater treatment plants, to identify protein hydrolyzing organisms in the mixed community. This microscopy-based method was later on extended to microbial communities involved in anaerobic digestion (Xia et al. 2011, 2012, 2015) and in colonizing the cow digestive tract (Xia et al. 2016). However, no studies have been published that convert this qualitative BODIPY FL casein staining into a fully developed protein hydrolysis assay.
This study aims to advance and provide proof-of-concept of the BODIPY FL casein protein hydrolysis assay as a full-fledged, high-throughput assay for application in wastewater treatment. Proof-of-concept for aerobic and anaerobic wastewater treatment systems will be provided through case studies on samples of a conventional municipal wastewater treatment plant (MWWTP Leuven), a high-rate municipal wastewater treatment plant (MWWTP Nieuwveer) and a lab-scale, two-stage, anaerobic reactor treating synthetic dairy wastewater.
Materials and methods
Protein hydrolysis assay
Protein hydrolysis was measured with the BODIPY FL casein substrate (EnzChek kit E6638, Thermofisher scientific, Waltham, Massachusetts, USA). The powder was resuspended in phosphate-buffered saline (0.0017 M KH2PO4, 0.005 M Na2HPO4, 0.150 M NaCl, pH 7.4) at a concentration of 1 g/L and stored as stock solution at − 20 °C in 20 μL aliquots. A BODIPY FL casein substrate working solution was prepared by diluting the stock solution to 10 mg/L with digestion buffer (10 mM Tris-HCl, pH 7.8, and 0.1 mM sodium azide) just before use. Sludge samples, less than 1 week old unless stated otherwise, were mixed and diluted to 1 g total solids (TS)/L with 10 mM Tris-HCl, pH 7.8. A Tris-HCl buffer at 50 mM for improved pH control was applied for MWWTP Nieuwveer samples only. Diluted sludge and the BODIPY FL casein working solution were incubated in triplicate or quadruplicate in a 96-well plate (art. no. 655090, Greiner bio one international GmbH, Kremsmünster, Austria) with a 100 μL/50 μL ratio, resulting in a final biomass-to-substrate ratio of 200:1 gTS/g. The plates were incubated at ambient temperature (23–27 °C) with moderate, orbital, on-line mixing at 6 mm amplitude in an Infinite M200 pro microplate reader (Tecan trading AG, Männedorf, Switzerland). The temperature exceeded 27 °C on some occasions during summer and annotated correspondingly in “Results”. Fluorescence intensity (arbitrary unit U) was determined every 10 min for at least 4 h, with excitation/emission at 485/520 nm. Further increases in the measuring frequency caused excessive BODIPY FL casein bleaching (data not shown). The first measurement was obtained after an initial mixing period of 10 min. All assays included substrate only and sludge only blanks in triplicate or quadruplicate. The full protocol for this assay is provided in Online resource 1, Protocol S1.
Sludge inactivation with a steam autoclave (Laboklav SHP 160 MSL, SHP steriltechnik AG, Detzel Schloss, Germany) was performed with MWWTP Leuven samples of December 2, 2019. To this end, a 150-mL sludge sample was autoclaved for 20 min at 121 °C and subsequently cooled down to room temperature. The autoclaved samples were stored at 4 °C and analyzed the day after. Sludge inactivation by freeze-thaw cycles was performed with the MWWTP Leuven sample of September 26, 2019. To this end, a 50-mL sludge sample was frozen at either − 20 °C or − 80 °C for at least 24 h. Afterwards, samples were defrosted slowly at 4 °C for at least 12 h prior to analysis. A second freeze-thaw cycle on the same samples was performed with the same method.
Reactor description and sampling
Samples were obtained from two full-scale MWWTPs and a lab-scale, two-stage, anaerobic reactor. A schematic of the full-scale plants and an overview of the operational performance at the time of sampling are provided in Fig. 1 and Online resource 1, Table S1, respectively.
Schematic overview of the a aerobic conventional municipal wastewater treatment plant Leuven and b aerobic high-rate municipal wastewater treatment plant Nieuwveer. Numbers in the bottom right corner indicate the number of tanks. Green diamonds indicate sampling points. Intermediate settling tanks and secondary settling tanks were either rectangular, as depicted below (6 intermediate, 6 secondary) or circular (1 intermediate, 3 secondary). Anaer = anaerobic, Anox = anoxic. *After the anaerobic zone, flows are diverted towards two carrousels, each with two subzones that can be aerated or anoxic
MWWTP Leuven is operated by Aquafin (Belgium) and has a design capacity of 135,000 population equivalents (P.E., 60 gBOD5/P.E./d). The incoming wastewater has a domestic origin with a minor fraction of industrial discharge (8% in 2018). The plant layout includes primary treatment (6 mm screens, oil and grease removal, sand trap), secondary treatment with anaerobic, aerobic, and anoxic zones and secondary sedimentation. Grab samples of 0.5 L were collected at six different locations in the morning of August 6, September 26, October 31, and December 2, 2019, transported to the lab within 1 h, and stored at 4 °C until the time of analysis. Samples were analyzed the next day for all samples, except for the October 31, 2019 sample (stored for 4 days).
MWWTP Nieuwveer is operated by Waterschap Brabantse Delta (the Netherlands) and functions as a two-stage, high-rate, activated sludge process, also denoted as an AB system. It has a design capacity of 362,000 P.E. with one P.E. defined as 150 gTOD/d. TOD takes into account oxygen demand for carbon and nitrogen removal (CTOD = CBOD5 + 4.18CNH4-N, Clifford and McGaughey 1982). This facility treats wastewater of domestic origin with a minor fraction of industrial discharge (< 10%). Primary treatment consists of coarse screens, oil and grease removal, and a sand trap. The two-stage process consists of a high-rate tank (3500 m3) with a desired hydraulic- (HRT) and sludge retention time (SRT) of 25 min and 0.6 day, respectively. Afterwards, sludge is allowed to settle in an intermediate settling tank with partial sludge recycle to the high-rate tank. The effluent from the intermediate settling is treated further in four parallel low-rate treatment tanks (three tanks 5400 m3 and one tank 12,000 m3). The low-rate tanks are comparable to a conventional activated sludge system and are followed by secondary settling. Settled sludge is recycled to the inlet of the low-rate tanks. Both the high-rate tank and low-rate tanks consist of a sequence of anoxic, facultative oxic, and oxic zones. More information on the plant layout and operational performance can be found in De Graaff et al. (2016) and Meerburg et al. (2016). Grab samples of 0.5 L were collected at 20 different locations in the morning of January 14, 2020, transported to the lab within 2 h, and stored at 4 °C until analyzed the day after.
The parallel, lab-scale, two-stage, anaerobic reactors were operated by Veolia Water Technologies Techno Center B.V. (Biothane, the Netherlands) for 200 days. The parallel reactors were fed with the same synthetic dairy wastewater, high in suspended solids, containing full fat milk (48 mL/L), granulated sugar (3.8 g/L), freeze-dried blood (1.9 g/L), sodium hydrogen carbonate (NaHCO3, 50 mM), and trace metals. Approximately 50% of the feed COD was non-soluble and it contained 3.7 g/L suspended solids. The parallel setups consisted of (i) an acidification tank (1 to 4.5 L, installed on day 72 in parallel setup 2) for partial hydrolysis of the feed and conversion of the soluble monomers to volatile organic acids, (ii) the reactor tank for additional hydrolysis, acidification and conversion to biogas (22 L), and (iii) the gravitational settling tank for biomass retention and recycling to the reactor tank. The reactor tank was inoculated with sludge from a full-scale anaerobic flocculent reactor system, while no inoculum was added to the acidification tank. Temperature control at 37 °C was applied for the reactor tank, while the acidification tank was operated at ambient conditions (23–26 °C). The first phase of the reactor experiment (days 0–50) was implemented to investigate the loading limits of a system with or without acidification tank, followed by a stabilization and recovery period from the high loading phase (days 50–100). From day 100 onwards, the focus was shifted to the study of an optimal HRT in the acidification tank. The biomass of the parallel acidification tank and reactor tanks was completely mixed and redistributed over the parallel setups on day 98 to ensure similar microbial communities at the start of this phase. Complete mixing and redistribution were repeated on day 114 for the acidification tank only. Fresh samples were shipped cooled, not frozen, and arrived within 24 h. Samples were stored at 4 °C until the time of analysis. All samples were analyzed within 6 days after sampling, except for samples on day 0 (11 days after sampling) and day 30 (13 days after sampling).
All environmental and operational data for the different reactor systems were obtained from the system operators and were determined according to standard methods (Greenberg et al. 1992).
Data analysis
Raw fluorescence intensities for the individual replicate wells (arbitrary unit U) were corrected with the average intensity of the substrate only and the average intensity of the sludge only blanks for every time point. Time was recalculated with t = 0 min as the moment of substrate addition to the first well in the plate. Manual filling of a full plate took approximately 3 min. A linear fit was calculated through the first 18 measurements, i.e., t = 0 to t = 200 min, with least squares regression in MS Excel™. Linear fit values were calculated for every replicate separately and normalized on a volatile solid (VS) basis. The coefficient of variation (CV) was calculated as the ratio of the sample standard deviation of the replicates over the mean of the replicates, expressed in percent values.
Results
Protein hydrolysis assay validation
Two experiments were conducted to test the assay validity. First, confirmation that measurements of protein hydrolysis rates are related to a biological action on the labeled BODIPY FL casein substrate was obtained in a validation experiment with samples of municipal wastewater treatment plant (MWWTP) Leuven. Steam autoclaving eliminated almost all proteolytic activity (Table 1). Values for the effluent sample were somewhat inflated through volatile solid (VS) normalization. Freeze-thaw cycles with freezing at − 20 °C or − 80 °C did not have a similar reduction effect. The protein hydrolysis rate never declined to less than 60% of the original value (Table 2).
Second, possible effects of sample storage at 4 °C were evaluated in an experiment with samples of MWWTP Leuven, obtained on August 6, 2019 (Fig. 2). Protein hydrolysis rates decline in an approximately linear fashion for the first 3 weeks of storage. This linear trend was confirmed through additional storage experiments over a 1-week period with samples obtained on September 26, 2019 and October 31, 2019. The average rate of decline was − 1% of the initial activity per additional day of storage in the first week for these additional samples (minimum + 0.5%, maximum − 2.1%, N = 19).
Evolution of protein hydrolysis rates during storage at 4 °C for raw influent (a), secondary treatment anaerobic zone (b), secondary treatment aerobic zone (c), and distribution point to secondary settling (d) at MWWTP Leuven, sampled on August 6, 2019. Protein hydrolysis rates are volatile solid normalized data rescaled to the average of the first measurement (value 100). Data points are individual values for all replicates. The sample collection date is day 0. Temperature exceeded 27 °C during protein hydrolysis rate determination in the first three experiments for every sample (Tmax = 29.9, 28.7, 32.1/30.7 on days 1, 2, 22/23)
Distribution of protein hydrolysis over space and time
One of the main application niches of protein hydrolysis assays is the determination of the activity profile over a treatment installation, i.e., spatial profiling, and follow-up of protein hydrolysis rates in a certain treatment zone over time. The latter is typically applied in research on factors influencing the metabolic activity of the microbial community, such as influent composition and load, temperature and pH. Proof-of-concept for the BODIPY FL casein assay is provided in this section with three different case studies aiming at profiling in different settings.
Full-scale municipal wastewater treatment
Protein hydrolysis rates were measured four times over the course of summer and autumn 2019 at MWWTP Leuven. Normalized values for protein hydrolysis rates of all replicates are shown in Fig. 3. Protein degradation was not limited to the secondary treatment stages, and already occurred in the raw influent and in the influent after screening. The protein hydrolysis rate in the effluent was low for all samples and varied from 6 to 8% of the average obtained for the three secondary treatment samples. The data showed no clear seasonal trend in protein degradation, although water temperatures in the biological treatment tank did decrease over time from 21.8 ± 1.1 °C on average in August to 14.2 ± 1.2 °C on average in December.
Protein hydrolysis rates for MWWTP Leuven, sampled throughout summer and autumn in 2019. Data points are individual values for all replicates (U/min/mg/VS). ○: August 6, 2019 (N = 6), ◆: September 26, 2019 (N = 6), ●: October 31, 2019 (N = 6), ◇: December 2, 2019 (N = 4). None of the influent or effluent samples was diluted, except for the raw influent and the influent after screening for October 31, 2019. Temperature exceeded 27 °C (Tmin = 21 °C, Tmax = 30 °C) during protein hydrolysis rate determination for the August 6, 2019 sample. Raw data for this figure are provided in Online resource 1, Table S2
Similarly, protein hydrolysis was determined for samples collected on January 14, 2020 at the high-rate MWWTP Nieuwveer. Normalized values for protein hydrolysis rates of all replicates are presented in Table 3. On average, protein hydrolysis rates in the high-rate stage were considerably lower than the values recorded for both low-rate stages (667 ± 70 versus 1248 ± 34 and 1215 ± 55, N = 9). The low-rate stage sludge recycle for tank 2 had a lower biomass concentration than the corresponding low-rate biological tank. This lower concentration was most probably related to sedimentation in the recycle ditch. The flow rate at the time of sampling was moderate, which could have allowed partial sludge settling. Furthermore, one outlier value was detected for the secondary settling tank connected to tank 4 (1168 U/min/mg/VS). This outlier was most probably related to washout sludge in the upper layers of the settling tank.
Lab-scale, two-stage anaerobic reactor
Protein hydrolysis rates were determined at nine time points throughout an experimental trial with two parallel two-stage, lab-scale, anaerobic reactors (Fig. 4). From day 100 onwards, one of the setups was subjected to variable hydraulic retention times in the acidification tank, by adapting the volume of the acidification tank, but not the volume of the reactor tank, while keeping the influent flow constant. Remarkably, the proportion of the total protein hydrolysis rate in the acidification tank increased significantly between day 107 and day 133 for the setup subjected to variable hydraulic loading.
Protein hydrolysis rates for two parallel anaerobic reactor setups. a Two-stage reactor with variable HRT in the acidification tank and b two-stage reactor with stable HRT in the acidification tank. Protein hydrolysis rates are averages over the replicates. AT = acidification tank, RT = reactor tank, *no acidification tank was installed, NA indicates that no sample was available. HRT values are presented as [HRT acidification tank; HRT reactor tank]. All samples were analyzed within 6 days after sampling, except for samples on day 0 (11 days after sampling) and day 30 (13 days after sampling). Temperature exceeded 27 °C during protein hydrolysis rate determination for samples on day 107 (Tmax = 30 °C) and day 133 (Tmax = 28.2 °C)
Discussion
Protein hydrolysis assay validation
Any enzyme assay should fulfill five fundamental requirements, being (i) a close approximation of reality through selection of the model substrate (ii) stability of the substrate and the enzyme prior to and throughout the assay, (iii) close contact between substrate and enzyme under the assay conditions, (iv) reproducible, sensitive measurement of product formation over time, and (v) adequate data normalization relevant for the research question (German et al. 2011).
First, it is widely acknowledged that casein is a suitable model substrate to measure the diversity of proteases in biological systems, as mentioned in the introduction. The hydrolytic action of different protease subtypes on BODIPY FL casein specifically has been established previously with protease preparations from different origins, such as snake venom (Price 2015), bacterial and fungal proteases (Ha et al. 2012), and yeast proteases (Hutter et al. 2005).
Second, substrate stability is ensured for BODIPY FL-based substrates as the BODIPY moiety has low fluorescence bleaching rates and is stable over a pH range from 2 to 11 (Schade et al. 1996). All BODIPY FL casein substrates in powder form or as concentrated stock were stored at − 20 °C according to the manufacturer’s instructions. Substrate only control samples did not show significant storage-induced changes in fluorescence (Online resource 1, Table S3). In contrast, sludge stability might differ depending on the sample origin. Protein hydrolysis rates declined slowly, in an approximately linear fashion in this study (Fig. 2). Proteases are floc-associated, either linked to the microbial cell membrane or encapsulated in the extracellular polymeric matrix (Frolund et al. 1995; Gessesse et al. 2003; Ni et al. 2017). This association stabilizes proteases to a large extent and most probably protects against fast degradation by proteolysis, as previously described in lake aquatic ecology (Kiersztyn et al. 2012). Analysis of fresh samples should remain the standard, as not all samples have a fully developed floc structure or the floc morphology is unknown beforehand.
Temperature and pH should remain constant as well throughout one assay and should be reproducible throughout a series of assays. Protein hydrolysis assays are typically performed with a buffered medium in wastewater treatment research (pH 7.5–8 with phosphate or Tris-HCl buffers), to enable comparison over different experiments and to avoid (i) issues with substrate aggregation, (ii) inhibitory effects of acidic conditions on the enzyme activity, and (iii) a mismatch between the assay pH and the actual process conditions, the latter often close to neutral pH (Cadoret et al. 2002; German et al. 2011; Gessesse et al. 2003). Moreover, buffer molarity should be adapted to the sample in question, as sludge samples can have a large natural buffering capacity. The 10 mM Tris-HCl buffer, suggested by Xia et al. (2007), did not provide a diluted sample pH close to 7.8 for all samples. Therefore, the buffer molarity was increased to 50 mM in the experiments for MWWTP Nieuwveer, with good results in terms of diluted sample pH (Online resource 1, Table S4). The assay temperature typically varies from ambient temperature to 37 °C in wastewater treatment research on proteases. Also here, the assay temperature should be adapted to resemble the process conditions (German et al. 2011). A distinct difference should be noted for aerobic treatment processes often operated at ambient temperature and anaerobic processes functioning optimally at 37 °C. Temperature control could not be applied in the experiments presented here. Therefore, results should be analyzed with this practical limitation in mind.
Third, a close contact between the BODIPY FL casein substrate and the enzymes encapsulated in the sludge was provided through moderate mixing in the intervals between fluorescence measurements. The rate of substrate transport towards the enzymes is determined by two opposing factors. On the one hand, diffusion limitations can slow down substrate transport, as casein is a stable micelle with a diameter of 50 to 500 nm (Fox and Brodkorb 2008). Cadoret et al. (2002) observed that azocasein degradation did not increase in activated sludge by prior floc dispersion with ultrasound or cation exchange resin. This observation led the authors to conclude that there was no diffusion limitation for the azocasein substrate in that case. On the other hand, the matrix of extracellular polymeric substances (EPS) in the floc can also serve as a substrate trap by adhesion forces, hindering the release of the substrate back into solution, which in turn leads to a driving force of substrate transport into the EPS matrix (Sivadon et al. 2019). Therefore, this assay incorporates not only the rate of substrate degradation but also the rate of substrate transport towards the protease enzyme for a given sludge sample.
Fourth, the measurement of product formation over time can be affected by fluorescence quenching in the sludge matrix and by fluorescent signal development derived from physico-chemical action on the substrate. Quenching through sludge-BODIPY interactions was not evaluated in this study, but could be studied in more detail with purified BODIPY FL labeled peptides or tailor-made labeled substrate analogs (Rezende et al. 2013). Excessive bleaching should be avoided by tailoring the measurement frequency to the fluorophore. Measurements at 5-min intervals, for example, were found to cause excessive bleaching with BODIPY FL casein in this study.
The measurement reproducibility can be analyzed with the coefficient of variation (CV) for the replicates. The CV ranged from 2 to 8.5% for most full-scale MWWTP samples, with five outliers in the dataset of 42 fresh samples shown in Fig. 3 and Table 3. These outliers included three of the effluent samples for MWWTP Leuven with CV 12.6%, 22.9%, and 89.7% for sampling on October 31, 2019, August 6, 2019, and December 2, 2019, respectively. The other outliers were a sample for one of the secondary settling tanks at MWWTP Breda, experiencing sludge washout (CV 75.1%) and the influent sample after screening, sand and grease removal at MWWTP Breda (CV 17.2%). A similar range was obtained for the lab-scale, two-stage anaerobic reactors (Q1–Q3 3–7%, N = 30), with five outliers. The reproducibility of the results presented here is in line with the study by Kreutz et al. (2016) with an L-alanine-aminopeptidase assay on a full-scale MWWTP. These authors defined an acceptable cutoff of 20% for the coefficient of variation of experimental quadruplicates. The sample heterogeneity is the main cause for outliers in reproducibility in this study, and not measurement variability. The sample heterogeneity cannot be reduced without introducing new possible errors. Sample homogenization prior to the assay, with ultrasound or high-speed mixing for example, drastically changes the original floc morphology and can cause cell lysis.
Fifth, data normalization is often performed in previous research on a volumetric basis [product units/time sample volume] and sometimes correlated later on with the sludge biomass concentration. A further complication is the measurement of product units, as these are defined differently for every assay substrate. In this study, data were normalized directly to the closest available approximation of active biomass, i.e., volatile solids [product units/time/volatile solids]. Product units are direct measurements of fluorescence intensity (arbitrary unit U).
Distribution of protein hydrolysis over space and time
Full-scale municipal wastewater treatment
Three common trends were observed in the results for MWWTP Leuven and MWWTP Nieuwveer. First, protein hydrolysis rates were similar in the aerated and anoxic zones of a biological treatment tank. This observation is somewhat surprising, as the electron acceptor conditions are different in anoxic and aerobic zones. However, a similar result was obtained with an azocasein assay on a lab-scale sequencing batch reactor with anaerobic-aerobic process conditions by Goel et al. (1998) and later confirmed with an azocasein and L-alanine aminopeptidase assay in a full-scale membrane bioreactor and a full-scale conventional activated sludge system by Gómez-Silván et al. (2013) and Kreutz et al. (2016), respectively. Sludge recirculation and hydrodynamic mixing appear to distribute the protein hydrolysis process evenly over interconnected reactor zones.
Second, the majority of the incoming protein was indeed hydrolyzed in the secondary treatment stage, as expected. The low-rate stage showed higher activities than the high-rate stage. To the authors’ knowledge, no previous studies have been performed on protein hydrolysis in full-scale, two-stage systems. Additional data are required to confirm this as a recurring observation.
Third, the specific, volatile solid normalized activity in the raw influent was at least 70% of the average activity in the biological treatment stages and even exceeded that activity in two sampling moments at MWWTP Leuven. The influent was often not analyzed in previous research, with two exceptions. Fischer et al. (2013) and Wiegand-Rosinus et al. (1995) report a tenfold lower volumetric activity in the influent compared to the biological treatment stage. Both authors obtained these values with an L-alanine aminopeptidase assay. When recalculating values to a volumetric activity, by multiplying the normalized values with the volatile solid concentration for influent and reactor samples, similar ratios of 10 to 12% for the influent versus biological treatment samples are obtained, except for the October 31, 2019 sample at MWWTP Leuven with a higher value (45%). This sample had a high influent solid concentration, suggesting a high influent organic load, for example due to diurnal patterns in emission of organic contaminants. A similar peak in hydrolytic activity related to a high influent organic load, measured as influent COD, was also observed by Fischer et al. (2013). The importance of the influent as a continuous source of active biomass immigrating into the wastewater treatment facility has only been recognized in recent years. The sewer system receives constant microbial inputs from domestic discharge and groundwater infiltration, which results in a highly dynamic sewer ecosystem in space and time (Frigon and Wells 2019). Although the net effect of immigration on the full microbial community in the treatment facility might be modest (Saunders et al. 2016), there is increasing evidence that immigration from the sewer system should not be neglected in specific cases such as nitrification at low temperatures (Jauffur et al. 2014). Therefore, it is suggested to incorporate influent samples in any future research on hydrolysis, especially for systems operated near their operational limits, e.g., high-rate activated sludge or low-temperature biological treatment.
Lab-scale, two-stage anaerobic reactor
The specific protein hydrolysis activities obtained in this trial are lower than the values obtained for full-scale aerobic MWW treatment facilities in this study, although the synthetic wastewater for the two-stage anaerobic reactors contained full fat milk as one of the main carbon sources. At present, it is unclear if this observation is related to a difference in overall microbial activity under aerobic and anaerobic conditions or related to other factors (Nybroe 1992).
The results for this trial should be analyzed with care, as a two-stage anaerobic reactor is dynamic in terms of biomass composition and metabolic activity. The complete lack of biomass retention and low hydraulic retention times in the acidification tank may result in rapid changes in protein hydrolysis rates. The results for day 107 indeed confirm this, with almost non-detectable (system 1) versus considerable activity (system 2) in the acidification tank, even though the parallel acidification tank and reactor tank contents were mixed and redistributed over system 1 and system 2 on day 98. The period of low activity for system 1 coincided with a low pH in the acidification tank, below 5.2 from day 102 to 120, and a steep increase in the levels of propionic acid, from 140 mg/L on day 1 to 1675 mg/L on day 12.
Nevertheless, it can be concluded that the operating conditions of the reactor systems can be adapted to increase the protein hydrolysis rate. On day 133, system 1 had developed a protein hydrolysis metabolic activity also in the acidification tank, through (i) complete mixing and redistribution of acidification tank contents over both parallel systems on day 114 and (ii) a decrease in the hydraulic retention time (HRT) for the acidification tank from day 114 onwards. The latter decrease in HRT also enabled a decrease in the concentrations of organic acids (5.3, 0.7, 0.4 gtVFA-COD/L on days 107, 128, and 136). High levels of organic acids can cause a pH drop, even when carbonate buffering is applied in the incoming wastewater, i.e., 50 mM NaHCO3 in this trial. A low pH most probably affects protein hydrolysis through physico-chemical effects. Milk casein is known to aggregate and sediment below a pH of 5.2 (Dalgleish and Corredig 2012), and therefore becomes unavailable for microbial hydrolysis in suspension.
In conclusion, the time course of protein hydrolysis in the two-stage anaerobic reactors indicates that a two-stage reactor setup is highly dynamic. A balance between hydraulic regime, buffering capacity, microbial activity, and operational pH should be maintained to ensure good performance in these anaerobic systems.
This study demonstrates that the BODIPY FL casein substrate can be applied successfully for fast and accurate measurement of protein hydrolysis in wastewater treatment systems. However, the current assay focuses only on proteins, while carbohydrates and lipids also comprise a large fraction of the incoming organic load in municipal wastewater treatment and in various industrial waste streams. Therefore, additional fluorescent substrates should be identified and integrated with the current approach. BODIPY FL DQ starch is a promising substrate for amylase activity and has been applied previously for microscopic studies of activated sludge (Xia et al. 2008) and high-throughput screening of fungal strains for amylase activity (Beneyton et al. 2016). Improvements in the throughput of this method could be obtained by automating not only the measurement, but also the sample processing steps such as sample dilution and mixing. Such a fully automated technique could then be integrated with other techniques such as BIOLOG physiological profiling (Button et al. 2016) or integrated multi-omics sample processing (Roume et al. 2013).
The ultimate goal of enzymatic screening efforts in wastewater treatment systems is to develop a mechanistic understanding of the dynamics in enzymatic activity in response to changing process conditions (Gómez-Silván et al. 2013; Hassard et al. 2020; Nybroe 1992). A mechanistic understanding would then lead to improved process control and biological performance. This goal can only be obtained through long-term measuring campaigns with a high sampling frequency, i.e., at least daily, in parallel with in-depth monitoring of relevant process parameters such as operating temperature, influent flow, influent composition and load, redox potential, pH and hydraulic and solid retention times in the system. Such a sampling campaign can only be executed if the throughput of the protein hydrolysis assay is sufficiently high.
References
Beneyton T, Wijaya IPM, Postros P, Najah M, Leblond P, Couvent A, Mayot E, Griffiths AD, Drevelle A (2016) High-throughput screening of filamentous fungi using nanoliter-range droplet-based microfluidics. Sci Rep 6:1–10. https://doi.org/10.1038/srep27223
Burgess J (2008) Hydrolytic enzymes in sewage sludge treatment: a mini-review. Water SA 34:343–350
Button M, Weber K, Nivala J, Aubron T, Muller RA (2016) Community-level physiological profiling of microbial communities in constructed wetlands: effects of sample preparation. Appl Biochem Biotechnol 178:960–973. https://doi.org/10.1007/s12010-015-1921-7
Cadoret A, Conrad A, Block JC (2002) Availability of low and high molecular weight substrates to extracellular enzymes in whole and dispersed activated sludges. Enzym Microb Technol 31:179–186. https://doi.org/10.1016/S0141-0229(02)00097-2
Charney J, Tomarelli RM (1947) A colorimetric method for the determination of the proteolytic activity of duodenal juice. J Biol Chem 171:501–505
Clifford DA, McGaughey LM (1982) Simultaneous determination of total nitrogen and total oxygen demand in aqueous samples. Anal Chem 54:1345–1350. https://doi.org/10.1021/ac00245a022
Dalgleish DG, Corredig M (2012) The structure of the casein micelle of milk and its changes during processing. Annu Rev Food Sci Technol 3:449–467. https://doi.org/10.1146/annurev-food-022811-101214
De Graaff MS, Van Den Brand TPH, Roest K, Zandvoort MH, Duin O, Van Loosdrecht MCM (2016) Full-scale highly-loaded wastewater treatment processes (A-stage) to increase energy production from wastewater: performance and design guidelines. Environ Eng Sci 33:571–577. https://doi.org/10.1089/ees.2016.0022
Felz S, Vermeulen P, van Loosdrecht MCM, Lin YM (2019) Chemical characterization methods for the analysis of structural extracellular polymeric substances (EPS). Water Res 157:201–208. https://doi.org/10.1016/j.watres.2019.03.068
Fischer K, Wolff B, Emmerling C (2013) Inter and intra plant variability of enzyme profiles including various phosphoesterases and sulfatase of six wastewater treatment plants. Bioresour Technol 137:209–219. https://doi.org/10.1016/j.biortech.2013.03.128
Fox PF, Brodkorb A (2008) The casein micelle: historical aspects, current concepts and significance. Int Dairy J 18:677–684. https://doi.org/10.1016/j.idairyj.2008.03.002
Frigon D, Wells G (2019) Microbial immigration in wastewater treatment systems : analytical considerations and process implications. Curr Opin Biotechnol 57:151–159. https://doi.org/10.1016/j.copbio.2019.02.021
Frolund B, Griebe T, Nielsen PH (1995) Enzymatic activity in the activated-sludge floc matrix. Appl Microbiol Biotechnol 755–761
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–1397. https://doi.org/10.1016/j.soilbio.2011.03.017
Gessesse A, Dueholm T, Petersen SB, Nielsen PH (2003) Lipase and protease extraction from activated sludge. Water Res 37:3652–3657. https://doi.org/10.1016/S0043-1354(03)00241-0
Goel R, Mino T, Satoh H, Matsuo T (1998) Enzyme activities under anaerobic and aerobic conditions in activated sludge. Water Res 32:2081–2088
Gómez-Silván C, Arévalo J, Pérez J, González-López J, Rodelas B (2013) Linking hydrolytic activities to variables influencing a submerged membrane bioreactor (MBR) treating urban wastewater under real operating conditions. Water Res 47:66–78. https://doi.org/10.1016/j.watres.2012.09.032
Greenberg AE, Clesceri LS, Eaton AD (Eds.) (1992) Standard methods for the examination of water and wastewater, 18th ed. American public health association (APHA) publications
Ha M, Bekhit AEDA, Carne A, Hopkins DL (2012) Characterisation of commercial papain, bromelain, actinidin and zingibain protease preparations and their activities toward meat proteins. Food Chem 134:95–105. https://doi.org/10.1016/j.foodchem.2012.02.071
Hashimoto W, Momma K, Maruyama Y, Yamasaki M, Mikami B, Murata K (2005) Structure and function of bacterial super-biosystem responsible for import and depolymerization of macromolecules. Biosci Biotechnol Biochem 69:673–692. https://doi.org/10.1271/bbb.69.673
Hassard F, Biddle J, Cartmell E, Coulon F, Stephenson T (2020) Biosolids recycling impact on biofilm extracellular enzyme activity and performance of hybrid rotating biological reactors. Sci Total Environ 706:135865. https://doi.org/10.1016/j.scitotenv.2019.135865
Haugland RP, Zhou M (1998) Dye labeled polymers as reagents for measuring polymer degradation. USA patent 5:719,031
Holt C (2016) Casein and casein micelle structures, functions and diversity in 20 species. Int Dairy J 60:2–13. https://doi.org/10.1016/j.idairyj.2016.01.004
Hutter KJ, Miedl M, Kuhmann B, Nitzsche F, Bryce JH, Stewart GG (2005) Detection of proteinases in Saccharomyces cerevisiae by flow cytometry. J Inst Brew 111:26–32. https://doi.org/10.1002/j.2050-0416.2005.tb00645.x
Jauffur S, Isazadeh S, Frigon D (2014) Should activated sludge models consider influent seeding of nitrifiers? Field characterization of nitrifying bacteria. Water Sci Technol 70:1526–1532. https://doi.org/10.2166/wst.2014.407
Jones LJ, Upson RH, Haugland RP, Panchuk-Voloshina N, Zhou M, Haugland RP (1997) Quenched BODIPY dye-labeled casein substrates for the assay of protease activity by direct fluorescence measurement. Anal Biochem 251:144–152. https://doi.org/10.1006/abio.1997.2259
Kiersztyn B, Siuda W, Chróst RJ (2012) Persistence of bacterial proteolytic enzymes in lake ecosystems. FEMS Microbiol Ecol 80:124–134. https://doi.org/10.1111/j.1574-6941.2011.01276.x
Kreutz JA, Bockenhuser I, Wacht M, Fischer K (2016) A 1-year study of the activities of seven hydrolases in a communal wastewater treatment plant: trends and correlations. Appl Microbiol Biotechnol 100:6903–6915. https://doi.org/10.1007/s00253-016-7540-6
Le C, Kunacheva C, Stuckey DC (2016) “Protein” measurement in biological wastewater treatment systems: a critical evaluation. Environ Sci Technol 50:3074–3081. https://doi.org/10.1021/acs.est.5b05261
Meerburg FA, Vlaeminck SE, Roume H, Seuntjens D, Pieper DH, Jauregui R, Vilchez-Vargas R, Boon N (2016) High-rate activated sludge communities have a distinctly different structure compared to low-rate sludge communities, and are less sensitive towards environmental and operational variables. Water Res 100:137–145. https://doi.org/10.1016/j.watres.2016.04.076
Ni H, Fan X, Guo H, Liang J, Li Q, Yang L (2017) Comprehensive utilization of activated sludge for the preparation of hydrolytic enzymes , polyhydroxyalkanoates , and water-retaining organic fertilizer. Prep Biochem Biotechnol 47:611–618. https://doi.org/10.1080/10826068.2017.1286599
Nybroe O (1992) Enzyme activities in waste water and activated sludge. Water Res 26:579–584. https://doi.org/10.1016/0043-1354(92)90230-2
Price JA (2015) Microplate fluorescence protease assays test the inhibition of select North American snake venoms’ activities with an anti-proteinase library. Toxicon 103:145–154. https://doi.org/10.1016/j.toxicon.2015.06.020
Raunkjær K, Hvitved-Jacobsen T, Nielsen PH (1994) Measurement of pools of protein, carbohydrate and lipid in domestic wastewater. Water Res 28:251–262. https://doi.org/10.1016/0043-1354(94)90261-5
Rezende LCDDL, Emery S, Emery F (2013) A review of the synthetic strategies for the development of BODIPY dyes for conjugation with proteins. Orbital Electron J Chem 5:62–83. https://doi.org/10.17807/orbital.v5i1.482
Roume H, Heintz-Buschart A, Muller EEL, Wilmes P (2013) Sequential isolation of metabolites, RNA, DNA, and proteins from the same unique sample, 1st ed, Methods in Enzymology Elsevier Inc https://doi.org/10.1016/B978-0-12-407863-5.00011-3
Saunders AM, Albertsen M, Vollertsen J, Nielsen PH (2016) The activated sludge ecosystem contains a core community of abundant organisms. ISME J 10:11–20. https://doi.org/10.1038/ismej.2015.117
Schade SZ, Jolley ME, Sarauer BJ, Simonson LG (1996) BODIPY-alpha-casein, a pH-independent protein substrate for protease assays using fluorescence polarization. Anal Biochem 243:1–7. https://doi.org/10.1006/abio.1996.0475
Sivadon P, Barnier C, Urios L, Grimaud R (2019) Biofilm formation as a microbial strategy to assimilate particulate substrates. Environ Microbiol Rep 11:749–764. https://doi.org/10.1111/1758-2229.12785
Twining SS (1984) Fluorescein isothiocyanate-labeled casein assay for proteolytic enzymes. Anal Biochem 143:30–34. https://doi.org/10.1016/0003-2697(84)90553-0
Wiegand-Rosinus M, Grollius H, Gerlizki E, Obst U (1995) Investigation of municipal waste water treatment with enzymatic activity tests in vivo. Vom Wasser 84:143–153
Xia Y, Kong Y, Nielsen PH (2007) In situ detection of protein-hydrolysing microorganisms in activated sludge. FEMS Microbiol Ecol 60:156–165. https://doi.org/10.1111/j.1574-6941.2007.00279.x
Xia Y, Kong Y, Nielsen PH (2008) In situ detection of starch hydrolyzing microorganisms in activated sludge. FEMS Microbiol Ecol 66:462–471. https://doi.org/10.1111/j.1574-6941.2008.00559.x
Xia Y, Massé DI, McAllister T a, Beaulieu C, Talbot G, Kong Y, Seviour R (2011) In situ identification of keratin-hydrolyzing organisms in swine manure inoculated anaerobic digesters. FEMS Microbiol Ecol 78:451–462. https://doi.org/10.1111/j.1574-6941.2011.01188.x
Xia Y, Massé DI, McAllister TA, Beaulieu C, Ungerfeld E (2012) Anaerobic digestion of chicken feather with swine manure or slaughterhouse sludge for biogas production. Waste Manag 32:404–409. https://doi.org/10.1016/j.wasman.2011.10.024
Xia Y, Wang DK, Kong Y, Ungerfeld EM, Seviour R, Massé DI (2015) Anaerobic digestibility of beef hooves with swine manure or slaughterhouse sludge. Waste Manag 38:443–448. https://doi.org/10.1016/j.wasman.2014.12.017
Xia Y, Kong Y, Huang H, Yang HE, Forster R, Mcallister TA (2016) In situ identification and quantification of protein-hydrolyzing ruminal bacteria associated with the digestion of barley and corn grain. Can J Microbiol 62:1063–1067. https://doi.org/10.1139/cjm-2016-0293
Acknowledgments
We gratefully acknowledge Waterschap Brabantse Delta (the Netherlands), Aquafin (Belgium), and Veolia Water Technologies Techno Center B.V. (Biothane, the Netherlands) for providing sludge samples and reactor operational data. We thank Johan Martens and Tom Bosserez for providing access to the Infinite M200 pro microplate reader and Koen Rummens for assistance in statistical analysis.
Funding
Pieter Van Gaelen was supported as doctoral candidate (aspirant) by the Research Foundation-Flanders (FWO–Vlaanderen; file number 28738).
Author information
Authors and Affiliations
Contributions
PVG conceived, designed, and conducted experiments, performed data analysis, and wrote the first draft of the manuscript. DS and IS supervised the project, reviewed the manuscript, and acquired funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 764 kb)
Rights and permissions
About this article
Cite this article
Van Gaelen, P., Springael, D. & Smets, I. A high-throughput assay to quantify protein hydrolysis in aerobic and anaerobic wastewater treatment processes. Appl Microbiol Biotechnol 104, 8037–8048 (2020). https://doi.org/10.1007/s00253-020-10751-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10751-4