Abstract
The extremophile red alga Galdieria sulphuraria was successfully grown immobilized in a twin-layer porous substrate bioreactor (TL-PSBR). A maximal biomass growth rate of 10 g dry weight m−2 day−1 was measured at a photon fluence rate of 200 μmol photons m−2 s−1 with addition of 1% CO2 and a temperature of 34 °C. Under these conditions, a maximal biomass value of 232 g m−2 was attained after 33 days of growth. Phycobilin productivity, however, was highest at a lower photon fluence rate of 100 μmol photons m−2 s−1 and reached a phycobilin value of 14 g m−2, a phycobilin content in the biomass of 63 mg g−1 and a phycobilin growth rate of 0.28 g m−2 day−1 for phycocyanin and 0.23 g m−2 day−1 for allophycocyanin. Addition of CO2 was essential to enhance growth and phycobilin production in G. sulphuraria and further optimization of the cultivation process in the TL-PSBR appears possible using a multi-phase approach, higher growth temperatures and optimization of nutrient supply. It is concluded that autotrophic cultivation of G. sulphuraria in a TL-PSBR is an attractive alternative to suspension cultivation for phycobilin production and applications in bioremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The water-soluble fluorescent phycobiliproteins (PBPs) are important components of the light-harvesting photosynthetic system, present only in cyanobacteria, red algae, glaucophytes and cryptophytes (Gantt 1980; Glazer 1994; Grossman et al. 1993). The main components of phycobiliproteins are c-phycocyanin, phycoerythrin and allophycocyanin. C-phycocyanin (C-PC) and allophycocyanin (A-PC) are used such as fluorescent markers in diagnostic histochemistry, as dyes in cosmetics and foods, and because of their antioxidant properties may have potential as therapeutic agents (Rimbau et al. 1999; Romay et al. 2003; Fernandéz-Rojas et al. 2014; Manirafasha et al. 2016; Li et al. 2019; Pagels et al. 2019) .
Commercially, the production of C-PC is almost exclusively associated with Arthrospira (Spirulina) platensis, a planktonic, filamentous cyanobacterium (Vernes et al. 2015; Xie et al. 2015; Dejsungkranont et al. 2017; Ho et al. 2018; Hsieh-Lo et al. 2019; Nwoba et al. 2019; Yu et al. 2019; Abalde et al. 1998; Eriksen 2007). The C-PC market is projected to reach US$97 million by 2019 (Santos et al. 2019), and C-PC trades for up to 500 US$ kg−1 for food-grade (Kannaujiya and Sinha 2016) and 10–50 US$ mg−1 for analytical grade (Kuddus et al. 2013). Since the C-PC production process in A. platensis depends on externally supplied light, the productivity of biomass, and therefore C-PC, is low in the open pond/raceway suspension cultures. In phototrophic outdoor cultures of A. platensis, C-PC productivities have been estimated to be on the order of 3–24 mg L−1 day−1 (Schmidt et al. 2005) with dry biomass concentrations usually not exceeding 1 g/L. In addition to low productivity due to seasonal daily fluctuations in temperature, light intensity and uneven mixing, open-pond cultivation also suffers from contamination by other microalgae, predators and pathogens (Richmond et al. 1990; Carbone et al. 2018, 2019; Richmond and Qiang 2010; Olivieri et al. 2014; Gargano et al. 2016; Osorio et al. 2019; Zuccaro et al. 2019).
The thermophilic unicellular red alga G. sulphuraria has recently emerged as a prospective alternative for production of C-PC (Carfagna et al. 2016, 2018; Castenholz and McDermott 2010; Cennamo et al. 2012; Iovinella et al. 2018; Ciniglia et al. 2017), especially when grown heterotrophically (Graverholt and Eriksen 2007; Sloth et al. 2017; Eriksen 2013; Eriksen 2018). G. sulphuraria grows heterotrophically with a large number of organic carbon sources at pH 1–2 (Gross and Schnarrenberger 1995; Gross 1999), where most contaminating organisms will not grow. High biomass densities (> 100 g/L) have been reported under these conditions (Schmidt et al. 2005). Although some isolates of G. sulphuraria maintain C-PC synthesis in the dark, the C-PC concentration is strongly reduced (10–25 mg/g dry biomass) compared to autotrophically grown cultures of both G. sulphuraria and A. platensis (8–15% C-PC in the dry biomass), almost cancelling the advantage of the higher biomass productivities of heterotrophic cultivation. A recent analysis used a two-step cultivation of G. sulphuraria combining heterotrophic growth with subsequent dilution and exposure to high light intensities to achieve a C-PC content of 13% in the dry biomass and a C-PC yield of 0.8 g/L (Wan et al. 2016). However, organic carbon had to be completely removed after heterotrophic cultivation and culture parameters and bioreactors needed to be changed, making this approach technically demanding.
Both A. platensis and G. sulphuraria as well as most other commercially used microalgae are grown in suspension cultures. However, G. sulphuraria and other thermophilic red algae that belong to the subdivision cyanidiophytina (Yoon et al. 2004; Pinto et al. 2007) thrive in geothermal volcanic areas at temperatures around 40 °C and at high sulphuric acid concentration associated with soil, rocks and in cryptoendolithic habitats (Gross et al. 1998; Ciniglia et al. 2004; Pinto 2007). Technical cultivation of G. sulphuraria in biofilm systems could open a new avenue of research.
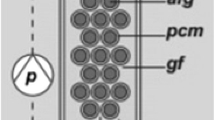
Porous substrate bioreactors (PSBR), especially of the twin-layer type (TL-PSBR; Nowack et al. 2005; Melkonian and Podola 2010), have recently attracted considerable attention as an alternative to low-density autotrophic suspension cultures (Podola et al. 2017; Pierobon et al. 2018; Zhuang et al. 2018). In brief, algae are immobilized by self-adhesion on a sheet-like (micro)porous substrate that is impermeable to the cells but permeable to the culture medium, the algae forming a biofilm. The culture medium is applied to the surface of the substrate layer opposite the biofilm, usually to a sheet-like (macro)porous source. The culture medium moves down the source layer by gravity flow and nutrients reach the biofilm by diffusion and convection through the substrate layer. The low energy demand for water circulation as well as the low water footprint and content of the biomass and associated ease of harvesting and processing of biomass make these systems attractive to many applications in bioremediation (Shi et al. 2007, 2014; Li et al. 2015, 2016; Piltz and Melkonian 2018), biotechnology and biorefinery processes (Podola et al. 2017; Wang et al. 2015). The low shear forces to which cells are exposed further enable the cultivation of a large diversity of microalgal species (Nowack et al. 2005; Naumann et al. 2013; Benstein et al. 2014; Kiperstok et al. 2017; Carbone et al. 2017a; Ekelhof and Melkonian 2017; Langenbach and Melkonian 2019). Here, we show that G. sulphuraria can be grown successfully in a bench-scale TL-PSBR to high biomass standing crops (230 g dry weight/m−2 growth area) and significant phycobilin amounts (14 g m−2). We evaluate the effects of different light intensities, addition of CO2 and frequency of exchange of culture medium on growth and C-PC/A-PC in production of G. sulphuraria in the TL-PSBR.
Materials and methods
Algal strain and culture medium
G. sulphuraria strain 064 ACUF (http://www.acuf.net) was chosen for the experiment. The stock culture grew in Galdieria medium (Gross and Schnarrenberger 1995) acidified by sulphuric acid at pH 1.5. Stock cultures were inoculated into a 250-ml glass column bioreactor and after 4 weeks, the scale-up was performed in 1 L Erlenmeyer flasks. Cultures were exposed to a photon fluence rate of 30 μmol photons m−2 s−1 with a light/dark cycle of 14/10 h. The temperature was adjusted with a transparent water bath to 34 °C.
Photobioreactor design and set up
The experiments were carried out in a bench-scale twin-layer system (TL-PSBR) as described by Shi et al. (2007) and Schultze et al. (2015).
A sodium discharge lamp (SON-T AGRO 400 W, Philips, Hamburg, Germany) was used to obtain the three light intensities (50 μmol m−2 s−1, 100 μmol m−2 s−1 and 200 μmol m−2 s−1) used in the experiments.
A quantum sensor (LI-190SA, LI-COR Biosciences GmbH, Bad Homburg, Germany) measured the photosynthetic active radiation (PAR); the light/dark cycle was again set to 14/10 h. Atmospheric CO2 (0.04%) or additional CO2 (1% v/v) were used for the experiments. The temperature was kept constant at 34 °C using an aquarium heater.
Inoculation on the twin-layer
When the microalgae achieved a sufficient cell density in suspension, the algae were harvested by centrifugation at 2000 rpm (Sorvall, RC5C) for 30 min and then immobilized on the twin-layer system using polycarbonate membranes (PC40, 0.4 μm pore size, 25 mm diameter, Whatman, Dassel, Germany) as the substrate layer. Microalgae were inoculated to a density of 10 g dry weight m−2 growth area by filtration.
Determination of biomass
During the first experiment, the biomass was harvested from the polycarbonate discs every 2 days in triplicate. In the next experiments, the biomass was harvested every 3 days in triplicate. Only biomass in the inoculated area was harvested; surplus biomass was scraped off (18 mm diameter). Then, the samples were lyophilized in a freeze dryer for 2 h and weighed in an analytical balance (Sartorius Bovenden, Germany).
Extraction and characterization of phycobilins
C-PC and A-PC were extracted after lyophilization from the dried biomass in the following way: the microalgae were removed from the disk and homogenized with quartz sand (Moraes et al. 2011); 4 ml of sodium acetate solution, pH 6.5 and 20 mM were added to the samples and the samples subjected to one freeze thaw cycle (Moon et al. 2014) at − 80 °C and 4 °C. After this step, the algae were centrifuged at 4 °C for 30 min at 10,000 rpm to remove cells debris. The blue-coloured supernatant contained the phycobiliproteins.
C-phycocyanin (C-PC) and allophycocyanin (A-PC) were measured spectrophotometrically using the following equations for quantification (Moon et al. 2014; Pan-Utai et al. 2018; Estrada et al. 2001):
where C-PC is c-phycocyanin, A-PC is allophycocyanin, C-EP and APC-EP are the purity of c-phycocyanin and allophycocyanin respectively, and A is the absorbance measured at the specified wavelength in quartz cuvettes.
Results
Here, we report, for the first time, growth of an extremophile red microalga in a TL-PSBR. Since no previous data on immobilized growth of G. sulphuraria existed, some basic parameters of growth were first tested such as varying light intensities and carbon dioxide addition (for technical reasons, the growth temperature was kept constant at 35 °C, which is considerably lower than the optimal growth temperature (42 °C) reported for this strain (Sloth et al. 2006).
Three sets of experiments were performed. In the first experiment, growth and phycobilin production were monitored over 14 days at two different carbon dioxide concentrations (atmospheric and 1% (v/v) and a fixed photon fluence rate of 100 μmol m−2 s−1. In the second set of experiments, over an extended experimental period of 30 days, different photon fluence rates (50, 100 and 200 μmol m−2 s−1) were compared at a fixed carbon dioxide concentration of 1% (v/v). Finally, the cultivation time was varied (14, 30 and 43 days) at a fixed photon fluence rate (100 μmol m−2 s−1) and carbon dioxide concentration (1%). In the latter experiment, nutrients were replenished more frequently (every 2 days instead of every 3 days) after 15 days of cultivation to avoid nutrient depletion.
Effect of CO2 addition on growth and phycobiliprotein production
G. sulphuraria strain 064 was tested in a TL-PSBR for 14 days in the presence of atmospheric CO2 (0.04%) or 1% CO2 (v/v, in the gas phase) at light intensities of 100 μmol m−2 s−1 (Fig. 1). Samples were taken every 2 days and the culture medium was exchanged every 3 days. During the experimental period, biomass growth was linear (Fig. 1). Addition of 1% CO2 had a significant positive effect on the biomass growth rate (~ 6 g m−2 day−1 with 1% CO2 compared to only 1.7 g m−2 day−1 at atmospheric CO2 levels; Fig. 1). After 14 days of growth, the biomass standing crop reached ~ 100 g dry weight m−2 with the addition of 1% CO2 (it should be noted that, on average, the water content in one m2 of a TL-PSBR is about 0.5–1 L during operation (Podola et al. 2017).
The light yield on biomass was larger in case of 1% CO2 (YX/PH = 0.68 g/mol) than in case of atmospheric CO2 (0.19 g/mol). The production of C-PC and A-PC also benefitted from the addition of CO2, with 3–4 times higher productivity (Fig. 1b–d) and on average 6–7 times higher pigment content in the biomass (Fig. 1c–e). Interestingly, C-PC and A-PC were present in similar amounts in the biomass (Fig. 1c–e).
Effect of different photon flux densities on biomass growth and phycobiliprotein production
To test how long linear growth of G. sulphuraria can be maintained in a TL-PSBR, the experimental period was extended to 30 days. Since the effect of elevated CO2 on growth depends on the photon fluence rate in TL-PSBRs (Schultze et al. 2015), also three different photon fluence rates at 1% CO2 concentration were tested.
The samples were taken every 3 days and the culture medium was exchanged every 3 days (Fig. 2). Depending on the photon fluence rate, the duration of linear growth varied: 15 days at 50 μmol m−2 s−1 (final biomass standing crop 107 g m−2), 22 days at 200 μmol m−2 s−1 (final biomass standing crop 225 g m−2) and 24 days at 100 μmol m−2 day−1(final biomass standing crop 190 g m−2). Biomass growth rates also varied with photon fluence rates being lowest (5.2 g m−2 day−1) at 50 μmol m−2 s−1 and highest (10 g m−2 day−1) at 200 μmol m−2 s−1 with the biomass growth rate at 100 μmol m−2 day−1 intermediate (7.5 g m−2 d−1). The biomass yield was almost constant at 50 and 100 μmol m−2 s−1 light intensity (0.92 and 0.91 g mol−1) and strongly decreased at 200 μmol m−2 s−1 (0.54 g mol−1), indicating the presence of a saturating light effect on photosynthesis and growth rate.
After 22–24 days, growth also ceased at the two higher photon fluence rates and the biomass density slightly decreased (Fig. 2).
Phycobiliprotein accumulation differed from biomass accumulation in that the highest phycobilin growth rate and phycobilin standing crop were observed at the intermediate photon fluence rate (100 μmol m−2 s−1), the lowest at the lowest photon fluence rate (50 μmol m−2-s−1) with the phycobilin accumulation intermediate at the highest photon fluence rate used (200 μmol m−2 s−1). The highest phycobiliproteins productivity was at 100 μmol photons m−2 s−1.
The highest C-PC value was achieved after 24 days (6.5 g m−2) while the highest A-PC value was achieved after 21 days (4.1 g m−2).
The highest percentage of phycobiliproteins in the biomass dry weight was determined at 100 μmol photons m−2 s−1: C-PC achieved 3.8% of dry weight and A-PC 2.3%. In the presence of 50 μmol photons m−2 s−1, the maximum C-PC percentage achieved 2.4% and A-PC 1.8% while at 200 μmol photons m−2 s−1, 1.8% and 1%.
After days 22–24, the phycobilin standing crop and concentration of phycobilin in the biomass dropped significantly (Fig. 2b–e).
Extending linear growth by increasing the frequency of nutrient replenishment
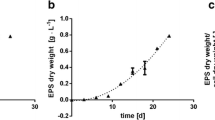
The previous experiment had shown that linear growth of G. sulphuraria in a TL-PSBR ceased around days 22–24 when the biomass density reached 200–220 g m−2. To investigate whether nutrient limitation could have been responsible for growth arrest and the subsequent drop in biomass and phycobilin standing crops, we extended the cultivation period to 42 days and exchanged the culture medium after 15 days of cultivation every 2 days (instead of every 3 days as in the previous experiments). The growth parameters were 100 μmol m−2 s−1 and addition of 1% CO2. More frequent exchange of the culture medium extended linear growth to 30 days over the 24 days in the previous experiment but the growth rate did not differ (Fig. 3a) compared to the previous experiment. The biomass standing crop reached 225 g m−2 at day 30 (Fig. 3a) compared to 190 g m−2 in the previous experiment. Nevertheless, growth ceased after day 33 (maximal biomass standing crop 232 g m−2) and the standing crop dropped to 210 g m−2 at the end of the experiment (Fig. 3a).
Similarly, phycobilin standing crop was higher than in the previous experiment and reached 7.39 g m−2 for C-PC at day 30 and 6.8 g m−2 for A-PC at day 33 (Fig. 3b–d). At day 30, the total phycobilin content in the dry biomass was 63 mg g−1. In this experiment, we also observed the highest productivities of C-PC (0.28 g m−2 day−1) and A-PC (0.23 g m−2 day−1). The drop in phycobilin standing crop after biomass growth ceased (after day 33) was less strong in this experiment than in the previous one. After the frequency of exchange of the culture medium was increased, the growth rates of the phycobilin also increased (after 6 days) despite the continuing linear biomass growth during this time (Fig. 3a, b, d). In consequence, phycobilin content in the biomass also increased between days 21 and 30 (Fig. 3c–e).
Purity level of C-PC and A-PC
In all experiments, the purity ratios of C-PC and A-PC were evaluated (see “Materials and methods” for details). The purity ratios were constant during the experiment but results were different for the two phycobiliproteins. Indeed, the purity ratio was higher for C-PC than A-PC (Fig. 4).
Discussion
G. sulphuraria showed a different behaviour in the TL-PSBR compared to other microalgae tested such as green algae or dinoflagellates (Benstein et al. 2014; Schultze et al. 2015). Indeed, G. sulphuraria in the natural environment grows at low light conditions under an atmosphere enriched in CO2 (Albertano et al. 2000; Cozzolino et al. 2000; Toplin et al. 2008; Del Mondo et al. 2019; Eren et al. 2018). So, the addition of CO2 (1%) in a TL-PSBR already enhanced growth at the low photon fluence rate of 50 μmol photons m−2 s−1 (compare Figs. 1a and 2a), whereas addition of CO2 in air usually does not have a measurable effect on biomass growth of microalgae at such low photon fluence rates (Langenbach and Melkonian 2019; Li et al. 2015).
However, G. sulphuraria achieved a good maximum biomass productivity of about 10 g m−2 day−1 at 200 μmol photons m−2 s−1 with addition of 1% CO2, comparable to that obtained in other microalgae such as Haematococcus pluvialis, Scenedesmus vacuolatus and Symbiodinum voratum using the same parameters and cultivation system (Carbone et al. 2017a; Kiperstok et al. 2017; Do et al. 2019; Langenbach and Melkonian 2019). Biomass growth at photon fluence rates exceeding 200 μmol m−2 s−1 was not tested in this study because it was found that phycobilin productivity was higher at the lower photon fluence rate of 100 μmol m−2 s−1. This result corroborates similar work on the LHC antenna carotenoid peridinin in the dinoflagellate Symbiodinium voratum, whose productivity was found to be highest at the same photon fluence rate in a TL-PSBR (Langenbach and Melkonian 2019) and has also been reported for phycobilin-containing algae (Graverholt and Eriksen 2007; Takano et al. 1995; Chen and Zhang 1997; Chen et al. 2017). It is well known that the amount of LHC antenna pigments (chlorophylls, carotenoids, phycobilin) strongly depends on the incident photon fluence rate (Müller et al. 2001). For phycobilin, the ratio of C-PC to A-PC also depends on the nutrient status of cells; under nitrogen limitation for example, C-PC is preferentially degraded (Yamanaka and Glazer 1980). In suspension cultures of G. sulphuraria, either of the two, A-PC (Graziani et al. 2013) or C-PC (Sørensen et al. 2013), was found to be dominant.
The advantages of growing microalgae immobilized in porous substrate bioreactors compared to suspension cultures have been recently summarized (Podola et al. 2017; Pierobon et al. 2018; Zhuang et al. 2018; Carbone et al. 2017b; Li et al. 2017). Since shear forces are minimized through cell immobilization and separation of the bulk culture medium from the immobilized algae by the microporous substrate layer, a large variety of microalgae, including shear-sensitive taxa, have been successfully grown in TL-PSBRs (e.g. Nowack et al. 2005; Ekelhof and Melkonian 2017; Langenbach and Melkonian 2019). Changing cultivation parameters such as culture medium, temperature and pH is easily achieved and does not involve interference with the cultivation process. Although the TL-PSBR is an open cultivation system, contaminations are usually not encountered during the cultivation period needed to obtain the maximal biomass standing crop. For G. sulphuraria, we have not seen contaminating organisms by light microscopy over the 42-day cultivation period under autotrophic culture conditions (results not shown); this was, of course, not unexpected as the pH had been adjusted to 1.5, providing a highly selective environment that minimized the contamination risk. However, when G. sulphuraria was grown mixotrophically in media enriched in organic carbon, fungi quickly developed in this open cultivation system (results not shown). Polycarbonate filters with a pore size of 0.2 μm were used to avoid permeation of the small cells of G. sulphuraria (2–3 μm diameter) through the substrate layer. For scale-up, other inexpensive types of substrate layers need to be chosen and tested for their suitability to maintain the G. sulphuraria biofilm coherent during growth.
In our experiment, the microalgae achieved the maximum growth rate at 200 μmol photons m−2 s−1, while in suspension culture, this organism grows better at lower level of light intensities (Seckbach 2007; Seckbach and Chapman 2010). This result corroborated data obtained from other algae grown in TL-PSBRs that showed high growth rates at very high photon fluence rates exceeding 1000 μmol m−2 s−1 (e.g. Schultze et al. 2015). This is linked to rapid attenuation of light in the subsurface layers of the biofilm, caused by efficient light absorption at the surface of the biofilm as measured with microsensors (Li et al. 2016). At the beginning of the experiment, the biofilm on the twin-layer system was relatively thin and most cells were exposed to higher photon fluence rates. In consequence, during the first 3 days of growth, the biomass growth rate was higher at 100 μmol photons m−2 s−1 than at 200 μmol m−2 s−1 (Fig. 2a). When the biofilm became thicker, the upper biofilm layers shaded the lower ones, thus reducing photoinhibition processes (Gross et al. 1998; Schultze et al. 2015) and allowing the biofilm to achieve higher grow rates at the higher light intensity.
These results suggest that the growth performances of G. sulphuraria should also be tested at photon fluence rates exceeding 200 μmol m−2 s−1 using a two-phase approach (Langenbach and Melkonian 2019) or even a three-phase approach: cultures could initially be grown for a few days at 100 μmol photons m−2 s−1 to minimize photoinhibition and then progressively shifted to higher photon fluence rates to enhance biomass growth. To optimize phycobilin production, it may be necessary to include a final third phase at the lower photon fluence rate and surplus nutrients (in particular nitrogen) to boost synthesis of phycobilin. Addition of CO2 was found to be necessary not only to enhance biomass growth but also phycobilin production. This result was in contrast to observations made in Arthrospira platentis, in which elevated CO2 concentration reduced biomass growth and C-PC concentration (Gordillo et al. 1998) and could be related to the contrasting habitats of both organisms.
Phycobilin concentrations under autotrophy are generally higher than those in heterotrophic condition (Ciniglia et al. 2014; Graziani et al. 2013). C-PC values obtained in this study thus exceeded the highest values obtained in heterotrophic cultivation (C-PC values of ~25 mg g−1 in Eriksen 2018; Sloth et al. 2006, 2017). However, higher phycobilin contents in the biomass than obtained here have been described in G. sulphuraria grown in Japanese hot springs supplemented with NH4. In this case, a C-PC value of ~ 100 mg g−1 was achieved but biomass standing crop was low (2.7 g L−1) in this open-pond system (Hirooka and Miyagishima 2016).
In conclusion, TL-PSBRs appear to be suitable to grow the thermo- and acidophilic red alga G. sulphuraria autotrophically to high biomass densities with acceptable phycobilin productivities and levels. Optimization of the biomass and phycobilin production process should be possible using a multi-phase approach with different photon fluence rates, optimal growth temperatures and manipulation of nutrient concentration, in particular nitrogen.
The possibility to produce phycobiliproteins from Galdieria sulphuraria in a twin-layer system has several advantages. (1) In a TL-PSBR, very high biomass densities exceeding those achievable by fermentation can be obtained, and harvesting the biomass as fresh weight thus does not require a pre-concentration step (Naumann et al. 2013; Wang et al. 2015; Gargano et al. 2016; Carbone et al. 2017a). (2) Despite the fact that the amount of phycocyanin produced by G. sulphuraria is no more or even less than that of other microalgae such as Porphyridium purpureum or A. platensis (Sosa-Hernández et al. 2019a; Coward et al. 2016; da Fontoura et al. 2018; Xie et al. 2015), the pigments show a very high level of purity, an indispensable factor for industrial production and cost effectiveness (Imbimbo et al. 2019; Graziani et al. 2013; Sørensen et al. 2013). Moreover, these results foster continuation of experiments with this microalga not only to optimize phycobiliprotein and biomass production but also to couple biomass production with other industrial applications such as wastewater treatment. (Sosa-Hernández et al. 2019b; da Silva et al. 2016; Rizwan et al. 2018; Centella et al. 2017; Minoda et al. 2015; Ju et al. 2016). Indeed, G. sulphuraria is considered one of the best algal candidates for phytoremediation of metals such as caesium, vanadium and uranium (Jalali et al. 2019; Fukuda et al. 2018) but also for removal of dissolved organic carbon and nutrients (Henkanatte-Gedera et al. 2017).
References
Abalde J, Betancourt L, Torres E, Cid A, Barwell C (1998) Purification and characterization of phycocyanin from the marine cyanobacterium Synechococcus sp. IO9201. Plant Sci 136:109–120
Albertano P, Ciniglia C, Pinto G, Pollio A (2000) The taxonomic position of Cyanidium, Cyanidioschyzon and Galdieria: an update. Hydrobiologia 433:137–143
Benstein RM, Cebi Z, Podola B, Melkonian M (2014) Immobilized growth of the peridinin-producing marine dinoflagellate Symbiodinium in a simple biofilm photobioreactor. Mar Biotechnol 16:621–628
Carbone DA, Olivieri G, Pollio A, Pinto G, Melkonian M (2017a) Growth and biomass productivity of Scenedesmus vacuolatus on a twin layer system and a comparison with other types of cultivations. Appl Microbiol Biotechnol 101:8321–8329
Carbone DA, Gargano I, Pinto G, De Natale A, Pollio A (2017b) Evaluating microalgal attachment to surfaces: a first approach towards a laboratory integrated assessment. Chem Eng Trans 57:73–78
Carbone DA, Gargano I, Chiaiese P, Pollio A, Marotta R, Olivieri G, Pinto G (2018) Scenedesmus vacuolatus cultures for possible combined laccase-like phenoloxidase activity and biodiesel production. Ann Microbiol 68:9–15
Carbone DA, Gargano I, Olivieri G, Marzocchella A, Andreozzi R, Marotta R, Spasiano D, Pinto G, Pollio A (2019) Light intensities maximizing photosynthesis and kinetics of photochemical steps in Graesiella emersonii under different cultivation strategies. Environ Eng Manag J 18:1527–1534
Carfagna S, Salbitani G, Bottone C, Vona V (2016) Galdieria sulphuraria as a possible source of food colorant. J Nutr Ecol Food Res 3:78–81
Carfagna S, Landi V, Coraggio F, Salbitani G, Vona V, Pinto G, Pollio A, Ciniglia C (2018) Different characteristics of C-phycocyanin (C-PC) in two strains of the extremophilic Galdieria phlegraea. Algal Res 31:46–52
Castenholz RW, McDermott TR (2010) The Cyanidiales: ecology, biodiversity, and biogeography. In: Seckbach J, Chapman DJ (eds) Red Algae in the Genomic Age. Springer, Dordrecht, The Netherlands, pp 357–371
Cennamo P, Marzano C, Ciniglia C, Pinto G, Cappelletti P, Caputo P, Pollio A (2012) A survey of the algal flora of anthropogenic caves of Campi Flegrei (Naples, Italy) archeological district. J Cave Karst Stud 74:243–250
Centella MH, Arevalo-Gallegos A, Parra-Saldivar R, Iqbal HM (2017) Marine-derived bioactive compounds for value-added applications in bio-and non-bio sectors. J Clean Prod 168:1559–1565
Chen F, Zhang Y (1997) High cell density mixotrophic culture of Spirulina platensis on glucose for phycocyanin production using a fed-batch system. Enzym Microb Technol 20:221–224
Chen X, Wu M, Yang Q, Wang S (2017) Preparation, characterization of food grade phycobiliproteins from Porphyra haitanensis and the application in liposome-meat system. LWT Food Sci Technol 77:468–474
Ciniglia C, Yoon HS, Pollio A, Pinto G, Bhattacharya D (2004) Hidden biodiversity of the extremophilic Cyanidiales red algae. Mol Ecol 13:1827–1838
Ciniglia C, Yang EC, Pinto G, Iovinella M, Vitale L, Yoon HS (2014) Cyanidiophyceae in Iceland: plastid rbcL gene elucidates origin and dispersal of extremophilic Galdieria sulphuraria and Galdieria maxima (Galdieriaceae, Rhodophyta). Phycologia 53:542–551
Ciniglia C, Iovinella M, Olivieri G, Cennamo P, Pollio A (2017) A potential use of the polyextremophilic microalga Galdieria sulphuraria (Cyanidiophyceae, Rodhophyta) in bio-recovery of rare metals. Phycologia Suppl 56:33
Coward T, Fuentes-Grünewald C, Silkina A, Oatley-Radcliffe DL, Llewellyn G, Lovitt RW (2016) Utilising light-emitting diodes of specific narrow wavelengths for the optimization and co-production of multiple high-value compounds in Porphyridium purpureum. Bioresour Technol 221:607–615
Cozzolino S, Caputo P, De Castro O, Moretti A, Pinto G (2000) Molecular variation in Galdieria sulphuraria (Galdieri) Merola and its bearing on taxonomy. Hydrobiologia 433:145–151
Dejsungkranont M, ChistiSarote Y, Sirisansaneeyakul S (2017) Optimization of production of C-phycocyanin and extracellular polymeric substances by Arthrospira sp. Bioprocess Biosyst Eng 40:1173–1188
Del Mondo A, Iovinella M, Petriccione A, Seth D, Cioppa D, Ciniglia C (2019) A spotlight on Rad52 in Cyanidiophytina (Rhodophyta): a relic in algal heritage. Plants 8(2):46
Do T, Ong B, Nguyen T, Melkonian M, Tran H (2019) Biomass and astaxanthin productivities of Haematococcus pluvialis in an angled twin-layer porous substrate photobioreactor: effect of inoculum density and storage time. Biology 8:68
Ekelhof A, Melkonian M (2017) Enhanced extracellular polysaccharide production and growth by microalga Netrium digitus in a porous substrate bioreactor. Algal Res 28:184–191
Eren A, Iovinella M, Yoon HS, Cennamo P, de Stefano M, de Castro O, Ciniglia C (2018) Genetic structure of Galdieria populations from Iceland. Polar Biol 41:1681–1691
Eriksen NT (2007) Production of phycocyanin — a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol 80:1–14
Eriksen NT (2013) Pigments from microalgae: a new perspective with emphasis on phycocyanin. In: Arlorio M (ed) Book of Abstracts and Proceedings of the 7th International Congress on Pigments in Foods. Aalborg University, Denmark, pp 37
Eriksen NT (2018) Heterotrophic production of phycocyanin in Galdieria sulphuraria. In: Durvasula RV, Subba Rao DV (eds) Extremophiles –from Biology to Biotechnology. CRC Press, Boca Raton, pp 87–101
Estrada JEP, Bescos PB, Fresno AMV (2001) Antioxidant activity of different fractions of Spirulina platensis protean extract. Farmaco 56:497–500
Fernandéz-Rojas B, Hernández-Juárez J, Pedraza-Chaverri J (2014) Nutraceutical properties of phycocyanin. J Funct Foods 11:375–392
da Fontoura PD, Radmann EM, Duarte JH, de Morais MG, Costa JAV (2018) Spirulina cultivated under different light emitting diodes: enhanced cell growth and phycocyanin production. Bioresour Technol 256:38–43
Fukuda SYA, Yamamoto R, Iwamoto K, Minoda A (2018) Cellular accumulation of caesium in the unicellular red alga Galdieria sulphuraria under mixotrophic conditions. J Appl Phycol 30:3057–3061
Gantt E (1980) Structure and function of phycobilisomes: light-harvesting pigment complexes in red and blue-green algae. Int Rev Cytol 56:45–80
Gargano I, Olivieri G, Andreozzi R, Marotta R, Marzocchella A, Pollio A (2016) Biodiesel production in outdoor cultures of Scenedesmus vacuolatus. Chem Eng Trans 49:397–402
Glazer AN (1994) Phycobiliproteins – a family of valuable, widely used fluorophores. J Appl Phycol 6:105–112
Gordillo FL, Jimenez C, Figueroa FL, Niell FX (1998) Effects of increased atmospheric CO2 and N supply on photosynthesis, growth and cell composition of the cyanobacterium Spirulina platensis (Arthrospira). J Appl Phycol 10:461–469
Graverholt OS, Eriksen NT (2007) Heterotrophic high cell-density fed-batch and continuous flow cultures of Galdieria sulphuraria and production of phycocyanin. Appl Microbiol Biotechnol 77:69–75
Graziani G, Schiavo S, Nicolai MA, Buono S, Fogliano V, Pinto G, Pollio A (2013) Microalgae as human food: chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Funct 4:144–152
Gross W (1999) Revision of comparative traits for the acid- and thermophilic red algae Cyanidium and Galdieria. In: Seckbach J (ed) Enigmatic Micro-organisms and Life in Extreme Environments. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 437–446
Gross W, Schnarrenberger C (1995) Heterotrophic growth of two strains of the acid-thermophilic red alga Galdieria sulphuraria. Plant Cell Physiol 36:4–9
Gross W, Kuver J, Tischendorf G, Bouchaala N, Busch W (1998) Cryptoendolithic growth of the red alga Galdieria sulphuraria in volcanic areas. Eur J Phycol 33:25–31
Grossman A, Schaefer MR, Chiang GG, Collier JL (1993) The phycobilisomes, a light-harvesting complex responsive to environmental conditions. Microbiol Rev 57:725–749
Henkanatte-Gedera SM, Selvaratnam T, Karbakhshravari M, Myint M, Nirmalakhandan N, Van Voorhies W, Lammers PJ (2017) Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: laboratory to field scale demonstration. Algal Res 24:450–456
Hirooka S, Miyagishima SY (2016) Cultivation of acidophilic algae Galdieria sulphuraria and Pseudochlorella sp. YKT1 in media derived from acidic hot springs. Front Microbiol 7:20–22
Ho SH, Liao JF, Chen CY, Chang JS (2018) Combining light strategies with recycled medium to enhance the economic feasibility of phycocyanin production with Spirulina platensis. Bioresour Technol 247:669–675
Hsieh-Lo M, Castillo G, Ochoa-Becerra M, Mojica L (2019) Phycocyanin and phycoerythrin: strategies to improve production yield and chemical stability. Algal Res 42:50–56
Imbimbo P, Romanucci V, Pollio A, Fontanarosa C, Amoresano A, Zarrelli A, Olivieri G, Monti DM (2019) A cascade extraction of active phycocyanin and fatty acids from Galdieria phlegraea. Appl Microbiol Biotechnol 103:9455–9464
Iovinella M, Eren A, Pinto G, Pollio A, Davis SJ, Cennamo P, Ciniglia C (2018) Cryptic dispersal in non-acidic environments from Turkey of Cyanidiophytina (Rhodophyta). Extremophile 22:713–723
Jalali F, Fakhar J, Zolfaghari A (2019) Investigation on biosorption of V (III), Ti (IV), and U(VI) ions from a contaminated effluent by a newly isolated strain of Galdieria sulphuraria. Separat Sci Technol (Philadelphia) 54:914
Ju X, Igarashi K, Miyashita K, Mitsuhashi H, Inagaki K, Fujii S, Sawada H, Kuwabara T, Minoda A (2016) Effective and selective recovery of gold and palladium ions from metal wastewater using a sulphothermophilic red alga, Galdieria sulphuraria. Bioresour Technol 211:759–764
Kannaujiya VK, Sinha RP (2016) An efficient method for the separation and purification of phycobiliproteins from a rice-field cyanobacterium Nostoc sp. strain HKAR-11. Chromatographia 79:335–343
Kiperstok AC, Sebestyén P, Podola B, Melkonian M (2017) Biofilm cultivation of Haematococcus pluvialis enables a highly productive one-phase process for astaxanthin production using high light intensities. Algal Res 21:213–222
Kuddus M, Singh P, Thomas G, Al-Hazimi A (2013) Recent developments in production and biotechnological applications of C-phycocyanin. Biomed Res Int 74:28–59
Langenbach D, Melkonian M (2019) Optimising biomass and peridinin accumulation in the dinoflagellate Symbiodinium voratum using a twin-layer porous substrate bioreactor. J Appl Phycol 31:21–28
Li T, Lin G, Podola B, Melkonian M (2015) Continuous removal of zinc from wastewater and mine dump leachate by a microalgal biofilm PSBR. J Hazard Mater 297:112–118
Li T, Piltz B, Podola B, Dron A, De Beer D, Melkonian M (2016) Microscale profiling of photosynthesis-related variables in a highly productive biofilm photobioreactor. Biotechnol Bioeng 113:1046–1055
Li T, Strous M, Melkonian M (2017) Biofilm-based photobioreactors: their design and improving productivity through efficient supply of dissolved inorganic carbon. FEMS Microbiol Lett 5:364–365
Li W, Su H, Yang P, Chen J, Liu N, Liu Q, Qin S (2019) Phycobiliproteins: molecular structure, production, applications, and prospects. Biotechnol Adv 37:340–353
Manirafasha E, Ndikubwimana T, Zeng X, Lu Y, Jing K (2016) Phycobiliprotein: potential microalgae derived pharmaceutical and biological reagent. Biochem Eng J 109:282–296
Melkonian M, Podola B (2010) Method and device for cultivating eukaryotic microorganisms or blue algae, and biosensor with cultivated eukaryotic microorganisms or blue algae. United States Patent No US 7:745, 20
Minoda A, Sawada H, Suzuki S, Miyashita S, Inagaki K, Yamamoto T, Tsuzuki M (2015) Recovery of rare earth elements from the sulpho-thermophilic red alga Galdieria sulphuraria using aqueous acid. Appl Microbiol Biotechnol 99:1513–1519
Moon M, Mishra S, Kim C, Suh W, Park M, Yang J (2014) Isolation and characterization of thermostable phycocyanin from Galdieria sulphuraria. Korean J Chem Eng 31:490–495
Moraes CC, Sala L, Cerveira GP, Kalil SJ (2011) C-phycocyanin extraction from Spirulina platensis wet biomass. Braz J Chem Eng 28:45–49
Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Naumann T, Çebi Z, Podola B, Melkonian M (2013) Growing microalgae as aquaculture feeds on twin-layers: a novel solid-state photobioreactor. J Appl Phycol 25:1413–1420
Nowack E, Podola B, Melkonian M (2005) The 96-well twin-layer system: a novel approach in the cultivation of microalgae. Protist 156:239–251
Nwoba E, Parleveit A, Laird D, Kamal A, Moheiman E (2019) Light management technologies for increasing algal photobioreactor efficiency. Algal Res 39:18–21
Olivieri G, Salatino P, Marzocchella A (2014) Advances in photobioreactors for intensive microalgal production: configurations, operating strategies and applications. J Chem Technol Biotechnol 89:178–195
Osorio JHM, Pinto G, Pollio A, Frunzo L, Nicolaas P, Lens L, Esposito G (2019) Start-up of a nutrient removal system using Scenedesmus vacuolatus and Chlorella vulgaris biofilms. Biores Bioproc 6:27–29
Pagels F, Guedes AC, Amaro H, Kijjoa H, Vasconcelos V (2019) Phycobiliproteins from cyanobacteria: chemistry and biotechnological applications. Biotechnol Adv 37:422–443
Pan-Utai W, Kahapana W, Iam S (2018) Extraction of C-phycocyanin from Arthrospira (Spirulina) and its thermal stability with citric acid. J Appl Phycol 30:231–242
Pierobon SC, Cheng X, Graham PJ, Nguyen B, Karakolis EG, Sinton D (2018) Emerging microalgae technology: a review. Sustain Energy Fuels 2:13–38
Piltz B, Melkonian M (2018) Immobilized microalgae for nutrient recovery from source-separated human urine. J Appl Phycol 30:421–429
Pinto G (2007) Cyanidiophyceae: looking back—looking forward. In: Seckbach J (ed) Algae and Cyanobacteria in Extreme Environments. Springer, Dordrecht, The Netherlands, pp 389–397
Pinto G, Ciniglia C, Cascone C, Pollio A (2007) Species composition of Cyanidiales assemblages in Pisciarelli (Campi Flegrei, Italy) and description of Galdieria phlegrea sp. nov. In: Seckbach J (ed) Algae and Cyanobacteria in Extreme Environments. Springer, Dordrecht, pp 487–502
Podola B, Li T, Melkonian M (2017) Porous substrate bioreactors: a paradigm shift in microalgal biotechnology? Trends Biotechnol 35:121–132
Richmond A, Qiang H (2010) Principles for efficient utilization of light for mass production of photoautotrophic microorganisms. Appl Biochem Biotechnol 65:649–658
Richmond A, Lichtenberg E, Stahl B, Vonshak A (1990) Quantitative assessment of the major limitations on productivity of Spirulina platensis in open raceways. J Appl Phycol 2:195–206
Rimbau V, Camins A, Romay C, Gonzalez R, Pallas M (1999) Protective effects of C-phycocyanin against kainic acid-induced neuronal damage in rat hippocampus. Neurosci Lett 276:75–78
Rizwan M, Mujtaba G, Memon SA, Lee K, Rashid N (2018) Exploring the potential of microalgae for new biotechnology applications and beyond: a review. Renew Sust Energ Rev 92:394–404
Romay CH, González R, Ledón N, Remirez D, Rimbau V (2003) C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci 4:207–216
Santos R, Santos P, Santos C, Dantas F, Teixera C (2019) Evaluation of the co-production of total carotenoids, C-phycocyanin and polyhydroxyalkanoates by Arthrospira platensis. Bioresour Technol Rep 7:30–35
Schmidt RA, Wiebe MG, Eriksen NT (2005) Heterotrophic high cell-density fed-batch cultures of the phycocyanin producing red alga Galdieria sulphuraria. Biotechnol Bioeng 90:77–84
Schultze LKP, Simon MV, Li T, Langenbach D, Podola B, Melkonian M (2015) High light and carbon dioxide optimize surface productivity in a twin-layer biofilm photobioreactor. Algal Res 8:37–44
Seckbach J (ed) (2007) Algae and Cyanobacteria in extreme environments. Springer, Dordrecht, The Netherlands
Seckbach J, Chapman DJ (eds) (2010) Red algae in the genomic age. Springer, Dordrecht, The Netherlands
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19:417–423
Shi J, Podola B, Melkonian M (2014) Application of a prototype-scale twin-layer photobioreactor for effective N and P removal from different process stages of municipal wastewater by immobilized microalgae. Bioresour Technol 154:260–266
da Silva VB, Moreira JB, de Morais MG, Costa JAV (2016) Microalgae as a new source of bioactive compounds in food supplements. Curr Opin Food Sci 7:73–77
Sloth JK, Wiebe MG, Eriksen NT (2006) Accumulation of phycocyanin in heterotrophic and mixotrophic cultures of the acidophilic red alga Galdieria sulphuraria. Enzym Microb Technol 38:168–175
Sloth JK, Jensen H, Pleissner D, Eriksen N (2017) Growth and phycocyanin synthesis in the heterotrophic microalga Galdieria sulphuraria on substrates made of food waste from restaurants and bakeries. Bioresour Technol 238:296–305
Sørensen L, Hantke A, Eriksen NT (2013) Purification of the photosynthetic pigment C-phycocyanin from heterotrophic Galdieria sulphuraria. J Sci Food Agric 93:2933–2938
Sosa-Hernández JE, Rodas Zuluaga LI, Castillo-Zacarías C, Rostro-Alanís M, de la Cruz R, Carrillo-Nieves D, Lovitt RW (2019a) A Light intensity and nitrogen concentration impact on the biomass and phycoerythrin production by Porphyridium purpureum. Mar Drugs 17:460
Sosa-Hernández JE, Romero-Castillo KD, Parra-Arroyo L, Aguilar-Aguila-Isaías MA, García-Reyes IE, Ahmed I, Iqbal H (2019b) Mexican microalgae biodiversity and state-of-the-art extraction strategies to meet sustainable circular economy challenges: high-value compounds and their applied perspectives. Mar Drugs 17:174–178
Takano H, Arai T, Hirano M, Matsunaga T (1995) Effects of intensity and quality of light on phycocyanin production by a marine cyanobacterium Synechococcus sp. NKBJ 042902. Appl Microbiol Biotechnol 43:1014–1018
Toplin JA, Norris TB, Lehr CR, McDermott TR, Castenholz RW (2008) Biogeographic and phylogenetic diversity of thermoacidophilic Cyanidiales in Yellowstone National Park, Japan and New Zealand. Appl Environ Microbiol 74:2822–2833
Vernes L, Granvillain P, Chemat F, Vian M (2015) Phycocyanin from Arthrospira platensis. Production, extraction and analysis. Curr Biotechnol 4:481–491
Wan I, Minxi U, Zhenyang W, Zhen Z, Jun W, Shulan L, Anquan Y, Yuanguang L (2016) A novel paradigm for the high-efficient production of phycocyanin from Galdieria sulphuraria. Bioresour Technol 218:272–278
Wang J, Liu J, Liu T (2015) The difference in effective light penetration may explain the superiority in photosynthetic efficiency of attached cultivation over the conventional open pond for microalgae. Biotechnol Biofuels 26:8–49
Xie Y, Jin Y, Zeng X, Chen J, Lu Y, Jing K (2015) Fed-batch strategy for enhancing cell growth and C-phycocyanin production of Arthrospira (Spirulina) platensis under phototrophic cultivation. Bioresour Technol 180:281–285
Yamanaka G, Glazer A (1980) Dynamic aspects of phycobilisome structure. Arch Microbiol 124:39–47
Yoon HS, Hackett JD, Ciniglia C, Pinto G, Bhattacharya D (2004) A molecular timeline for the origin of photosynthetic eukaryotes. Mol Biol Evol 21:809–818
Yu J, Hancui H, Xiadoan H, Congchun W, Ting Z, Yuhan L. Roger R, Hongli Z (2019) Continuous cultivation of Arthrospira platensis for phycocyanin production in large-scale outdoor raceway ponds using microfiltered culture medium. Bioresour Technol 287:121420
Zhuang L, Dawei Y, Zhang J, Fei L, Yin W, Tian Z, Guo H, Hong Y (2018) The characteristics and influencing factors of the attached microalgae cultivation: a review. Renew Sust Energ Rev 94:1110–1119
Zuccaro G, Steyer JP, Van Lis R (2019) The algal trophic mode affects the interaction and oil production of a synergistic microalga-yeast consortium. Bioresour Technol 237:608–617
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants and animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carbone, D.A., Olivieri, G., Pollio, A. et al. Biomass and phycobiliprotein production of Galdieria sulphuraria, immobilized on a twin-layer porous substrate photobioreactor. Appl Microbiol Biotechnol 104, 3109–3119 (2020). https://doi.org/10.1007/s00253-020-10383-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10383-8