Abstract
The potential of probiotics for treating ulcerative colitis (UC) has attracted increasing attention. However, more studies are still needed to guide physicians on the proper selection and use of probiotics. Here, we propose that combination of multiple probiotics with different functions can reduce intestinal inflammation. In this study, the effects of probiotics (Lactobacillus reuteri, Bacillus coagulans, Bifidobacterium longum, and Clostridium butyricum) on the physiology and histopathology of colon were evaluated in a dextran sulfate sodium (DSS)-induced colitis mouse model. The combined species, as well as the species individually, were tested and compared with sulfasalazine (SASP) and two Chinese herbal therapies. Results show that the functions of the four probiotic strains were different in regulating intestinal immunity and barrier function. The four-species probiotic cocktail was more effective than the species individually and anti-inflammatory drugs in repairing the dysbiosis of mucosal microbial ecology and reducing intestinal inflammation. The multi-strain probiotic mixture increased the proportion of beneficial bacteria and decreased the proportion of pro-inflammatory bacteria in the colonic mucosa. In addition, probiotic mixture significantly enhanced the expression of IL-10 and intestinal barrier function. These results suggest that a combination of multiple probiotics with different functions has synergistic effects and can restore the balance of interactions between microorganisms and immunological niches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease that affects the colon. UC can induce colorectal cancer and a variety of extra-intestinal inflammatory manifestations and complications, which require high-cost treatments and result in great damage to human health and the economy (Neurath 2014; Ungaro et al. 2017). Unhealthy lifestyle and environmental pollution are important risk factors for UC. The incidence of UC is increasing worldwide and has become a global public health threat.
The current therapeutic aim for UC is to induce and maintain remission, thereby preventing colectomy and colorectal cancer (Ungaro et al. 2017). The first-line drugs in UC treatment are aminosalicylates, corticosteroids, biological drugs, and immunosuppressants. However, these drugs are not effective in a large number of UC patients, and some may even cause serious adverse effects (Deltenre et al. 1999; Ek and Rosenborg 2017; Ransford and Langman 2002). Therefore, the development of new therapeutic strategies is urgent. The potential of probiotics for UC treatment has attracted increasing attention. Studies have shown that administration of certain probiotic strains can reduce intestinal inflammation and injury, improve the intestinal microbial ecology, and prevent the development of colitis-associated adenocarcinoma (Fitzpatrick et al. 2012; Jang et al. 2018; Je et al. 2018; Talero et al. 2015). Clinical studies have shown that probiotics can alleviate the symptoms of UC (Tursi et al. 2010) and maintain clinical remission (Yoshimatsu et al. 2015). Even so, more research is still need to guide physicians on the proper selection and use of probiotics.
In traditional Chinese medicine, Massa Medicata Fermentata (MMF) and Euphorbia humifusa Willd. are used to treat gastrointestinal diseases. MMF, a fermented traditional Chinese medicine, can relieve ethanol-induced acute gastric injury in rats (Shin et al. 2013) and neomycin-induced colon injury and disorders in gut microbiota (Bose et al. 2013). However, it is not clear whether MMF has therapeutic effects for UC. E. humifusa has anti-inflammatory, astringent, and hemostatic effects and is used to treat diarrhea and hematochezia (Li et al. 2015; Luyen et al. 2014; Shang et al. 2018). However, the effects of E. humifusa on intestinal microbiota have not been reported.
UC is characterized by dysregulation of the intestinal barrier function and immune system. The weakened intestinal barrier and immune system are important risk factors for the initiation and progression of UC (McGuckin et al. 2009; Ramos and Papadakis 2019). It has been shown that anti-inflammatory cytokines (such as IL-10 and TGFβ) play an important role in the control of UC (Neurath 2014). In addition, studies have confirmed that dysbiosis of the intestinal microbiota is involved in the pathogenesis of UC (Sartor and Wu 2017; Zhang et al. 2017). Compared with healthy individuals, in UC patients and experimental colitis animals, microbial diversity and beneficial bacteria (such as Lactobacillus, Clostridium clusters IV, and XIVa and Bifidobacterium) are decreased, while pro-inflammatory bacteria (such as Escherichia coli, Fusobacterium, and Ruminococcus gnavus) are increased (Nishida et al. 2018; Ramos and Papadakis 2019; Zhang et al. 2017). Previous research have reported that intervention with intestinal commensal microorganisms, probiotics, or diets could restore the balance of the interplay between gut microbiome and immunological niches, thereby alleviating intestinal inflammation and damage (Burrello et al. 2018; Ganji-Arjenaki and Rafieian-Kopaei 2018; Pagliari et al. 2017; Zou et al. 2018). Therefore, repairing the disturbed intestinal microecology and regulating the intestinal immune system and barrier function may contribute to the treatment of UC.
Here, we assessed the effects of four probiotic strains (Lactobacillus reuteri RAM0101, Bacillus coagulans RAM1202, Bifidobacterium longum CICC6197, and Clostridium butyricum RAM0216) on the symptoms, histopathology, immune response, and barrier function of colon in a dextran sulfate sodium (DSS)-induced colitis mouse model. The four-species probiotic cocktail, as well as the species individually, were tested and compared to anti-inflammatory drugs. Sequencing of the 16S rRNA gene V3–V4 region and full-length 16S rRNA were performed to analyze the microbial composition of the colonic mucosa. Compared with clinical anti-inflammatory drugs, the multi-strain probiotic mixture was more effective in restoring intestinal microbial ecology and alleviating intestinal inflammation and injury. The effect of the multi-strain probiotics depends on the synergy of different functions of the four probiotic strains.
Materials and methods
Preparation of medications and probiotics
The MMF and extracts of E. humifusa used in this study were processed as follows. Stir-fried MMF was pulverized and sifted through a 100-mesh sieve. Dry E. humifusa was pulverized, and then, water (HW), 70% ethanol (H70E), and absolute ethanol extracts (HE) were prepared as follows: E. humifusa powder was soaked in 10 volumes of water, 70% ethanol, or absolute ethanol, respectively, for 30 min and then boiled with a reflux condenser for 3 h. The supernatant was separated by centrifugation, and the sediment was extracted once again. Then, the supernatant was concentrated by rotary evaporation and lyophilization successively. The MMF powder and E. humifusa extracts (HW, H70E, and HE) were stored at − 30 °C before use.
The bacterial strains L. reuteri RAM0101 (CGMCC No. 17853), B. coagulans RAM1202 (CGMCC No. 17852), and C. butyricum RAM0216 (CGMCC No. 17854) were isolated from the colon of a weaned pig and have been deposited in the China General Microbiological Culture Collection Center (CGMCC). B. longum CICC6197 was purchased from the China Center of Industrial Culture Collection (CICC). The culture conditions of the probiotics are described in Supplementary Material. The antibiotic sensitivity, hemolytic characteristic, and toxin production of these probiotic strains were examined to determine their safety (data not shown).
DSS-induced ulcerative colitis model and treatment
Specific pathogen free (SPF) C57BL/6N mice (male, 6 weeks old, 18–22 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (http://www.vitalriver.com/). Mice were fed a basal diet (Keao Xieli Feed Co., Ltd., Beijing, China) and housed in a controlled environment with feed and water ad libitum. After a 1-week adaptation, mice were given 2.5% (w/v) DSS (molecular weight = 36,000–50,000 Da, MP Bio, Canada) in the drinking water (n = 10 per group) to induce colitis. The induction of colitis and drug intervention was performed simultaneously.
At the outset, the protective effects of sulfasalazine (SASP), MMF, the E. humifusa extracts, and the probiotic mixture on DSS-induced colitis were analyzed. Healthy mice were treated with 400 μL of PBS (Con group) by gavage once a day. Colitis mice were treated with equal volumes of PBS (DSS group), SASP solution (SASP group, 150 mg/kg/day), the MMF suspension (MMF group, 1000 mg/kg/day), HW (HW group, 858 mg/kg/day), H70E (H70E group, 702 mg/kg/day), HE (HE group, 702 mg/kg/day), or a mixture of the four probiotic strains (Promix group) by gavage once a day. The four probiotics were suspended in PBS and mixed at a ratio of 1:1:1:1 (a total of approximately 4 × 109 colony-forming unit (CFU) per mouse). During an 8-day trial, body weight, diarrhea, hematochezia, and mortality were recorded daily, and disease activity index (DAI) scores were calculated according to Supplemental Table S1.
In the follow-up experiment, the functional differences of the four probiotic strains were analyzed. For the Con, DSS, and Promix groups, the dosage regimen was the same as described above. For L. reuteri RAM0101 (Lr group), B. coagulans RAM1202 (Bc group), B. longum CICC6197 (Bf group), and C. butyricum RAM0216 (Cb group), the four strains were used alone, and the dose was approximately 1 × 109 CFU per mouse. The preparation process of the probiotics was as described above. During a 6-day trial, body weight, diarrhea, and hematochezia were recorded daily, and DAI scores were calculated according to Supplemental Table S1.
The animal experiment was approved by the Institutional Animal Care and Use Committee of Jilin University (IACUC). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Histological analysis
Colonic tissues were processed according to a previous study (Wang et al. 2018) for histopathology analysis. Scoring of the Nancy index (Marchal-Bressenot et al. 2016), inflammatory cell infiltration, and destruction of the mucosal structure (Burrello et al. 2018) was performed according to the criteria described in Supplemental Table S2.
Quantification of gene expression in colonic mucosa by reverse transcription-quantitative PCR
Total RNA was extracted from colonic tissues using the TaKaRa MiniBEST Universal RNA Extraction kit (TaKaRa, Dalian, China), and then, cDNA was generated using the PrimeScriptTM reagent kit with gDNA Eraser (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The mRNA levels of Tnf-α, Cox-2, Ifn-γ, Il-1β, Il-6, Il-10, Tgfβ, Il-17a, Il-22, Reg3γ, lysozyme, Mmp-3, Mmp-9, Muc-2, Zo-1, occludin, and claudin-1 genes were measured by quantitative PCR (qPCR). Gapdh was used as the reference gene. The primer sequences are described in Supplemental Table S3. qPCR was performed using 2× RealStar Green Fast Mixture with ROX II (GenStar, Beijing, China), and data were acquired in an Applied Biosystems 7500 Real-Time PCR Systems (Thermo Fisher Scientific, Foster City, CA). The PCR and amplification conditions were the optimum conditions recommended by the manufacturer’s instructions. The fold change of the target genes was calculated by the 2−∆∆Ct formula.
Western blot analysis
Western blot analysis was performed as described by Gao et al. (2017). β-Actin was used as the reference. Antibodies specific for β-actin (ab8227, 1:1000), occludin (ab167161, 1:5000), and claudin-1 (ab129119, 1:1000) were purchased from Abcam Ltd. (Shanghai, China).
Quantification of organic acids in intestinal contents
Concentrations of lactate, acetate, propionate, and butyrate in the colonic contents were quantified by high-performance liquid chromatography (HPLC). Sample pretreatment and derivatization was performed as previously described (Wang et al. 2018).
DNA extraction, sequencing, and data processing
Details of the experiments and data processing about sequencing of 16S rRNA gene V3–V4 region and full-length 16S rRNA gene are described in the Supplementary Material.
Statistical analysis
The data are expressed as the mean values ± SEM. Statistical analyses were performed in SPSS Statistics 17.0 (IBM, New York, USA) using the data generated above. Multiple comparisons were conducted using one-way analysis of variance (ANOVA). A value of p < 0.05 was considered statistically significant. Figures were plotted with GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. Heatmaps of bacterial relative abundance were made using R version 3.5.3 (https://www.r-project.org/) with the heatmap package. Spearman correlation analysis was performed in SPSS Statistics 17.0, and the heatmaps of the correlation analysis were plotted using MetaboAnalyst 4.0 (Chong et al. 2018) (https://www.metaboanalyst.ca/faces/home.xhtml).
Accession numbers
High-throughput sequencing data have been submitted to the NCBI Sequence Read Archive (SRA) under accession numbers SRP200458, SRP200588, and SRP200476.
Results
Promix alleviated DSS-induced colitis more effectively than anti-inflammatory drugs
In order to test the potential of traditional Chinese medicine (MMF and E. humifusa) and probiotics for UC treatment, the effects of the four-species probiotic cocktail, MMF, and extracts of E. humifusa on DSS-induced intestinal inflammation and injury were analyzed and compared with clinical anti-inflammatory drug SASP.
In this study, the combination of the four probiotic strains (Promix group) effectively alleviated DSS-induced colitis, as indicated by the decreased weight loss (p = 0.004), DAI (p = 0.012), mortality, shortening of the colon (p < 0.001), and atrophy of the cecum (Fig. 1). The HW reduced diarrhea (p < 0.05), hematochezia (p < 0.05), and mortality (Fig. 1b–e), but it did not protect against weight loss and shortening of the colon (Fig. 1a and g). SASP, MMF, H70E, and HE had little protective effects on colitis and even exacerbated the weight loss compared with the DSS treatment alone.
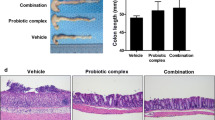
Effects of the probiotic mixture and anti-inflammatory drugs on DSS-induced colitis. a The body weight, b diarrhea, c hematochezia, d DAI scores, and e survival rate of mice were recorded daily. f and g The length of the colon was measured at sacrifice (day 8). Data represent mean values ± SEM (n = 10 per group). Statistical analysis was performed using one-way ANOVA with LSD. a, b Different letters indicate significant differences (p < 0.05)
Scoring of the Nancy index, inflammatory cell infiltration (histopathological analysis), and structural damage (goblet cell loss, ulceration, granulation tissue, and red blood cell migration) was used to evaluate the histopathology of the colon (Fig. 2a–d). DSS induced acute and severe inflammatory cell infiltration and mucosal structural damage in the colon. Promix and HW markedly decreased the Nancy index (p < 0.05), inflammatory cell infiltration (p < 0.001), and mucosal structural damage (p < 0.001). Although SASP treatment decreased inflammatory cell infiltration (p = 0.059), it did not protect against the damage of the intestinal epithelium. H70E and HE treatment maintained the structural integrity of the intestinal epithelium (p < 0.001) but did not reduce inflammatory cell infiltration or the Nancy index. MMF had no protective effects on tissue damage and inflammation. The RT-qPCR results showed that the expression of genes related to pro-inflammatory cytokines, which were dramatically upregulated in the DSS group, was significantly reduced in the Promix group (Fig. 2e–g). Furthermore, as shown by western blotting, the Promix restored the levels of occludin and claudin-1, which were decreased by DSS treatment (Fig. 2h–j). Together, these results suggest that the multi-strain probiotic mixture effectively protects against intestinal inflammation and injury caused by DSS.
Effects of the probiotic mixture and anti-inflammatory drugs on the histopathology and gene expression of the colon. a H & E staining (scale bare is 100 μm). b Nancy index, c inflammatory cell infiltration, and d destruction of the mucosal structure were scored according to H & E staining. e–g The expression of genes related to pro-inflammatory cytokines was measured by RT-qPCR. h Western blot analysis of Occludin and Claudin-1 proteins in the colon. i and j Statistical results of the western blot of Occludin and Claudin-1 proteins in the colon. Data represent mean values ± SEM (n = 10 per group). Statistical analysis was performed using one-way ANOVA with LSD. a, b Different letters indicate significant differences (p < 0.05)
The probiotic mixture alleviated colitis more effectively than single probiotics
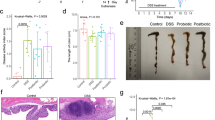
The above results confirmed that the combination of the four probiotics effectively protected against DSS-induced intestinal inflammation and damage. Next, we asked whether the effects of single probiotics are equal to those of the multi-strain mixture. Therefore, we compared the effects of single probiotics and probiotic combination on DSS-induced colitis. The physiological manifestations (Fig. 3a–e) showed that the treatment with the individual probiotics relieved the symptoms of colitis to different degrees. For instance, in addition to Bf (p = 0.059), Lr, Bc, and Cb significantly reduced the weight loss of mice at day 6 (p < 0.01, Fig. 3a). All four probiotic strains delayed the occurrence of diarrhea and hematochezia in the early stage of the trial and reduced the DAI scores from day 4 (p < 0.01, Fig. 3d), especially Bc and Cb (Fig. 3b). On the sacrifice (day 6), Lr (p = 0.05) and Cb (p = 0.004) significantly relieved the shortening of the colon and atrophy of the cecum. Compared with the incomplete effects of the single probiotics, the Promix robustly alleviated the manifestations of colitis in the mice, as indicated by the reduced weight loss (p < 0.01), diarrhea (p < 0.05), hematochezia (p < 0.01), DAI scores (p < 0.001), and shortening of the colon (p < 0.001). Furthermore, histopathological analysis of the colon (Fig. 3f) showed that the probiotic mixture protected against inflammatory cell infiltration (p = 0.014) and damage to the colonic mucosa (p = 0.003) and reduced the Nancy index (p = 0.004) more effectively than single probiotics. The above results show that the combination of the four probiotics is more effective than any one of the single probiotics.
Effects of single probiotics and the probiotics in combination on DSS-induced colitis. a The body weight, b diarrhea, c hematochezia, and d DAI scores of mice were recorded daily. e The length of the colon was measured at sacrifice (day 6). f H & E staining (scale bare is 100 μm). The Nancy index, inflammatory cell infiltration, and destruction of the mucosal structure were scored according to H & E staining. Data represent mean values ± SEM (n = 10 per group). Statistical analysis was performed using one-way ANOVA with LSD. a, b Different letters indicate significant differences (p < 0.05)
The probiotic mixture improved the microbial ecology of the colonic mucosa
Since the alteration of intestinal microorganisms directly affects the progression of colitis (Zhang et al. 2017), we next analyzed whether repair of the intestinal microbial ecology is involved in the reduction of intestinal inflammation.
First, the effects of the probiotic mixture and anti-inflammatory drugs on the intestinal microbial composition were compared. The partial least squares-discriminant analysis (PLS-DA) at the operational taxonomic unit (OTU) level revealed significant differences in the microbial composition of each treatment group (Supplemental Fig. S1). A detailed taxonomic analysis showed that DSS decreased the relative abundance of Firmicutes, Actinobacteria, and Deferribacteres and increased the relative abundance of Proteobacteria and Cyanobacteria (Fig. 4a and b). In addition, DSS significantly reduced the proportion of core microbiota (Burrello et al. 2018) (Bacteroidales S24-7 group, Lachnospiraceae, Lactobacillaceae, Ruminococcaceae, Rikenellaceae, Bifidobacteriaceae, and Erysipelotrichaceae), which averaged 85% in healthy mice (Con group) but only 69.4% in the DSS mice (Fig. 4e). According to previous studies (Mahalhal et al. 2018; Mukhopadhya et al. 2012), the alteration of the microbial composition suggested that DSS caused dysbiosis of colonic mucosal microbiota. HW intensified the changes in the proportions of these bacteria on the basis of DSS treatment and promoted the expansion of the pro-inflammatory bacteria Rhodospirillaceae (Proteobacteria). SASP increased the relative abundance of taxa belonging to Proteobacteria, including Enterobacteriaceae, Desulfovibrionaceae, and Aeromonadaceae (Fig. 4c and d), which have been reported to be increased in colitis mice and inflammatory bowel disease (IBD) patients (Mukhopadhya et al. 2012; Zhou et al. 2018). Taken together, these results show that HW and SASP might exacerbate the dysbiosis caused by DSS. In contrast, the Promix not only reduced the proportions of pro-inflammatory bacteria but also increased the proportions of beneficial bacteria Bifidobacterium (Schroeder et al. 2018) and Blautia (Tong et al. 2018) (Fig. 4c and d). Moreover, the Promix protected against the reduction of core microbiota in the colonic mucosa (Fig. 4e) and increased the relative abundance of Lactobacillus and Bifidobacterium in the cecal contents (Supplemental Fig. S1g and h). In summary, the multi-strain probiotic mixture is more beneficial than anti-inflammatory drugs for the restoration of the mucosal microecology.
Microbial composition of the colonic mucosa by 16S rRNA gene V3–V4 region sequencing. a and b Histogram of microbial composition at the phylum level. c Histogram of microbial composition at the family level. d Relative abundance of bacteria at the family and genus levels. e Relative abundance of the core microbiota. Data represent mean values ± SEM (n = 6 per group). Statistical analysis was performed using one-way ANOVA with LSD. a, b Different letters indicate significant differences (p < 0.05)
Next, full-length 16S rRNA sequencing was used in an effort to better assess the differences between the effects of single probiotics and probiotic combination on mucosal microbiota. A total of 163,989 circular consensus sequencing (CCS) sequences were obtained from 21 samples (n = 3 per group). Next, 147,890 optimized CCS sequences were generated after quality control and filtration (7042 optimized CCS sequences per sample on average) for subsequent analysis, and the rarefaction curve showed that the sequencing data volume was sufficient (Supplemental Fig. S2a). Lr, Bc, Bf, Cb, and Promix increased the microbial diversity, which was decreased by DSS (Fig. 5b). Generally, intestinal microbial diversity is decreased in IBD patients (Sartor and Wu 2017), and high microbial diversity helps maintain intestinal homeostasis. Therefore, probiotics are beneficial for the repair of the microbial ecology. At the phylum level (Fig. 5a and c), all probiotics recovered the proportions of Bacteroidetes and Deferribacteres compared with the DSS group. The single probiotic groups (Lr, Bc, Bf, and Cb) tended to have an increased relative abundance of Cyanobacteria and Proteobacteria, which are associated with inflammation (Broadhurst et al. 2012; Mahalhal et al. 2018; Mukhopadhya et al. 2012). The increased proportions of these bacteria may be detrimental to the restoration of intestinal homeostasis, but the expansion of such microorganisms was limited in the Promix group. At the genus level, probiotics inhibited the expansion of Mucispirillum (belonging to Deferribacteres) induced by DSS (Fig. 5d and e). Mucispirillum, which colonizes the mucus layer, is associated with intestinal fibrosis and colitis (Jacob et al. 2018; Robertson et al. 2005; Rooks et al. 2014). In addition, the Promix increased the relative abundance of the anti-inflammatory bacteria Akkermansia, Blautia, Lactobacillus, and Bacillus coagulans (Fig. 5e).
Microbial composition of the colonic mucosa by full-length 16S rRNA sequencing. a and c Histogram of microbial composition at the phylum level. b Shannon index and Simpson index. d and e Histogram of microbial composition at the genus level. Data represent mean values ± SEM (n = 3 per group). Statistical analysis was performed using one-way ANOVA with LSD. a, b Different letters indicate significant differences (p < 0.05)
The probiotic mixture upregulated the expression of genes related to IL-10 and intestinal barrier function
The alteration of intestinal microbiota is closely related to the homeostasis of the immune system and the intestinal barrier. Therefore, the expression of genes related to inflammation (Tnf-α, Cox-2, Ifn-γ, Il-1β, Il-6, Il-10, Tgfβ, Il-17a, and Il-22), antimicrobial peptides (Reg3γ and lysozyme), tissue degradation (Mmp-3 and Mmp-9), and intestinal barrier function (Muc-2, occludin, claudin-1, and Zo-1) was analyzed (Fig. 6).
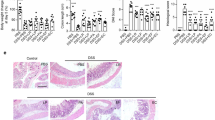
Effects of probiotic strains on inflammation and barrier function of the colon. a and b The expression of genes related to inflammation, antimicrobial peptide, degradation of tissues, and intestinal barrier function measured by RT-qPCR. c Western blot analysis of Occludin and Claudin-1 proteins in the colon. Data represent mean values ± SEM (n = 10 per group). Statistical analysis was performed using one-way ANOVA with LSD. a, b Different letters indicate significant differences (p < 0.05)
Compared with the Con group, the DSS group showed significantly increased expression of Tnf-α (p < 0.001), Ifn-γ (p = 0.087), Il-1β (p = 0.002), Il-6 (p < 0.001), Il-22 (p = 0.028), Reg3γ (p = 0.009), Mmp-3 (p < 0.001), and Mmp-9 (p < 0.001) and decreased the expression of Tgfβ (p = 0.032). In addition, the DSS group also showed reduced levels of occludin and claudin-1 (Fig. 6b and c). These results suggest that DSS treatment leads to severe dysfunction of the intestinal immune system and barrier. Compared with the DSS group, the Lr group reduced levels of Il-6 (p = 0.018), Mmp-3 (p < 0.001), and Mmp-9 (p = 0.062) and the expression of other genes was minimally affected. The Bc group reduced expression of Il-1β (p = 0.004), Il-6 (p < 0.001), Mmp-3 (p < 0.001), and Mmp-9 (p = 0.002) and increased expression of occludin (p = 0.003). The Bf group decreased expression of Cox-2 (p = 0.064), Il-6 (p = 0.006), Mmp-3 (p < 0.001), and Mmp-9 (p = 0.012) and increased expression of Tgfβ (p = 0.046) and Il-17a (p = 0.035). The Cb group reduced expression of Il-1β (p = 0.022), Il-6 (p = 0.007), and Mmp-3 (p < 0.001) and increased expression of Muc-2 (p = 0.014). These results suggest that the four probiotic strains have different effects on the regulation of mucosal immunity and barrier function.
Compared with single probiotics, the combination of the four probiotics was more efficient in reducing intestinal inflammation and injury and showed the synergy of the four probiotic strains. As shown in Fig. 6, the Promix reduced the expression of pro-inflammatory cytokines more robustly than single probiotics and significantly increased the expression of Il-10 (p = 0.037). In addition, although the single probiotics barely affected the expression of claudin-1, the Promix significantly increased the mRNA level of claudin-1 (p = 0.039). Furthermore, the Promix preserved the occludin and claudin-1 protein levels more effectively than the single probiotics, as shown by western blotting (Fig. 6c).
Probiotics had a limited impact on the concentrations of organic acids in the intestinal contents
Producing organic acids is one of the important functions of probiotics. Organic acids can enhance intestinal barrier integrity and affect the function and differentiation of intestinal immune cells (Lee et al. 2018; Sun et al. 2017). Therefore, we analyzed whether the protective effects of the probiotics are directly related to the regulation of organic acid concentrations in the intestinal tract. Compared with the Con group, the DSS group showed an increased concentration of lactate and decreased concentrations of acetate, propionate, and butyrate (Fig. 7). Although the probiotics protected against colitis, they did not significantly affect the concentrations of organic acids compared with the DSS treatment. Instead, the Lr and Bf groups showed significantly reduced concentrations of butyrate compared with the DSS group; butyrate has been reported to help enhance intestinal homeostasis (Sun et al. 2018). These results suggest that the probiotic-mediated protection against intestinal inflammation and damage might not depend on alterations in the concentrations of organic acids. For example, exopolysaccharides, soluble proteins, or pili from Lactobacillus and Bifidobacterium have been shown to improve intestinal homeostasis (Castro-Bravo et al. 2018; Duranti et al. 2016; Yan et al. 2007). Therefore, more studies are needed to explain the mechanisms of probiotics for the treatment of UC.
Effects of probiotics on the concentration of organic acids in the colonic contents. a Lactate, b acetate, c propionate, and d butyrate concentrations were measured by HPLC. Data represent mean values ± SEM (n = 10 per group). Statistical analysis was performed using one-way ANOVA with LSD. a, b Different letters indicate significant differences (p < 0.05)
Discussion
The potential of probiotics for the treatment of multiple diseases, such as diabetes (Bordalo Tonucci et al. 2017), intestinal inflammation (Ganji-Arjenaki and Rafieian-Kopaei 2018), and allergies (Hajavi et al. 2019), has attracted increasing attention. However, more studies are required to clarify the differences in the mechanisms and guide the use of probiotics. In this study, the effects of four probiotic strains (L. reuteri RAM0101, B. coagulans RAM1202, B. longum CICC6197, and C. butyricum RAM0216) on intestinal inflammation and damage were analyzed in a DSS-colitis mouse model. The results showed that the effects of the four probiotic strains were different on the regulation of intestinal immunity, barrier function, and microbial ecology. The combination of the four probiotic strains was more effective than any of the single probiotic strain and anti-inflammatory drugs at improving the intestinal homeostasis.
Currently, the first-line therapy for mild to moderate UC is aminosalicylates, such as 5-aminosalicylic acid and SASP (Ungaro et al. 2017). In this study, SASP only alleviated the shortening of the colon and inhibited the DSS-induced upregulation of pro-inflammatory cytokines; it failed to protect against weight loss, DAI, and the destruction of the intestinal epithelium. SASP is a first-line drug for UC treatment and has proven effects. In this study, SASP did not protect against DSS-induced colitis, which may indicate the limitations of DSS-induced colitis mouse model. Extracts of E. humifusa (HW, H70E, and HE) maintained the structural integrity of the colon and effectively decreased inflammation, which might be due to the effects of tannins (de Jesus et al. 2012) that are abundant in E. humifusa. However, extracts of E. humifusa did not prevent weight loss or shortening of the colon. However, these results do not negate the value of E. humifusa in the treatment of enteritis because traditional Chinese medicine usually involves the use of multiple drugs, resulting in a complex combination of synergistic chemicals. Of all the treatments, Promix was the most effective in protecting against intestinal inflammation and damage, indicating the therapeutic potential of the probiotic mixture for UC.
A stable and healthy intestinal microecosystem is the foundation required for the homeostasis of the intestinal barrier and immune system. It has been reported that dysbiosis of the intestinal microbiota is closely related to the initiation and progression of UC (Ananthakrishnan et al. 2018; Sartor and Wu 2017; Zhang et al. 2017). In this study, DSS caused dysbiosis of the colonic mucosal microbiota. The dysbiosis of the intestinal microbiota may lead to an imbalance of the interplay between the intestinal microbiome and immunological niches and the loss of nutritional niches and functional genes of the microbiota. SASP and HW exacerbated the changes in microbial abundance caused by DSS, both at the phylum and family level. Although SASP and HW relieved the symptoms of colitis, in the long run, the influence of SASP and HW on the intestinal microbiota was not conducive to the recovery of intestinal homeostasis. In contrast, the Promix inhibited the expansion of harmful bacteria, increased the relative abundance of beneficial bacteria, and restored the commensal bacteria to a normal level. Previous studies reported that fecal microbiota transplantation (FMT) or probiotics (VSL#3) could stimulate immune cells by restoring healthy microbial ecology, thereby reducing intestinal inflammation and damage (Burrello et al. 2018; Di Giacinto et al. 2005). Therefore, the regulation of the intestinal microbial ecology by the multi-strain probiotic mixture is one of the keys to the restoration of intestinal homeostasis.
Intact and well-functioning intestinal epithelial monolayers are the basis of intestinal homeostasis (Ramos and Papadakis 2019). Maintaining the function and structural integrity of the intestinal barrier is critical for preventing the initiation and progression of intestinal inflammation. Glycoprotein, encoded by the Muc-2 gene, is the main component of mucous layer, which provides a physical and chemical barrier against microorganisms. The deletion of the Muc-2 gene or deficiency of the mucus layer is associated with a variety of immune-related diseases and spontaneous colitis (McGuckin et al. 2009; Turner 2009). In this study, the expression of Muc-2 was upregulated in the Cb, Bc, and Promix groups, which would strengthen the colonic mucous layer and resist the invasion of microbiota. In addition to the mucous layer, increased permeability of the tight junctions accelerates the development of inflammatory diseases (Turner 2009). In this study, DSS decreased the levels of occludin and claudin-1. Bc increased the mRNA level of the occludin gene. Promix increased the mRNA and protein levels of the occludin and claudin-1 genes, which are crucial for the recovery of tight junctions. Probiotics have been reported that enhancing the intestinal barrier function is one of the important factors to relieve the symptoms of diseases (Bron et al. 2017; Schroeder et al. 2018). Therefore, the probiotic combination used in this study may be effective in prevention and treatment of UC by enhancing the intestinal barrier function. Further clinical trials will be conducted to verify the efficacy and safety of the probiotic mixture.
Probiotics such as Lactobacillus (Gao et al. 2015), Bifidobacterium (Jang et al. 2018), Bacillus coagulans (Mu and Cong 2019), and Clostridium butyricum (Cai et al. 2016; Zhang et al. 2009) can regulate the intestinal immune response when used alone or in combination (Di Giacinto et al. 2005). However, it has not been reported whether these probiotics work by affecting the same immune responses. The results of this study showed that the effects of the four probiotic strains on the immune response are different (Fig. 8). For example, only Bf significantly increased the expression of TGFβ and IL-17a, indicating that Bf may promote the repair of damaged intestines through the Act1-mediated signaling pathway (Song et al. 2015). These results suggest that different probiotics could specifically interact with different immunological niches (Fig. 8b). The dysregulation of intestinal microbiota in IBD patients and experimental colitis animals may result in the imbalance of interactions between microorganisms and immunological niches. The combination of probiotics with different immunologic functions would complement the unsaturated immunological niches. That is, we suppose, why the multi-strain probiotic mixture was more effective in reducing inflammation than the single probiotics.
Overview of the effects of different probiotics on the intestinal immune response and barrier integrity. a Heatmap of physiological indexes affected by different probiotic strains. The source and function of the physiological indexes were described according to previous reviews (Kmiec et al. 2017; McGuckin et al. 2009; Neurath 2014). b Sketch map of the interactions between different probiotic strains and immunological niches. In the inflammatory state, the integrity of the intestinal barrier is reduced, leading to the transfer of antigens into the gut lamina propria. The antigens are detected by antigen-presenting cells (such as dendritic cells and macrophages) and activate a range of immune responses, including the release of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IFN-γ, IL-17a, and COX-2) and inflammatory cell infiltration. The damage to the intestinal barrier and invasion of antigens induces the production of IL-22, which stimulates the secretion of antimicrobial peptides (such as Reg3γ). Persistent and active inflammation induces myofibroblasts to secrete MMP-3 and MMP-9 and exacerbates intestinal epithelial injury. The four probiotics function differently in the regulation of the intestinal immune response and intestinal barrier. The combination of the four probiotics reduces the expression of pro-inflammatory cytokines more robustly than single probiotics and enhances the expression of IL-10 and intestinal barrier proteins. The blue lines show the effects of single probiotic strain, and the black lines show the effects of the multi-strain probiotic mixture. The dotted lines indicate the presumed immune pathways. DCs, dendritic cells; NK, natural killer cells; IELs, intraepithelial lymphocytes; IECs, intestinal epithelial cells; ILCs, innate lymphoid cells. Em dash “—,” not applicable
IL-10 is an important anti-inflammatory cytokine. A genetic polymorphism of IL-10 is one of the key risk factors for UC, and the insufficiency of IL-10 or the IL-10 receptor (IL-10R) leads to severe spontaneous colitis (Neurath 2014). It has been reported that FMT, probiotics, and SCFAs reduce intestinal inflammation through IL-10-mediated immune pathways (Burrello et al. 2018; Di Giacinto et al. 2005; Sun et al. 2018). These studies showed that enhancing the expression of IL-10 is one of the keys to control intestinal inflammation. In this study, the probiotic mixture significantly increased the expression of IL-10, which was crucial for the reduction of intestinal inflammation caused by DSS.
IL-22 is thought to be involved in the repair of intestinal damage. IL-22 also induces intestinal epithelial cells (IECs) to secrete antimicrobial molecules (such as defensins and REG), which in turn affect the colitogenic microbiota (Neurath 2014). In this study, DSS treatment increased the expression of IL-22 and Reg3γ, which were decreased by Promix treatment. Spearman correlation analysis showed that IL-22 and Reg3γ were positively correlated with pathological injury, levels of inflammation, MMP-3, and MMP-9, while they were negatively correlated with the expression of claudin-1, occludin, ZO-1, and lysozyme (Supplemental Fig. S3). Considering that reducing the invasion of microorganisms can reduce inflammation, the increased expression of IL-22 might be caused by the destruction of the intestinal barrier and the stress response of intestinal immune system induced by invading microorganisms. In this study, Promix significantly inhibited the expression of IL-22 and Reg3γ, reflecting the protective effects of the multi-strain probiotic mixture on intestinal barrier integrity and the prevention of antigen invasion.
Understanding of the relationships between gut microbes and disease have benefited from the development of high-throughput sequencing technology. To date, short-read sequencing technology has been widely used to analyze the microbial communities of various environments. However, due to the limitation of sequencing length, the short-read sequencing has low phylogenetic resolution and accuracy (Myer et al. 2016; Singer et al. 2016; Whon et al. 2018). In this study, a large number of OTUs generated by 16S rRNA gene V3–V4 region sequencing could only be classified at the family level, and some OTUs belonging to the same taxa showed a completely different trend (Supplemental Fig. S4). These contrasting results blurred the relationships between microorganisms and colitis. To better analyze the influence of single and multi-strain probiotics on the intestinal microbial composition and to show the relationships between bacteria and colitis, we implemented the full-length 16S rRNA sequencing to analyze the microbial composition of the colonic mucosa. The full-length 16S rRNA sequencing showed higher phylogenetic resolution than the short-read sequencing technology. The proportion of unclassified OTUs decreased significantly, and almost all of the OTUs were classified into genus taxa. At the species level, the relative abundance of L. reuteri and B. coagulans was accurately distinguished (Fig. 5e and Supplemental Fig. S2c), which was consistent with the expected results. On this basis, the microorganisms significantly related to the physiology of the colitis mice were identified through Spearman correlation analysis (Supplemental Fig. S2b). However, the PacBio sequencing technology still has some shortcomings, such as a higher error rate and higher level of deletions than the short-read sequencing platform (Whon et al. 2018). And the results obtained by the PacBio sequencing technology are quite different from those obtained by other high-throughput sequencing technologies (Myer et al. 2016; Singer et al. 2016; Whon et al. 2018). Therefore, more studies are needed to determine the optimal point between full-length 16S rRNA sequencing and short-read sequencing.
In summary, our results revealed the functional differences of four probiotic strains in the regulation of intestinal immunity, barrier function, and microbiota. Moreover, the combination of the four probiotics showed synergistic effects of the individual probiotics and was more effective than clinical anti-inflammatory drugs or any of the four individual probiotics in alleviating DSS-induced colitis. The probiotic mixture alleviated colitis by restoring the mucosal microbial ecology and increasing the expression of IL-10 and intestinal barrier function. The multi-strain probiotic mixture reduced invasion of the deep intestinal tissues by bacteria and maintained the host microorganism balance. This study confirmed the safety and effectiveness of the probiotic mixture and explained the advantages of multi-strain probiotics in disease treatment.
References
Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, Vavricka SR, Fiocchi C (2018) Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol 15(1):39–49. https://doi.org/10.1038/nrgastro.2017.136
Bordalo Tonucci L, Dos Santos KM, De Luces Fortes Ferreira CL, Ribeiro SM, De Oliveira LL, Martino HS (2017) Gut microbiota and probiotics: focus on diabetes mellitus. Crit Rev Food Sci Nutr 57(11):2296–2309. https://doi.org/10.1080/10408398.2014.934438
Bose S, Han KW, Lee MJ, Kim H (2013) Intestinal protective effects of herbal-based formulations in rats against neomycin insult. Evid Based Complement Alternat Med 2013:161278. https://doi.org/10.1155/2013/161278
Broadhurst MJ, Ardeshir A, Kanwar B, Mirpuri J, Gundra UM, Leung JM, Wiens KE, Vujkovic-Cvijin I, Kim CC, Yarovinsky F, Lerche NW, McCune JM, Loke P (2012) Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS Pathog 8(11):e1003000. https://doi.org/10.1371/journal.ppat.1003000
Bron PA, Kleerebezem M, Brummer RJ, Cani PD, Mercenier A, MacDonald TT, Garcia-Rodenas CL, Wells JM (2017) Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr 117(1):93–107. https://doi.org/10.1017/S0007114516004037
Burrello C, Garavaglia F, Cribiu FM, Ercoli G, Lopez G, Troisi J, Colucci A, Guglietta S, Carloni S, Guglielmetti S, Taverniti V, Nizzoli G, Bosari S, Caprioli F, Rescigno M, Facciotti F (2018) Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat Commun 9(1):5184. https://doi.org/10.1038/s41467-018-07359-8
Cai M, Zeng L, Li LJ, Mo LH, Xie RD, Feng BS, Zheng PY, Liu ZG, Liu ZJ, Yang PC (2016) Specific immunotherapy ameliorates ulcerative colitis. Allergy Asthma Clin Immunol 12:37. https://doi.org/10.1186/s13223-016-0142-0
Castro-Bravo N, Wells JM, Margolles A, Ruas-Madiedo P (2018) Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Front Microbiol 9:2426. https://doi.org/10.3389/fmicb.2018.02426
Chong J, Soufan O, Li C, Caraus I, Li SZ, Bourque G, Wishart DS, Xia JG (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46(W1):W486–W494. https://doi.org/10.1093/nar/gky310
de Jesus NZ, de Souza FH, Gomes IF, de Almeida Leite TJ, de Morais Lima GR, Barbosa-Filho JM, Tavares JF, da Silva MS, de Athayde-Filho PF, Batista LM (2012) Tannins, peptic ulcers and related mechanisms. Int J Mol Sci 13(3):3203–3228. https://doi.org/10.3390/ijms13033203
Deltenre P, Berson A, Marcellin P, Degott C, Biour M, Pessayre D (1999) Mesalazine (5-aminosalicylic acid) induced chronic hepatitis. Gut 44(6):886–888. https://doi.org/10.1136/Gut.44.6.886
Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M (2005) Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol 174(6):3237–3246. https://doi.org/10.4049/jimmunol.174.6.3237
Duranti S, Gaiani F, Mancabelli L, Milani C, Grandi A, Bolchi A, Santoni A, Lugli GA, Ferrario C, Mangifesta M, Viappiani A, Bertoni S, Vivo V, Serafini F, Barbaro MR, Fugazza A, Barbara G, Gioiosa L, Palanza P, Cantoni AM, de’ Angelis GL, Barocelli E, de’ Angelis N, van Sinderen D, Ventura M, Turroni F (2016) Elucidating the gut microbiome of ulcerative colitis: bifidobacteria as novel microbial biomarkers. FEMS Microbiol Ecol 92(12):fiw191. https://doi.org/10.1093/femsec/fiw191
Ek S, Rosenborg S (2017) Mesalazine as a cause of fetal anemia and hydrops fetalis: a case report. Medicine 96(50):e9277. https://doi.org/10.1097/MD.0000000000009277
Fitzpatrick LR, Small JS, Greene WH, Karpa KD, Farmer S, Keller D (2012) Bacillus coagulans GBI-30, 6086 limits the recurrence of Clostridium difficile-Induced colitis following vancomycin withdrawal in mice. Gut Pathog 4(1):13. https://doi.org/10.1186/1757-4749-4-13
Ganji-Arjenaki M, Rafieian-Kopaei M (2018) Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol 233(3):2091–2103. https://doi.org/10.1002/jcp.25911
Gao C, Major A, Rendon D, Lugo M, Jackson V, Shi Z, Mori-Akiyama Y, Versalovic J (2015) Histamine H2 Receptor-mediated suppression of intestinal inflammation by probiotic Lactobacillus reuteri. mBio 6(6):e01358–e01315. https://doi.org/10.1128/mBio.01358-15
Gao X, Xie Q, Kong P, Liu L, Sun S, Xiong B, Huang B, Yan L, Sheng J, Xiang H (2017) Polyphenol- and caffeine-rich post-fermented Pu-er tea improves diet-induced metabolic syndrome by remodeling intestinal homeostasis in mice. Infect Immun 86(1):e00601–e00617. https://doi.org/10.1128/IAI.00601-17
Hajavi J, Esmaeili SA, Varasteh AR, Vazini H, Atabati H, Mardani F, Momtazi-Borojeni AA, Hashemi M, Sankian M, Sahebkar A (2019) The immunomodulatory role of probiotics in allergy therapy. J Cell Physiol 234(3):2386–2398. https://doi.org/10.1002/jcp.27263
Jacob N, Jacobs JP, Kumagai K, Ha CWY, Kanazawa Y, Lagishetty V, Altmayer K, Hamill AM, Von Arx A, Sartor RB, Devkota S, Braun J, Michelsen KS, Targan SR, Shih DQ (2018) Inflammation-independent TL1A-mediated intestinal fibrosis is dependent on the gut microbiome. Mucosal Immunol 11(5):1466–1476. https://doi.org/10.1038/s41385-018-0055-y
Jang SE, Jeong JJ, Kim JK, Han MJ, Kim DH (2018) Simultaneous amelioratation of colitis and liver injury in mice by Bifidobacterium longum LC67 and Lactobacillus plantarum LC27. Sci Rep 8(1):7500. https://doi.org/10.1038/s41598-018-25775-0
Je IG, Lee DG, Jeong DG, Hong D, Yoon JM, Moon JS, Park S (2018) The probiotic, ID-JPL934, attenuates dextran sulfate sodium-induced colitis in mice through inhibition of proinflammatory cytokines expression. J Med Food 21(9):858–865. https://doi.org/10.1089/jmf.2017.4152
Kmiec Z, Cyman M, Slebioda TJ (2017) Cells of the innate and adaptive immunity and their interactions in inflammatory bowel disease. Adv Med Sci 62(1):1–16. https://doi.org/10.1016/j.advms.2016.09.001
Lee YS, Kim TY, Kim Y, Lee SH, Kim S, Kang SW, Yang JY, Baek IJ, Sung YH, Park YY, Hwang SW, Eunju O, Kim KS, Liu SQ, Kamada N, Gao N, Kweon MN (2018) Microbiota-derived lactate accelerates iIntestinal stem-cell-mediated epithelial development. Cell Host Microbe 24(6):833–846. https://doi.org/10.1016/j.chom.2018.11.002
Li ZJ, Guo X, Dawuti G, Aibai S (2015) Antifungal activity of ellagic acid in vitro and in vivo. Phytother Res 29(7):1019–1025. https://doi.org/10.1002/ptr.5340
Luyen BTT, Tai BH, Thao NP, Eun KJ, Cha JY, Xin MJ, Lee YM, Kim YH (2014) Anti-inflammatory components of Euphorbia humifusa Willd. Bioorg Med Chem Lett 24(8):1895–1900. https://doi.org/10.1016/j.bmcl.2014.03.014
Mahalhal A, Williams JM, Johnson S, Ellaby N, Duckworth CA, Burkitt MD, Liu X, Hold GL, Campbell BJ, Pritchard DM, Probert CS (2018) Oral iron exacerbates colitis and influences the intestinal microbiome. PloS One 13(10):e0202460. https://doi.org/10.1371/journal.pone.0202460
Marchal-Bressenot A, Scherl A, Salleron J, Peyrin-Biroulet L (2016) A practical guide to assess the Nancy histological index for UC. Gut 65(11):1919–1920. https://doi.org/10.1136/gutjnl-2016-312722
McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G (2009) Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis 15(1):100–113. https://doi.org/10.1002/ibd.20539
Mu Y, Cong Y (2019) Bacillus coagulans and its applications in medicine. Benef Microbes 10(6):679–688. https://doi.org/10.3920/BM2019.0016
Mukhopadhya I, Hansen R, El-Omar EM, Hold GL (2012) IBD-what role do Proteobacteria play? Nat Rev Gastro Hepat 9(4):219–230. https://doi.org/10.1038/nrgastro.2012.14
Myer PR, Kim M, Freetly HC, Smith TPL (2016) Evaluation of 16S rRNA amplicon sequencing using two next-generation sequencing technologies for phylogenetic analysis of the rumen bacterial community in steers. J Microbiol Methods 127:132–140. https://doi.org/10.1016/j.mimet.2016.06.004
Neurath MF (2014) Cytokines in inflammatory bowel disease. Nat Rev Immunol 14(5):329–342. https://doi.org/10.1038/nri3661
Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A (2018) Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 11(1):1–10. https://doi.org/10.1007/s12328-017-0813-5
Pagliari D, Gambassi G, Piccirillo CA, Cianci R (2017) The intricate link among gut “immunological niche,” microbiota, and xenobiotics in intestinal pathology. Mediators Inflamm 2017:8390595. https://doi.org/10.1155/2017/8390595
Ramos GP, Papadakis KA (2019) Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc 94(1):155–165. https://doi.org/10.1016/j.mayocp.2018.09.013
Ransford RA, Langman MJ (2002) Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut 51(4):536–539. https://doi.org/10.1136/gut.51.4.536
Robertson BR, O’Rourke JL, Neilan BA, Vandamme P, On SL, Fox JG, Lee A (2005) Mucispirillum schaedleri gen. nov., sp. nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents. Int J Syst Evol Microbiol 55(Pt 3):1199–1204. https://doi.org/10.1099/ijs.0.63472-0
Rooks MG, Veiga P, Wardwell-Scott LH, Tickle T, Segata N, Michaud M, Gallini CA, Beal C, van Hylckama-Vlieg JE, Ballal SA, Morgan XC, Glickman JN, Gevers D, Huttenhower C, Garrett WS (2014) Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J 8(7):1403–1417. https://doi.org/10.1038/ismej.2014.3
Sartor RB, Wu GD (2017) Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology 152(2):327–339 e4. https://doi.org/10.1053/j.gastro.2016.10.012
Schroeder BO, Birchenough GMH, Stahlman M, Arike L, Johansson MEV, Hansson GC, Backhed F (2018) Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 23(1):27–40 e7. https://doi.org/10.1016/j.chom.2017.11.004
Shang X, Miao X, Yang F, Li B, Guo X, Pan H, Zhang Y, Zhang J (2018) The anti-diarrheal activity of the non-toxic dihuang powder in mice. Front Pharmacol 9:1037. https://doi.org/10.3389/fphar.2018.01037
Shin IS, Jeon WY, Shin HK, Cha SW, Lee MY (2013) Banhabaekchulchunma-tang, a traditional herbal formula attenuates absolute ethanol-induced gastric injury by enhancing the antioxidant status. BMC Complement Altern Med 13:170. https://doi.org/10.1186/1472-6882-13-170
Singer E, Bushnell B, Coleman-Derr D, Bowman B, Bowers RM, Levy A, Gies EA, Cheng JF, Copeland A, Klenk HP, Hallam SJ, Hugenholtz P, Tringe SG, Woyke T (2016) High-resolution phylogenetic microbial community profiling. ISME J 10(8):2020–2032. https://doi.org/10.1038/ismej.2015.249
Song X, Dai D, He X, Zhu S, Yao Y, Gao H, Wang J, Qu F, Qiu J, Wang H, Li X, Shen N, Qian Y (2015) Growth factor FGF2 cooperates with interleukin-17 to repair intestinal epithelial damage. Immunity 43(3):488–501. https://doi.org/10.1016/j.immuni.2015.06.024
Sun M, Wu W, Liu Z, Cong Y (2017) Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol 52(1):1–8. https://doi.org/10.1007/s00535-016-1242-9
Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, Chen F, Xiao Y, Zhao Y, Ma C, Yao S, Carpio VH, Dann SM, Zhao Q, Liu Z, Cong Y (2018) Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun 9(1):3555. https://doi.org/10.1038/s41467-018-05901-2
Talero E, Bolivar S, Avila-Roman J, Alcaide A, Fiorucci S, Motilva V (2015) Inhibition of chronic ulcerative colitis-associated adenocarcinoma development in mice by VSL#3. Inflamm Bowel Dis 21(5):1027–1037. https://doi.org/10.1097/MIB.0000000000000346
Tong X, Xu J, Lian F, Yu X, Zhao Y, Xu L, Zhang M, Zhao X, Shen J, Wu S, Pang X, Tian J, Zhang C, Zhou Q, Wang L, Pang B, Chen F, Peng Z, Wang J, Zhen Z, Fang C, Li M, Chen L, Zhao L (2018) Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional Chinese herbal formula: a multicenter, randomized, open label clinical trial. mBio 9(3):e02392–e02317. https://doi.org/10.1128/mBio.02392-17
Turner JR (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9(11):799–809. https://doi.org/10.1038/nri2653
Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA, Modeo ME, Rodino S, D’Amico T, Sebkova L, Sacca N, Di Giulio E, Luzza F, Imeneo M, Larussa T, Di Rosa S, Annese V, Danese S, Gasbarrini A (2010) Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol 105(10):2218–2227. https://doi.org/10.1038/ajg.2010.218
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J-F (2017) Ulcerative colitis. Lancet 389(10080):1756–1770. https://doi.org/10.1016/s0140-6736(16)32126-2
Wang YB, Xie QH, Sun S, Huang BJ, Zhang Y, Xu Y, Zhang SM, Xiang HY (2018) Probiotics-fermented Massa Medicata Fermentata ameliorates weaning stress in piglets related to improving intestinal homeostasis. Appl Microbiol Biotechnol 102(24):10713–10727. https://doi.org/10.1007/s00253-018-9438-y
Whon TW, Chung WH, Lim MY, Song EJ, Kim PS, Hyun DW, Shin NR, Bae JW, Nam YD (2018) The effects of sequencing platforms on phylogenetic resolution in 16S rRNA gene profiling of human feces. Sci Data 5:180068. https://doi.org/10.1038/sdata.2018.68
Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB (2007) Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132(2):562–575. https://doi.org/10.1053/j.gastro.2006.11.022
Yoshimatsu Y, Yamada A, Furukawa R, Sono K, Osamura A, Nakamura K, Aoki H, Tsuda Y, Hosoe N, Takada N, Suzuki Y (2015) Effectiveness of probiotic therapy for the prevention of relapse in patients with inactive ulcerative colitis. World J Gastroenterol 21(19):5985–5994. https://doi.org/10.3748/wjg.v21.i19.5985
Zhang HQ, Ding TT, Zhao JS, Yang X, Zhang HX, Zhang JJ, Cui YL (2009) Therapeutic effects of Clostridium butyricum on experimental colitis induced by oxazolone in rats. World J Gastroenterol 15(15):1821–1828. https://doi.org/10.3748/wjg.15.1821
Zhang SL, Wang SN, Miao CY (2017) Influence of microbiota on intestinal immune system in ulcerative colitis and its intervention. Front Immunol 8:1674. https://doi.org/10.3389/fimmu.2017.01674
Zhou Y, Xu ZZ, He Y, Yang Y, Liu L, Lin Q, Nie Y, Li M, Zhi F, Liu S, Amir A, González A, Tripathi A, Chen M, Wu GD, Knight R, Zhou H, Chen Y, Ercolini D (2018) Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems 3(1):e00188–e00117. https://doi.org/10.1128/mSystems.00188-17
Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, Kumar MV, Gewirtz AT (2018) Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 23(1):41–53.e4. https://doi.org/10.1016/j.chom.2017.11.003
Funding
This work was supported by the Science and Technology Development Planning of Jilin [20180201078YY].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The animal experiment was approved by the Institutional Animal Care and Use Committee of Jilin University (IACUC). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 791 kb).
Rights and permissions
About this article
Cite this article
Wang, Y., Xie, Q., Zhang, Y. et al. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl Microbiol Biotechnol 104, 335–349 (2020). https://doi.org/10.1007/s00253-019-10259-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10259-6