Abstract
Cell physiology parameters are essential aspects of biological processes; however, they are difficult to determine on-line. Dielectric spectroscopy allows the on-line estimation of viable cells and can provide important information about cell physiology during culture. In this study, we investigated the dielectric property variations in Kluyveromyces marxianus SLP1 and Saccharomyces cerevisiae ERD yeasts stressed by 5-hydroxymethyl-2-furaldehyde and 2-furaldehyde during aerobic growth. The dielectric properties of cell permittivity, specific membrane capacitance (Cm), and intracellular conductivity (σIn) were considerably affected by furan aldehydes in the same way that the cell population, viability, cell size, substrate consumption, organic acid production, and respiratory parameters were. The yeasts stressed with furan aldehydes exhibited three physiological states (φ): adaptation, replicating, and nonreplicating states. During the adaptation state, there were small and stable signs of permittivity, Cm, and σIn; additionally, no cell growth was observed. During the replicating state, cell growth was restored, and the cell viability increased; in addition, the permittivity and σIn increased rapidly and reached their maximum values, while the Cm decreased. In the nonreplicating state, the permittivity and σIn were stable, and Cm decreased to its minimum value. Our results demonstrated that knowing dielectric properties allowed us to obtain information about the physiological state of the cells under control and stressed conditions. Since the permittivity, Cm, and σIn are directly associated with the physiological state of the yeast, these results should contribute to a better understanding of the stress response of yeasts and open the possibility to on-line monitor and control the physiological state of the cell in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The yeasts have evolved to be able to survive severe and sudden changes in their external environment. In nature, yeasts must deal with diverse fluctuations in conditions such as temperature, osmolarity, pH, the presence of toxic chemicals, and the provision and depletion of nutrients; actually, minimal changes in external conditions can cause several disturbances in the intracellular environment.
For the optimum growth, function, and synthesis of bioproducts, the yeasts must have specific and critical balanced internal conditions, for example, internal pH. Cellular disturbances cause stress in the yeast that may affect metabolic fluxes and cellular structures such as membranes and alter the synthesis of macromolecules such as lipids, proteins, and nucleic acids; all these modifications provoke damage in the yeast function (Gibson et al. 2007). Cellular growth under stress conditions requires maintenance of the internal environment to address these unfavorable situations. The yeast enters a phase of adaptation and responds quickly by synthesizing molecules that allow it to attenuate or repair the damage caused by stress. These adaptations are multiple and involve adaptation at the physiological and genetic levels. Cell physiology is most commonly described by the term “physiological state (φ)”; the physiological state can be defined by different variables that provide important information about the cellular state. These variables include growth and metabolic rates, including their ratios; concentrations and metabolic fluxes; degrees of substrate/nutrient limitations; cellular morphology (shape, the degree of aggregation, size); membrane state; and dielectric properties (membrane conductivity, intracellular conductivity) (Ansorge et al. 2010). In other words, the physiological state φ for a cell population is defined as the cellular properties that affect the fermentative activity and the characteristics of the cell population and reflect changes in the conditions of a culture.
Cell physiology and viability are essential aspects of biological processes and are very difficult to determine. The monitoring of cell physiology and viable cells on-line can allow control of the growth rate, monitor the critical phases during culturing, and identify deviations during the process. The study of dielectric properties (capacitance) in the cell has a potential application for on-line estimation of viable cells (based on the loss of the integrity of the membrane); therefore, it can provide important information about the cellular physiology during cultivation (Opel et al. 2010; Tibayrenc et al. 2011). Dielectric spectroscopy has been applied in biotechnological processes to determine viable biomass (Carvell and Dowd 2006), cell death (Patel and Markx 2008), and stress response (Tibayrenc et al. 2011) and to study the physiological state of yeasts (Farías-Álvarez et al. 2018). In recent years, one of the most studied biotechnological processes is the production of second-generation bioethanol, which is produced from nonfood raw materials such as lignocellulosic material (Gupta and Verma 2015 ). In the conversion of biomass into biofuels, the lignocellulosic material must be pretreated, and during this step, several toxic compounds such as furan aldehydes are generated, affecting the physiological parameters of microorganisms used in the fermentation. 2-Furaldehyde (furfural) and 5-hydroxymethyl-2-furaldehyde (HMF) are the most representative aldehyde inhibitor compounds and are composed of a furan ring and an aldehyde functional group, which is toxic to the cell (Liu et al. 2008). Yeasts can metabolize these furan aldehydes into less toxic compounds by aldehyde reductases. HMF is biotransformed to furan-2,5-dimethanol (FDM) and furfural to furanmethanol (FM) (Liu 2011). In the particular case of furfural and HMF, these compounds can inhibit cell growth and metabolite production, and they negatively affect the fermentative capacities of the yeast such as the volumetric ethanol yield and productivity (Sandoval-Nuñez et al. 2017; Flores-Cosío et al. 2018). Currently, most of the studies performed to understand the furan aldehydes and phenolic and organic acid effects in yeast are oriented toward Saccharomyces cerevisiae. However, to the best of our knowledge, there is not enough information about the dynamic variations in dielectric properties associated with the tolerance, response mechanisms, and physiological state of a promising yeast for industrial applications, Kluyveromyces marxianus, when it is introduced to stress conditions with furfural and HMF. Therefore, in this contribution, the measurements with a dielectric spectroscopy probe were used to evaluate and compare the effects of furfural and HMF on the cell effective membrane conductance (permittivity), specific membrane capacitance (Cm), and intracellular conductivity (σIn) to better understand the physiological changes in the yeasts K. marxianus and S. cerevisiae.
Materials and methods
Microorganisms
The strains used in this study were the commercial strain S. cerevisiae Ethanol Red® (fermentis) (Lesaffre, France) (ERD) and the strain K. marxianus NRRL Y-50883 (SLP1), which was isolated from Agave salmiana juice in Charcas, San Luis Potosí, and belongs to the Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco AC (CIATEJ AC) yeast collection. Yeast strains were stored on YPD agar medium at 4 °C.
Culture medium
A defined mineral culture medium was used, containing 20.0 g L−1 glucose as the only carbon source; a mixture of salts composed of (g L−1) 1.49 Na2HPO4·2H2O, 3.0 K2HPO4, 3.0 (NH4)·2SO4, and 1.0 glutamic acid; 2.5 mL L−1 vitamin solution composed of (mg L−1) 1.0 aminobenzoic acid, 125.0 mesoinositol, 5.0 nicotinic acid, 5.0 pantothenic acid, 5.0 pyridoxine, 5.0 thiamine HCl, and 0.012 biotin; and 1 mL L−1 oligoelement solution containing (mg L−1) 410.0 MgCl2·6H2O, 19.2 ZnCl2, 0.61 CuCl2·2H2O, 4.45 MnCl2·4H2O, 0.5 CoCl2·6H2O, 17.37 CaCl2, 11.66 FeCl2·4H2O, 0.36 (NH4)6Mo7O24·4H2O, and 3.0 H3BO3. Glucose was prepared in a 700 g L−1 stock. Glucose and salts were sterilized by autoclave (121 °C for 15 min). Oligoelements and vitamins were sterilized by filtration (0.20 μm).
Inoculum and bioreactor conditions
Inocula were generated in Erlenmeyer flasks (500 mL) containing mineral medium incubated in a rotary shaker for 12 h at 250 rpm, 30 °C, and an initial pH of 4.5. Batch fermentations were performed in duplicate in a 3-L Applikon® Biotechnology (JG Delft, The Netherlands) bioreactor containing a final work volume of 1.5 L of mineral medium and sterilized at 121 °C for 15 min. An ADI 1030 Biocontroller (Applikon) was used to control agitation with the following parameters: 500 rpm, pH 4.5, temperature 30 °C, and aeration 1 vvm. To maintain the pH at the setpoint, 1 M NaOH or H3PO4 was used. The fermenter was inoculated with 10% of the total reactor volume, resulting in a population of 20 × 106 cells mL−1. The cell population was measured by a Neubauer chamber.
Cell inhibitor conditions
Sterilized HMF and furfural were added to the medium after inoculation at concentrations of 27.75 mM HMF and 15.61 mM furfural to promote stress conditions without killing the yeasts. This concentration of furan aldehydes was selected based on results obtained in a previous work (Flores-Cosío et al. 2018). Control cultures were not supplemented with furan aldehydes, although they were grown under the same operational and nutritional conditions. Inhibitors were sterilized by filtration (0.20 μm). All reagents and compounds used in this study were provided by Sigma-Aldrich® (St. Louis, MO, USA). Two replicated experiments were carried out for each set of conditions. Specific growth rate (μmax), substrate consumption rate (rs), HMF consumption rate (rsh), and furfural consumption rate (rsf) were calculated in the exponential phase.
Dielectric spectroscopy measurements
A Fogale® nanotech (Nines, France) dielectric spectroscopy probe attached to an EVO® Box was used to detect cellular viability on-line. Frequency spectra were acquired over 6 min; the system measured the capacitance values at 17 different frequencies (from 0.3 to 10 MHz) during a frequency scan. The permittivity, the dielectric increment (△ε), the characteristic frequency (fc), and α (also known as Cole-Cole α) were obtained directly from EVO 400 software (Fogale® nanotech—Ver. 2.5.16). Specific membrane capacitance (Cm), and intracellular conductivity (σIn) were calculated through the equations described in Tibayrenc et al. (2011). For the cell inhibitor conditions, both inhibitors were added after inoculation. Prior to the addition of HMF and furfural, the spectroscopy probe signal was allowed to stabilize with the inoculum for 15 min, and a zero signal was marked in the software.
Determination of glucose, organic acids, and inhibitors
Quantification of glucose was performed by high-performance liquid chromatography (HPLC) using an Agilent (Santa Clara, CA, USA) HPLC (1220 Infinity) equipped with a refractive index monitor. Separations were performed on a Bio-Rad (Hercules, CA, USA) Aminex HPX-87H column (300 mm × 7.8 mm, 9 μm) at 50 °C using 5 mM H2SO4 as the mobile phase (0.5 mL min−1) for 30 min. For organic acids (acetic acid, formic acid, levulinic acid, malic acid, pyruvic acid, and succinic acid), the same column and conditions were used, and compounds were quantified by a wavelength UV detector at 210 nm. Furfural and HMF were analyzed using an Agilent (Santa Clara, CA, USA) Zorbax Eclipse Plus C18 column (4.6 mm × 250 mm, 5 μm) at 25 °C in a mobile phase consisting of (A) 2.5% formic acid in water and (B) 100% methanol. A gradient from 0 to 48% B was performed for 55 min at a flow rate of 0.8 mL min−1. Peaks were quantified using several UV wavelengths (262.5 nm, 275.5 nm, 295.5 nm, and 342.5 nm).
O2 and CO2 exhaust gas analysis
The consumption of O2 and the production of CO2 were measured on-line from the analysis of gases exhausted at the reactor outlet with BlueSens (Herten, Germany) gas analyzers, and the data were obtained with BACV software (Ver. 7.6.2.1). The O2 concentration in the atmosphere was taken as the initial concentration (21%).
Cell size and cell viability
The cell size was measured using a CASY® cell counter Model TT from Roche Innovatis AG (Reutlingen, Germany). Cell suspensions were diluted 1 × 102–1 × 104 in an electrolyte/buffer (CASYton®) before measurement. To analyze the results, the CASY Excel® 2.4 software was used. To validate the cellular viability, colony forming units (CFUs) were counted, and the growth was carried out in YPD medium.
Lipid profile in cell membrane
For lipid profile analysis of the cell membrane, lipid extraction was carried out according to the protocol proposed by Matyash et al. (2008) with some modifications (see supplemental material). Phospholipid quantification was carried out by high-performance thin layer chromatography (HPTLC) (see supplemental material). As standards, phosphatidylethanolamine (PE-P7943), phosphatidylcholine (PC-P3556), and phosphatidylinositol (PI-P0639) were used. All standards were acquired from Sigma-Aldrich® (St. Louis, MO, USA).
Results
Cell dielectric properties and kinetic parameters
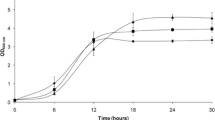
To investigate the dielectric property variations in the yeasts K. marxianus SLP1 and S. cerevisiae ERD during aerobic growth, cell permittivity was measured on-line with the Fogale® (Nines, France) dielectric spectroscopy probe during batch cultures under control and stressed conditions. Figure 1a1, b1 depict the permittivity signal for S. cerevisiae and K. marxianus under control conditions, respectively, where the behavior was similar to that obtained with conventional techniques. The same behavior was observed even in the presence of furan aldehydes (Fig. 1a2, b2). Under stressed conditions, during the lag phase, low permittivity (< 1 pF cm−1) values were determined by the spectroscopy probe. Additionally, dielectric properties such as Cm and σIn could be calculated with the dielectric spectroscopy probe. The Cm was associated with glucose uptake, so in control conditions, at the beginning of the fermentation, the parameter Cm had a value of approximately 12 and 10 (μF cm−2) for the strains ERD and SLP1, respectively (Fig. 1a1, b1). Cm decreased in magnitude as the fermentation carried on, apparently at a similar rate as glucose decreased (Fig. 2); the transfer or assimilation of nutrients indicated that the membrane was active. Finally, the parameter Cm remained constant when glucose was almost consumed (Fig. 1a1, b1). However, the parameter Cm was drastically affected by furan aldehydes in both yeasts, showing a decrease of 70% in magnitude compared with that under control conditions at the beginning of the kinetics (Fig. 1a2, b2). However, the value of Cm slowly increased when furan aldehydes begin to be metabolized, reaching a maximum value of 4.4 μF cm−2 for SLP1 at 4 h, while the value for ERD only reached 2.1 μF cm−2, half of the value for SLP1.
In the control culture, at time 0 h, both strains had an initial σIn of 2 mS cm−1, which suddenly increased at 2 h to a maximum of 15 mS cm−1 and 16.5 mS cm−1 for ERD and SLP1, respectively. Afterwards, the magnitude of σIn decreased with time and remained constant until the stationary phase, with values of 7 mS cm−1 for ERD and 5.5 mS cm−1 for SLP1 (Fig. 1a1, b1). During stress, inhibitors had a direct effect on σIn; in the beginning, both strains presented low values for σIn. This result is in concordance with the cell population profile during the lag phase; then, σIn increases in magnitude when furfural is totally consumed and until total glucose depletion, to then decrease for the rest of the kinetics experiment (Fig. 1a2, b2). For SLP1, the σIn after 24 h showed a null signal (Fig. 1b2). As mentioned earlier, both yeast strains decreased in Cm and σIn in the presence of inhibitors compared with the control conditions; however, while ERD did not present changes in Cm and σIn in the lag phase, SLP1 showed an increase in Cm.
To validate the physiological changes detected by dielectric spectroscopy, the physiological and kinetics parameters (cell population, specific growth rate, substrate consumption rate, HMF consumption rate, furfural consumption rate, organic acid production, cell size dispersion, and respiratory capacity) were determined in aerobic batch fermentations under control and stressed conditions. Figure 2a1, b1 depict the kinetic behavior of the cell population and glucose consumption of the strains ERD and SLP1 under control conditions. Both strains began to grow almost from the inoculation time, and no lag phase was observed. ERD and SLP1 uptook glucose from the initial fermentation time and consumed it in approximately 7 h, matching the time when the biomass reached the maximum growth. Figure 2a2, b2 show the behavior of the same strains and parameters when 25.75 mM HMF and 15.61 mM furfural were added into the bioreactor. At first, the addition of furan aldehydes inhibited cell growth, prolonging the lag phase in both strains compared with that in control fermentations; however, the yeasts were able to reduce both inhibitors, and once inhibitors were reduced, the cellular growth was restored. One of the main effects observed was that the cell growth decreased 30% and 11% for ERD and SLP1, respectively (Fig. 2a2, b2). Glucose consumption was hampered by the effect of the inhibitors, which was more evident for K. marxianus; however, this strain showed a higher inhibitor reduction rate and, consequently, a shorter lag phase than S. cerevisiae (Table 1). The cell population and CFUs (Supplemental Fig. S1) in both yeasts behaved similarly to the permittivity signal under control and stressed conditions.
Organic acid production, O2 uptake, and CO2 evolution
Under control conditions, O2 was consumed from the beginning of fermentation, reaching its minimum concentration at 8 h for both K. marxianus SLP1 and S. cerevisiae ERD yeasts, although the O2 consumption was small for SLP1. The same trend was found for the CO2 production; ERD yeast produced a more significant percentage than SLP1 (Fig. 3a1, b1). In the presence of inhibitors, yeast respiration was limited since O2 consumption was not detected until furfural was reduced (Fig. 3a2, b2). On the other hand, the maximum CO2 production was observed at the exponential phase and declined later, indicating that most of the glucose in the medium was depleted.
For organic acids, in control cultures, ERD and SLP1 produced succinic acid from the exponential phase (approximately 0.5 g L−1), but it was metabolized during the stationary phase; only SLP1 was able to completely degrade succinic acid after 12 h (Fig. 4a1, b1). Small concentrations of malic acid that were quickly consumed were also found (data not shown). The yeast ERD doubled the succinic acid concentration under the stress scenario and after furfural reduction compared with the control conditions, reaching a maximum value of 0.88 g L−1 at 24 h. On the other hand, the succinic acid concentration produced by SLP1 was 0.31 g L−1 at 24 h, one-third of that produced by ERD. Acetic acid was only generated in stressed cultures after the consumption of furfural (Fig. 4a2, b2). The malic acid concentration detected during the growth kinetics was lower than the succinic acid concentrations, and the maximum malic acid concentration was 6.64 mg L−1 for ERD and 3.81 mg L−1 for SLP1 (data not shown).
Cell size dispersion
The cell diameter variation was determined for K. marxianus SLP1 and S. cerevisiae ERD. Under control conditions, the cell diameter for both strains increased during the exponential phase, and when the culture reached the stationary phase, the diameter gradually decreased until it remained constant; at the stationary phase, the average mean diameter was 4.41 μm for ERD and 3.94 μm for SLP1 (Supplemental Fig. S1). Under inhibitory conditions, both strains maintained their cell size during the lag phase; subsequently, when the inhibitors were reduced, an increase in cell diameter was observed due to the restoration of cell growth. The mean average diameter for both strains during the stationary phase was higher in the presence of furan aldehydes than under the control conditions, presenting values of 4.94 μm and 3.99 μm for ERD and SLP1, respectively (Supplemental Fig. S1). Additionally, during the lag phase caused by furan aldehydes, a greater dispersion of cell size (with smaller subpopulation) was found for both strains (Supplemental Fig. S2).
Evaluation of membrane phospholipids
Under control conditions, no membrane phospholipids were detected for S. cerevisiae ERD, while for K. marxianus SLP1, only PE was detected (Table 2). ERD and SLP1 showed changes in the membrane phospholipid profile caused by furan aldehydes. Under inhibitory condition, only PE was observed and it was the condition that presented the highest concentrations of this phospholipid for both strains (Table 2).
Discussion
The efficiency of bioprocesses depends on the level of understanding and controlling the physiological state of the cell population. In the specific case of the production of second-generation ethanol, the productivity is strongly affected by changes in the cellular state caused by inhibitory compounds such as HMF and furfural. In this study, at the beginning of the cultures, the cell growth was vulnerable to the inhibitors, which increased the interval of the lag phase compared with that of control cultures (Fig. 1). Similar results have already been reported by other authors (Liu et al. 2004; Ma and Liu 2010; Sehnem et al. 2013). Kinetic parameters were also affected in both strains by furan aldehydes; however, K. marxianus SLP1 showed a better capacity to biotransform both inhibitors; this response has already been reported in previous work (Flores-Cosío et al. 2018), and it is suggested that the strain SLP1 may be better adapted to stressful environments because it was isolated from the fermentation process of mezcal, which is carried out under hostile environmental conditions such as high concentrations of initial sugars, high or low temperatures, and the presence of growth inhibitors (saponins). Additionally, under inhibitory conditions, some differences regarding the organic acid production between S. cerevisiae ERD and K. marxianus SLP1 were found; for example, ERD produced twice the succinic acid concentration that SLP1 did. The production of succinic and acetic acids contributed to maintaining the redox balance of the cell; however, only acetic acid has been associated with the reduction of furan aldehydes (Flores-Cosío et al. 2018).
Dielectric properties. (a) S. cerevisiae ERD. (b) K. marxianus SLP1. (1) control, (2) 27.75 mM HMF and 15.61 mM furfural; symbols denote permittivity ( ), specific membrane capacitance (Cm, ○), intracellular conductivity (σIn,
), specific membrane capacitance (Cm, ○), intracellular conductivity (σIn,  ), glucose depletion (⎯⎯⎯), HMF depletion (— —), and furfural depletion (- -)
), glucose depletion (⎯⎯⎯), HMF depletion (— —), and furfural depletion (- -)
The inhibition and restoration of growth after the reduction of inhibitors were clearly detected and observed through the permittivity. Diverse studies have reported changes in the dielectric properties of cells caused by several factors (Patel and Markx 2008; Tibayrenc et al. 2011). Patel et al. (2008) studied cell permittivity variations in S. cerevisiae exposed to different lethal environmental stresses, and they concluded that large changes in capacitance (due to cell death) directly affect the permeability of the membrane and, consequently, the conductivity of the membrane and the internal conductivity. When the cell membrane is compromised, the membrane capacitance decreases, as observed in this research, furan aldehydes decrease permittivity, suggesting damage to the cell membrane. Therefore, the effect of furan aldehydes on membrane phospholipid composition was analyzed.
Phospholipids of the cell membrane are an important component for the fluidity and permeability of the membrane; they are sensitive to changes in the chemical and physical properties of the environment. The changes found in this study on membrane phospholipids showed an increase in the levels of PC and PE caused by furan aldehydes; the entry of these compounds into the cell alters the membrane composition and consequently also inhibits growth. PC is very important in the protection of cells and can provide higher membrane permeability (Xia and Yuan 2009). On the other hand, PE plays an important role in membrane fusion/fission events (Siegel and Epand 1997). An increase in PE has been observed, causing a greater curvature in the membrane that facilitates its fluidity (Van Der Rest et al. 1995). The increase in PE and PC levels by furan aldehydes suggests greater membrane fluidity, making the insertion of the inhibitors into membrane bilayers easier. In a previous work, it has been reported that the addition of inhibitors changes the composition of cell membrane phospholipids and disturbs the fluidity of the membrane (Xia and Yuan 2009), which is consistent with the one observed in this research.
A second dielectric property that presented changes with furan aldehydes was Cm. Cm is the membrane capacitance per unit area and refers to the ability of the plasma membrane to accumulate electrical charge. It has been shown that variations in membrane fluidity can change the Cm (Ferris et al. 1990); however, the changes observed in this research by furan aldehydes may be influenced by other important cellular factors. Previous researches have indicated that changes in Cm are largely associated with cell membrane morphology such as microvilli, ruffles, and folds (Dopp et al. 2000; Zimmermann et al. 2008; Wang et al. 2012) and can be interpreted as a measure of wrinkles (level of folding of the cellular membrane); due to these characteristics, Cm may reflect the complexity of cell surface morphology (Patel and Markx 2008; Patel et al. 2008). Other studies have mentioned that apoptosis (Pethig and Talary 2007), expression of plasma proteins (Zimmermann et al. 2008), and viral infections (Ansorge et al. 2007) can influence the values of Cm due to processes directly related to the cell membrane morphology. Because furan aldehydes disturb and affect the entire cell, it is suggested that the changes in Cm observed in this investigation are mainly attributed to membrane damage and metabolic changes caused by HMF and furfural.
The variations in intracellular conductivity (σIn) could indicate an exchange of material between the cytoplasm and the medium; however, no changes were observed in the conductivity of the medium until several hours after the steady state was reached, indicating that this variation was not caused by ionic leakage. In a previous work performed by Tibayrenc et al. (2011), σIn was used as a stress-sensitive cell state indicator. The increase in σIn during the exponential phase suggests that the cells are active. The decrease in σIn agrees with the total consumption of the carbon source and is reaching the stationary phase, which occurs when the nutrients of the medium have been exhausted and there is no cell division. Under stressful conditions, σIn was significantly affected in ERD and SLP1 yeasts. As previously observed during the adaptation phase, the cells are committed to reducing the furan aldehydes in the medium, so there is no growth, and the σIn is also consequently affected. This behavior was reflected in low values of σIn, which indicate low cellular activity and possible cellular damage caused by the furan aldehydes. It has been reported that furfural causes an accumulation of reactive oxygen species (ROS) and generates cellular damage, including damage to the mitochondria, the vacuoles, and the actin cytoskeleton (Allen et al. 2010). In other investigations, induced morphological changes, late apoptotic/necrotic cells, and thermal stress caused a decrease in σIn (Labeed et al. 2006; Ansorge et al. 2010; Tibayrenc et al. 2011). Tibayrenc et al. (2011) mentioned that other possible causes that can lead to a variation in the σIn are due to the cytoplasmic electrical properties and the production of certain components that are capable of affecting the viscosity of the cytosol and the mobility of the ions, such as the accumulation of trehalose and glycogen; however, more research is required to correlate the σIn variations with physiological events.
In addition to the changes in the dielectric properties, changes in the cell size of the yeasts were also observed. Size dispersion is an important characteristic of the adaptation process that influences almost all aspects of cell physiology, resulting in its role as a valuable indicator of physiological state. It can be an important selective trait for survival under constantly changing environments and nutrient limitation. The maintenance of cell size requires homeostasis between macromolecule synthesis and degradation and, in multicellular systems, is closely linked to nutrient and growth factor availability. Yeast cell age, progression through the cell cycle, and even process conditions are some of the effectors that can cause a change in yeast cell size. Various authors have shown the importance of cell size measurements in studying the response of yeast cells under different types of stress, such as heat stress (Tibayrenc et al. 2010), osmotic stress (Kiehl et al. 2011), oxidative stress (Belo et al. 2005), and ethanol stress (Ma et al. 2013). In this study, we observed that under stressful conditions such as a lack of carbon source or a furan aldehydes presence, size dispersion increased, and the average cellular size decreased. In the stationary phase, various subpopulations were found under control conditions, which we attributed to the lack of nutrients. This behavior was also observed in the presence of inhibitors. In the presence of a rich source of nutrients, the cells maintained a high level of macromolecular synthesis to promote growth and increase size. Conversely, limitations in the nutritional environment restrain protein synthesis to conserve crucial metabolites and consequently reduce size. Under conditions of poor nutrition, cells can adapt to maintain growth and continue to survive, although at a smaller size. On the other hand, the increase in size dispersion observed during the lag phase caused by the furan aldehydes could be due to the damage caused by the inhibitors. For both strains during the lag phase, the subpopulations with a smaller cell size increased approximately 20% in abundance in comparison with their abundance under control conditions (Supplemental Fig. S1). Tibayrenc et al. (2011) found an increasing subpopulation of significantly smaller cells when cells were submitted to thermal stress and observed the same results after adding acetate (5 g L−1) or furfural (52 mM).
Figure 4 shows all the physiological changes that furan aldehydes cause to yeasts and their correlation with dielectric properties, and Supplemental Table S1 shows the different physiological states that were detected using the dielectric properties. Three physiological states were observed under the stress with furan aldehydes; we defined them as the adaptation, replicating, and nonreplicating states. During the adaptation state, there are low and stable signals of permittivity, Cm, and σIn; the cells in this state show growth, and substrate/oxygen consumption stopped; additionally, there is a decrease in cell size and low viability. In the replicating state, cell growth and substrate/oxygen consumption are restored while cell viability increases; the permittivity and σIn increase rapidly, while the Cm decreases. Finally, in the nonreplicating state, the permittivity stabilizes and reaches its maximum values, Cm is also stabilized but has a very low signal, and σIn reaches its maximum values; however, unlike the permittivity, the σIn decreases rapidly and subsequently stabilizes.
Cells under stress conditions undergo changes in their physiology, and the cells stressed with furan aldehydes are able to grow after a long period of adaptation. However, the yeasts showed altered metabolism (reduced metabolic and growth rates, production of metabolites) that produces deviations in the normal processes of the cell. This research showed that furan aldehydes cause considerable changes in the dielectric properties of the cell and that these changes have a direct correspondence with the physiological state of the yeast, which was validated by conventional techniques. The present study, using two biotechnologically interesting yeasts, S. cerevisiae ERD and K. marxianus SLP1, contributes to the knowledge of the correlation of the physiological state of the yeasts under control and stress conditions and on-line monitoring by dielectric spectroscopy. The results presented here can be useful in the monitoring and control of bioprocesses to manage the environmental conditions to know, for instance, the adaptive responses of yeasts to stress, to follow yeast growth and physiological state on-line, and even to adjust the medium composition to increase metabolite productivities.
References
Allen SA, Clark W, McCaffery JM, Cai Z, Lanctot A, Slininger PJ, Liu ZL, Gorsich SW (2010) Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol Biofuels 3:2
Ansorge S, Esteban G, Schmid G (2007) On-line monitoring of infected Sf-9 insect cell cultures by scanning permittivity measurements and comparison with off-line biovolume measurements. Cytotechnology 55:115–124. https://doi.org/10.1007/s10616-007-9093-0
Ansorge S, Esteban G, Schmid G (2010) Multifrequency permittivity measurements enable on-line monitoring of changes in intracellular conductivity due to nutrient limitations during batch cultivations of CHO cells. Biotechnol Prog 26:272–283. https://doi.org/10.1002/btpr.347
Belo I, Pinheiro R, Mota M (2005) Morphological and physiological changes in Saccharomyces cerevisiae by oxidative stress from hyperbaric air. J Biotechnol 115:397–404. https://doi.org/10.1016/j.jbiotec.2004.09.010
Carvell JP, Dowd JE (2006) On-line measurements and control of viable cell density in cell culture manufacturing processes using radio-frequency impedance. Cytotechnology 50:35–48
Dopp E, Jonas L, Nebe B, Budde A, Knippel E (2000) Dielectric changes in membrane properties and cell interiors of human mesothelial cells in vitro after crocidolite asbestos exposure. Environ Health Perspect 108:153–158
Farías-Álvarez LJ, Gschaedler-Mathis A, Sánchez-Ortiz AF, Femat R, Cervantes-Martínez J, Arellano-Plaza M, Zamora-Pedraza C, Amillastre E, Ghommidh C, Herrera-López EJ (2018) Xanthophyllomyces dendrorhous physiological stages determination using combined measurements from dielectric and Raman spectroscopies, a cell counter system and fluorescence flow cytometry. Biochem Eng J 136:1–8. https://doi.org/10.1016/j.bej.2018.04.016
Ferris LE, Davey CL, Kell DB (1990) Evidence from its temperature dependence that the β-dielectric dispersion of cell suspensions is not due solely to the charging of a static membrane capacitance. Eur Biophys J 18:267–276. https://doi.org/10.1007/BF00188039
Flores-Cosío G, Arellano-Plaza M, Gschaedler-Mathis A, Amaya-Delgado L (2018) Physiological response to furan derivatives stress by Kluyveromyces marxianus SLP1 in ethanol production. Rev Mex Ing Quim 17:189–202
Gibson BR, Lawrence SJ, Leclaire JPR, Powell CD, Smart KA (2007) Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol Rev 31:535–569. https://doi.org/10.1111/j.1574-6976.2007.00076.x
Gupta A, Verma JP (2015) Sustainable bio-ethanol production from agro-residues: a review. Renew Sust Energ Rev 41:550–567. https://doi.org/10.1016/j.rser.2014.08.032
Kiehl TR, Shen D, Khattak SF, Jian Li Z, Sharfstein ST (2011) Observations of cell size dynamics under osmotic stress. Cytometryt A 79:560–569. https://doi.org/10.1002/cyto.a.21076
Labeed FH, Coley HM, Hughes MP (2006) Differences in the biophysical properties of membrane and cytoplasm of apoptotic cells revealed using dielectrophoresis. Biochim Biophys Acta 1760:922–929. https://doi.org/10.1016/j.bbagen.2006.01.018
Liu ZL (2011) Molecular mechanisms of yeast tolerance and in situ detoxification of lignocellulose hydrolysates. Appl Microbiol Biotechnol 90:809–825. https://doi.org/10.1007/s00253-011-3167-9
Liu ZL, Slininger PJ, Dien BS, Berhow MA, Kurtzman CP, Gorsich SW (2004) Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5-bis-hydroxymethylfuran. J Ind Microbiol Biotechnol 31:345–352. https://doi.org/10.1007/s10295-004-0148-3
Liu ZL, Moon J, Andersh BJ, Slininger PJ, Weber S (2008) Multiple gene-mediated NAD(P)H-dependent aldehyde reduction is a mechanism of in situ detoxification of furfural and 5-hydroxymethylfurfural by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 81:743–753. https://doi.org/10.1007/s00253-008-1702-0
Ma M, Liu ZL (2010) Comparative transcriptome profiling analyses during the lag phase uncover YAP1, PDR1, PDR3, RPN4, and HSF1 as key regulatory genes in genomic adaptation to the lignocellulose derived inhibitor HMF for Saccharomyces cerevisiae. BMC Genomics 11:660. https://doi.org/10.1186/1471-2164-11-660
Ma M, Han P, Zhang R, Li H (2013) Ultrastructural changes of Saccharomyces cerevisiae in response to ethanol stress. Can J Microbiol 59:589–597. https://doi.org/10.1139/cjm-2012-0745
Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D (2008) Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res 49:1137–1146. https://doi.org/10.1194/jlr.D700041-JLR200
Opel CF, Li J, Amanullah A (2010) Quantitative modeling of viable cell density, cell size, intracellular conductivity, and membrane capacitance in batch and fed-batch CHO processes using dielectric spectroscopy. Biotechnol Prog 26:1187–1199. https://doi.org/10.1002/btpr.425
Patel P, Markx GH (2008) Dielectric measurement of cell death. Enzyme Microb Tech 43:463–470. https://doi.org/10.1016/j.enzmictec.2008.09.005
Patel PM, Bhat A, Markx GH (2008) A comparative study of cell death using electrical capacitance measurements and dielectrophoresis. Enzyme Microb Tech 43:523–530. https://doi.org/10.1016/j.enzmictec.2008.09.006
Pethig R, Talary M (2007) Dielectrophoretic detection of membrane morphology changes in Jurkat T-cells undergoing etoposide-induced apoptosis. IET Nanobiotechnol 1:2–9
Sandoval-Nuñez D, Arellano-Plaza M, Gschaedler A, Arrizon J, Amaya-Delgado L (2017) A comparative study of lignocellulosic ethanol productivities by Kluyveromyces marxianus and Saccharomyces cerevisiae. Clean Technol Envir Policy 20:1–9. https://doi.org/10.1007/s10098-017-1470-6
Sehnem NT, da Silva MA, Leite FCB, de Barros PW, de Morais MA, Ayub MAZ (2013) 5-Hydroxymethylfurfural induces ADH7 and ARI1 expression in tolerant industrial Saccharomyces cerevisiae strain P6H9 during bioethanol production. Bioresour Technol 133:190–196. https://doi.org/10.1016/j.biortech.2013.01.063
Siegel DP, Epand RM (1997) The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: implications for membrane fusion mechanisms. Biophys J 73:3089–3111. https://doi.org/10.1016/S0006-3495(97)78336-X
Tibayrenc P, Preziosi-Belloy L, Roger JM, Ghommidh C (2010) Assessing yeast viability from cell size measurements? J Biotechnol 149:74–80. https://doi.org/10.1016/j.jbiotec.2010.06.019
Tibayrenc P, Preziosi-Belloy L, Ghommidh C (2011) On-line monitoring of dielectrical properties of yeast cells during a stress-model alcoholic fermentation. Process Biochem 46:193–201. https://doi.org/10.1016/j.procbio.2010.08.007
Van Der Rest ME, Kamminga AH, Nakano A, Anraku Y, Poolman B, Konings WN (1995) The plasma membrane of Saccharomyces cerevisiae: structure, function, and biogenesis. Microbiol Rev 59:304–322
Wang X, Becker FF, Gascoyne PR (2012) Membrane dielectric changes indicate induced apoptosis in HL-60 cells more sensitively than surface phosphatidylserine expression or DNA fragmentation. Biochim Biophys Acta 1564:412–420. https://doi.org/10.1158/0008-5472.CAN-10-4002.BONE
Xia JM, Yuan YJ (2009) Comparative lipidomics of four strains of Saccharomyces cerevisiae reveals different responses to furfural, phenol, and acetic acid. J Agric Food Chem 57:99–108. https://doi.org/10.1021/jf802720t
Zimmermann D, Zhou A, Kiesel M, Feldbauer K, Terpitz U, Haase W, Schneider-Hohendorf T, Bamberg E, Sukhorukov VL (2008) Effects on capacitance by overexpression of membrane proteins. Biochem Biophys Res Commun 369:1022–1026. https://doi.org/10.1016/j.bbrc.2008.02.153
Funding
This study was funded by the “Fondo de Sustentabilidad Energética” Projects 245750 and 249564 CONACYT-SENER. Flores-Cosío Guillermo received a grant from CONACYT, México.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 592 kb).
Rights and permissions
About this article
Cite this article
Flores-Cosío, G., Herrera-López, E.J., Arellano-Plaza, M. et al. Dielectric property measurements as a method to determine the physiological state of Kluyveromyces marxianus and Saccharomyces cerevisiae stressed with furan aldehydes. Appl Microbiol Biotechnol 103, 9633–9642 (2019). https://doi.org/10.1007/s00253-019-10152-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10152-2





 ), glucose depletion (⎯⎯⎯), HMF depletion (— —), and furfural depletion (- -)
), glucose depletion (⎯⎯⎯), HMF depletion (— —), and furfural depletion (- -)
 ), O2 (⊕), acetic acid (△), succinic acid (
), O2 (⊕), acetic acid (△), succinic acid ( ), glucose depletion (⎯⎯⎯), HMF depletion (— —), and furfural depletion (- -)
), glucose depletion (⎯⎯⎯), HMF depletion (— —), and furfural depletion (- -)