Abstract
Ulcerative colitis (UC) is a chronic relapsing disease. Treatment of UC would benefit from specific targeting of therapeutics to the intestine. Previous studies have demonstrated that bovine lactoferricin and lactoferrampin have bactericidal, anti-inflammatory, and immunomodulatory effects. Here, we investigated whether oral administration of a bovine lactoferricin–lactoferrampin (LFCA)-encoding Lactococcus lactis (LL-LFCA) strain could alleviate experimental colitis. LFCA derived from LL-LFCA inhibited the growth of Escherichia coli and Staphylococcus aureus in vitro. In mice, administration of LL-LFCA decreased the disease activity index and attenuated dextran sulfate sodium (DSS)–induced body weight loss and colon shortening. LL-LFCA treatment also ameliorated DSS-induced colon damage, inhibited inflammatory cell infiltration, significantly decreased myeloperoxidase activity, and ameliorated DSS-induced disruption of intestinal permeability and tight junctions. In addition, 16S rDNA sequencing showed that LL-LFCA reversed DSS-induced gut dysbiosis. The production of proinflammatory mediators in serum and the colon was also reduced by administration of LL-LFCA. In vitro, LFCA derived from LL-LFCA decreased the messenger RNA expression of proinflammatory factors. The underlying mechanisms may involve inhibition of the nuclear factor kappa B (NF-κB) pathway. The results demonstrate that LL-LFCA ameliorates DSS-induced intestinal injury in mice, suggesting that LL-LFCA might be an effective drug for the treatment of inflammatory bowel diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is a group of chronic conditions of the colon and small intestine that includes Crohn’s disease and ulcerative colitis (UC) (Liu et al. 2016a). UC is characterized by acute abdominal pain, weight loss, diarrhea, and even hematochezia, which can severely reduce the quality of life (Gao et al. 2017). Although some drugs have been tested in preclinical and clinical models of IBD, their therapeutic potential is limited and their clinical application remains unrealized, as there is a significant cohort of patients with refractory disease as well as others who cannot tolerate the treatments or the severity of the side effects (Shigemori et al. 2015). Therefore, therapeutic options that are safer and more effective for the prevention and treatment of UC are needed.
Although the precise etiology of the disease is unknown, accumulating evidence indicates that inappropriate and sustained mucosal inflammatory responses play a key role in UC (Sun et al. 2015a). The overproduction of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, IL-1β, and IL-12, in the intestine prolongs the inflammatory cascade and may impair mucosal barrier function and increase intestinal permeability (Carvajal et al. 2017; Price et al. 2010). A disproportionately large number of first-degree relatives of patients with IBD have increased intestinal permeability (Kang et al. 2017). This suggests that barrier dysfunction may be an early defect in IBD. In addition, previous studies have demonstrated decreased expression of junction complex proteins in the intestinal mucosa of patients with IBD (Vancamelbeke and Vermeire 2017).
The major function of the intestinal epithelial barrier, which is composed of intestinal epithelial cells and tight junctions (TJs), is to defend against the passage of toxins and pathogenic organisms present in the intestinal lumen (Chon et al. 2010). Once the barrier is disrupted, toxic luminal antigens may pass into the lamina propria, and the resulting innate immune response aggravates the release of multiple cytokines, which appear to initiate and accelerate inflammation in UC (Deng et al. 2018a). Therefore, maintaining the integrity of the structure and function of the intestinal barrier should alleviate the development of inflammation or accelerate the resolution of inflammation.
Pioneer’s studies have demonstrated the successful use of food-grade lactic acid bacteria (LAB) for the oral delivery of anti-inflammatory molecules to inflamed intestines (Martin et al. 2014; Wang et al. 2016). This approach is based on the local synthesis and delivery of therapeutic molecules by viable recombinant LAB (recLAB) in situ. Delivery of antimicrobial peptides (AMPs) that protect the host by suppressing the propagation of a wide range of harmful bacteria can prevent inflammation in mouse models of colitis (Wong et al. 2017).
AMPs are cationic amphiphilic molecules with antimicrobial activity. They include defensins, cathelicidins, antimicrobial lactoferrin (LF) peptides, and other molecules (Patel and Akhtar 2017). Bovine lactoferricin (Lfcin B) and lactoferrampin (Lfampin) are two antimicrobial peptides derived from the innate immunity factor LF (Sinha et al. 2013). Lfcin B is a 25-amino acid peptide (LF amino acids 17–41) that has more potent activity against bacteria than LF (Gifford et al. 2005). Lfampin comprises residues 268–284 in the N1 domain of LF and also shows substantially higher activity than LF (Van der Kraan et al. 2004). Recent reports have suggested that a chimera of Lfcin B and Lfampin has stronger activity and a broader antimicrobial spectrum than their constituent peptides or the combination of these peptides (Bolscher et al. 2012; Ruangcharoen et al. 2017). In addition to its antimicrobial activity, the chimera has also been reported to be involved in maintaining intestinal homeostasis (Tang et al. 2009; Tang et al, 2012). Here, we joined Lfcin B and Lfampin with a flexible linker (GGGS)2. The resulting peptide was designated lactoferricin–lactoferrampin (LFCA).
The use of antibiotic resistance markers in recLAB can cause safety problems (O’Connor et al. 2007; Toomey et al. 2010). Lactococcus lactis AMJ1543 is an alanine racemase [alr] gene-deficient L. lactis that is derived from L. lactis MG1363. L. lactis is influential in the alleviation of colitis (Jones & Foxx-Orenstein 2007). Moreover, the use of L. lactis vector would allow for a more cost-effective and direct delivery of therapeutics to the site of inflammation. L. lactis that is genetically modified to secrete a heterologous protein (such as IL-27, IL-10, and transforming growth factor β1) is perhaps the most promising application of this strategy and has been demonstrated in several studies to be effective in treating gastrointestinal inflammation (Hanson et al. 2014; Rebeca et al. 2014; Bermudez-Humaran et al. 2015). Therefore, in this study, we used the L. lactis Δalr strain AMJ1543 (alr is a food-grade complementary auxotrophic screening marker) as a mucosal delivery vehicle and developed a recombinant strain of L. lactis AMJ1543 secreting biologically active LFCA (LL-LFCA).
We explored whether direct delivery of LFCA using L. lactis AMJ1543 could ameliorate dextran sulfate sodium (DSS)–induced experimental colitis in mice and if it could be a useful strategy for the treatment of IBD.
Materials and methods
Reagents and cell culture
Reagent-grade DSS salt (MW 36–50 kDa, Cat. no. 160110) was purchased from MP Biomedicals (USA). Myeloperoxidase (MPO) and diamine oxidase (DAO) activity assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (China). Limulus amebocyte lysate reagent was purchased from GenScript (LAL test; USA). 4′,6-Diamidino-2-phenylindole (DAPI) was purchased from Invitrogen (USA). All restriction enzymes and T4 DNA ligase were purchased from New England Biolabs (USA). Bovine serum albumin (BSA) was purchased from Roche Diagnosis, Ltd. (China). Lipopolysaccharide (LPS) from Escherichia coli serotype O55:B5 was purchased from Sigma-Aldrich (USA). M-MLV Reverse Transcriptase was purchased from Promega (USA). Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-6, IL-1β, and IL-12 were purchased from eBioscience (USA). Primary monoclonal antibodies against IκB, p65, p-IκB, p-p65, lamin A, and β-actin were purchased from Cell Signaling Technology, Inc. (USA); antibodies against zonula occludens-1 (ZO-1) were obtained from Proteintech (USA); and claudin-2 and E-cadherin antibodies were purchased from Abcam (USA). All other chemicals used in this study were reagent grade and were purchased from Sigma. Synthetic LFCA (sLFCA) was synthesized (with 90% purity) by Genewiz Biological Technology Company, Ltd. (China).
RAW264.7 and Caco-2 cells were obtained from the Cell Bank of the Chinese Academic of Sciences (China) and were cultured in Dulbecco’s modified Eagle’s medium (Gibco, USA) supplemented with 10% fetal bovine serum (Sijiqing, China), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a 95% humidified atmosphere containing 5% CO2.
Bacterial strains and growth conditions
L. lactis AMJ1543 is an alanine racemase (alr)-deficient bacterium derived from L. lactis MG1363, which was kindly provided by NIZO Food Research (Ede, The Netherlands). The bacteria were cultured in M17 medium containing 0.5% glucose and 200 μg/mL d-alanine overnight at 30 °C without shaking. The genetically modified strain of L. lactis AMJ1543, LL-LFCA, was grown in M17 supplemented with 0.5% glucose. Escherichia coli CVCC10141 and Staphylococcus aureus CVCC25923 were cultured in Luria–Bertani (LB) broth for 12 h to reach saturation (≥ 1.0 × 108 colony forming units (CFU) per mL).
Construction of LL-LFCA
The recombinant plasmid pAMJ1653-LFCA was constructed as follows: LFCA consists of Lfcin B (GenBank accession no. AY569034.1), and Lfampin joined with a flexible linker (GGGS)2 (supplement Fig. S1a). The DNA encoding LFCA was synthesized by Genewiz Biological Technology Company, Ltd. The plasmid pMD-LFCA was digested with BspQI and BglII restriction enzymes to release a fragment encoding LFCA with compatible ends. The fragment was ligated into pAMJ1653, which carries the alanine racemase gene (kindly provided by NIZO Food Research), at the BspQI and BglII sites using T4 DNA ligase (supplement Fig. S1b). The pAMJ1653-LFCA was then electrotransformed into competent L. lactis AMJ1543 cells, which were plated on M17 plates without d-alanine for positive selection as previously described (Liu et al. 2012), to generate pAMJ1653-LFCA/LL AMJ1543 (LL-LFCA).
Characterization of LFCA expression in L. lactis AMJ1543
Recombinant LFCA-expressing L. lactis AMJ1543 (LL-LFCA) and L. lactis AMJ1543 transformed with an empty plasmid (LL-control) were cultured in M17 medium containing 0.5% glucose for 24 h. The cultures were centrifuged at 10,000×g for 5 min, and the culture supernatant and cell pellet were collected. The supernatant was mixed with 100 μL of 100% trichloroacetic acid (TCA) and incubated for 10 min on ice to precipitate the proteins, which were used to prepare a 20-fold concentration as previously described (Nandakumar et al. 2003). The supernatant protein concentrate and cell pellet were analyzed by 18% tricine–SDS-PAGE and western blotting using anti-bovine lactoferrin polyclonal antibodies (prepared by our laboratory and diluted 1:500), as the primary antibody, and horseradish peroxidase (HRP)–conjugated goat anti-mouse IgG (1:4000), as the secondary antibody. The concentration of secreted LFCA was quantitated as previously described (Zhou et al. 2018). The cell lysate and culture supernatant of L. lactis AMJ1543 transformed with the empty plasmid (LL-control) were included as controls.
E. coli and S. aureus growth inhibition assay
The killing activity of the lysate of LL-LFCA was determined by a colony culture assay as described previously (Ruangcharoen et al. 2017). Briefly, E. coli CVCC10141 and S. aureus CVCC25923 (1 × 106 CFU) were grown in the presence or absence of bacterial lysate from LL-LFCA (10.0 μg of LFCA protein) in LB medium at 37 °C for 6 h, 12 h, 18 h, and 24 h; bacterial lysate from LL-control was used as a negative control. The incubated mixture was serially diluted in physiological saline and plated on LB agar. Colonies were counted after incubation at 37 °C for 24 h. The percent killing or inhibition was calculated using the formula [1 − (CFU sample / CFU control)] × 100%. Each assay was performed on three separate occasions, each with triplicate determinations. After treatment with LFCA protein for 24 h, the E. coli CVCC10141 and S. aureus CVCC25923 cell pellets were treated as previously described (Flores-Villasenor et al. 2010) and observed using transmission electron microscopy with a model JEM-1230 microscope (JEOL, Japan) operated at 100 kV. sLFCA was used as a positive control.
Animals and experimental design
The study was approved by the Animal Care and Use Committee of Northeast Agricultural University and was performed in accordance with institutional rules. Five-week-old female C57BL/6 mice (weight, 20–25 g) were purchased from Changchun Yisi Animal Research Institute (China). The mice were bred and maintained under specific pathogen-free conditions for a minimum of 1 week before the experiment. The mice were randomly assigned to the control (CON), DSS-treated, DSS+LL-LFCA–treated, and DSS+LL-control–treated groups. Acute colitis was induced by the oral administration of 3.5% DSS (w/v) in fresh tap water ad libitum for 7 days (n = 12, per group) as described previously (Wirtz et al. 2007). No major differences in water consumption were detected between groups. LL-LFCA and LL-control were administered intragastrically on days 1–7 at a concentration of 5 × 109 CFU in 200 μL of phosphate-buffered saline (PBS).
L. lactis localization
LL-LFCA was stained with carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) as described previously (Silva RT et al. 2014). The CFDA-SE–stained LL-LFCA was administered to healthy C57BL/6 mice by oral gavage. Intestinal mucous was collected from the jejunum, ileum, and colon at 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, and 24 h after treatment. The intestinal mucous was diluted with PBS, and the fluorescence signal was assessed by flow cytometry using a FACSCalibur device (BD Biosciences, USA).
Immunohistochemistry
The expression of LFCA of the colonic tissues was assessed as previously described (Li et al. 2018). In brief, 3-μm sections of paraffin-embedded tissues were cut and baked in the oven followed by dewaxing deparaffinized to unmask antigens. Sections were blocked with BSA for 1 h. For LFCA staining, tissue sections were first incubated with anti-bovine LF polyclonal antibodies (prepared by our laboratory and diluted 1:50) overnight at 4 °C. HRP-labeled anti-mouse secondary antibody was then used for incubating the washed sections for 30 min. Subsequently, immunoreactivity was visualized by staining with diaminobenzidine (DAB) and examining by light microscopy. Finally, sections were counterstained with hematoxylin, dehydrated, mounted, and analyzed.
Assessment of disease activity and histological analysis
Animals were euthanized with an overdose of diethyl ether on day 8 and were analyzed for colitis by standard parameters as described previously (Han et al. 2015). The Rachmilewitz disease activity index (DAI) was calculated by using a previously established scoring system (Kasinathan et al. 2018). The distal portion of the colon was used for histological analysis, and the evaluation criteria were as previously described (Yin et al. 2018).
Measurement of MPO activity
Neutrophil infiltration into the inflamed colonic mucosa was quantified by assessing MPO activity using the O-dianisidine method. Protein extracts from colonic were used to assess MPO levels according to the manufacturer’s instructions.
Measurement of DAO and LPS levels and ELISA quantification of cytokines
Blood was collected from the retro-orbital plexus at euthanasia in a BD Microtainer (Becton Dickinson, USA). Hemolysis-free sera were obtained after centrifugation. DAO activity in sera was measured using an assay kit according to the manufacturer’s instructions. Serum LPS levels were determined using limulus amebocyte lysate according to the manufacturer’s instructions. TNF-α, IL-6, IL-1β, and IL-12 levels in serum were quantified by ELISA according to the manufacturer’s instructions.
Quantitative real-time PCR analysis
Tissues and cells were broken down and lysed by using a TissueLyser 24 (Jingxin, China). Total RNA was isolated with TRIzol reagent and messenger RNA (mRNA) was reverse transcribed into complementary DNA (cDNA) using M-MLV Reverse Transcriptase. The cDNA was analyzed by real-time quantitative PCR using an ABI 7500 apparatus (Thermo Fisher Scientific, USA). The primers in the reaction are shown in Supplemental Table S1. β-Actin was selected as the reference gene due to its uniform expression in each sample, and the relative mRNA expression was calculated by the 2−ΔΔCt method.
Preparation of nuclear extracts and whole cell lysates
Nuclear protein extracts were prepared according to the modified method as described previously (Yin et al. 2018). Whole cell lysates were prepared as described previously (Ren et al. 2018).
Western blotting
Total proteins were extracted from colon tissues or RAW264.7 macrophage cells that were lysed in radioimmunoprecipitation assay (RIPA) buffer containing the protease inhibitor phenylmethylsulfonyl fluoride. Proteins were separated and subjected to western blotting as described previously (Yin et al. 2018). Protein bands were detected immunologically using antibodies against nuclear factor kappa B (NF-κB) p65 (1:3000), NF-κB p-p65 (1:3000), IκB-α (1:2000), p-IκB-α (1:1000), ZO-1 (1:1000), E-cadherin (1:1000), and claudin-2 (1:1000). All blots were stripped and reprobed with β-actin (1:4000) or lamin A (1:2000) antibodies to confirm equal protein loading.
Immunofluorescence staining
Caco-2 cell monolayers were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 1% BSA. Then, the monolayers were incubated with primary antibodies against ZO-1 (1:50), E-cadherin (1:100), or claudin-2 (1:100) overnight at 4 °C. The cells were subsequently incubated with the appropriate secondary fluorescent-conjugated antibody for 1 h. Cell nuclei were stained with DAPI. Fluorescence was examined by fluorescence microscopy using a ZOE™ Fluorescent Cell Imager (Bio-Rad, Singapore).
Microbiome analysis
Microbial DNA was extracted from mouse cecal samples using the E.Z.N.A. Stool DNA Kit (Omega Bio-tek, USA) according to the manufacturer’s protocol. The V3–V4 region of the eukaryotic 16S rDNA gene was amplified using the primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 806R (5′-GGACTACHVGGGTATCTAAT-3′). Each amplified product was concentrated by solid-phase reversible immobilization and quantified by electrophoresis using a model 2100 Bioanalyzer (Agilent, USA). After quantifying the DNA concentration using a NanoDrop spectrophotometer, each sample was diluted to 1 × 109 mol/μL in Tris-EDTA buffer and pooled. Then, 20 μL of the pooled mixture was sequenced with the MiSeq sequencing system (Illumina, USA) according to the manufacturer’s instructions. The resulting reads were analyzed as previously described (Liu et al, 2018; Ren et al. 2018). All 16S ribosomal RNA (rRNA) sequencing data were submitted under accession no. SRP188494. (The CON group corresponds to the Z group of the raw data).
Statistical analyses
Mean ± SD values were calculated, and the statistical significance of differences was analyzed using one-way analysis of variance (ANOVA) followed by multiple comparisons between groups using Tukey’s post hoc test. Differences with p values < 0.05 were considered significant, and the significance is reported as *p ≤ 0.05 and **p ≤ 0.01. All calculations were performed using SPSS 19.0 software (SPSS, Inc., USA).
Result
Generation of a L. lactis AMJ1543 strain producing LFCA, and bioactivity of LFCA
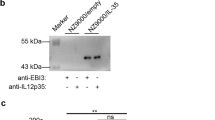
In this study, we engineered recombinant L. lactis AMJ1543 strains with a plasmid expressing LFCA (LL-LFCA) and verified that the growth curves of LL-LFCA and LL-control were similar, indicating that the expression of LFCA did not impede the growth of L. lactis AMJ1543 (Supplemental Fig. S2). Immunoblotting of LL-LFCA cell lysates and supernatant confirmed the expression and secretion of LFCA by the detection of a 4.6-kDa band (Fig. 1a, b). The expression level of LL-LFCA was higher in culture medium at 16 h than at 12 h. However, there was no difference in expression between 16 and 24 h (Fig. 1c, d).
Characterization of the recombinant L. lactis Δalr AMJ1543 strain producing LFCA (LL-LFCA). Protein production and secretion were analyzed by western blotting, which resolved LFCA as an immunoreactive 4.6-kDa band (panels a and b), and ELISA (panels c and d). LL-LFCA and LL-control were cultured for 24 h, and LFCA levels were assayed in the cell lysates and 20-fold TCA-concentrated culture supernatant. Synthetic LFCA (sLFCA) was used as a positive control for the LFCA immunoblot. Data are presented as the means ± SD. **p < 0.01 vs. the supernatant from LL-control
The capacity of LL-LFCA cell lysates to inhibit the growth of E. coli and S. aureus strains was determined. LL-LFCA lysates, but not the LL-control lysates, significantly inhibited the growth of E. coli and S. aureus (49.23% and 64.43%, respectively) when cultured with the lysate for 24 h (Fig. 2a, b), which was similar to the levels of inhibition induced by sLFCA. Negative staining and electron microscopy revealed that LL-LFCA cell lysates damaged the morphology of E. coli and S. aureus, and bacteria treated with LL-LFCA or sLFCA lysate contained more atypical vesicles, protrusions, and filamentations than untreated cells (control) and cells treated with LL-control cell lysate (LL-control; Fig. 2c).
LFCA produced by LL-LFCA is bioactive. a, b Bar graph showing the effects of lysates (10 μg protein) from LL-LFCA cells on the growth of E. coli CVCC10141 and S. aureus CVCC25923. c Ultrastructural damage in bacteria treated with cell lysates (10 μg protein) from LL-LFCA. Control, bacteria treated with PBS; LL-control, bacteria treated with cell lysates from LL-control; LL-LFCA, bacteria treated with cell lysates from LL-LFCA; sLFCA, bacteria treated with 10 μg of synthetic LFCA. Cells were analyzed by electron microscopy. d LL-LFCA localization in healthy mice. Samples of the intestinal mucosa of the jejunum, ileum, and colon were collected from mice treated with CFDA-SE–stained LL-LFCA (CFDA-SE-LL-LFCA) for 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, and 24 h, and the fluorescence signal was assessed by flow cytometry. Data are presented as the means ± SD. **p < 0.01 vs. the LL-control group. e Protein expression levels of LFCA in the colonic mucosa was detected by immunohistochemistry
LL-LFCA is localized to the jejunum, ileum, and colon in mice
Data concerning the localization of the LL-LFCA stained with CFDA-SE in sections of gastrointestinal tract tissue are presented in Fig. 2d. Compared with 2 h after administration, colonization of the LL-LFCA in the jejunum and ileum was significantly reduced with time. Especially at 24 h and 6 h after administration, the abundance of colonization of the LL-LFCA in the jejunum and ileum was not significantly different compared to that of control mice. However, at 24 h after administration, LL-LFCA still colonized the colon, and the abundance of colonization was significantly more than that in the control. The expression of LFCA by the LL-LFCA in the colonic mucosa was tested by immunohistochemistry. LFCA was expressed in the LL-LFCA group, but not in the control and LL-control groups (Fig. 2e).
LL-LFCA ameliorates clinical symptoms and inflammation of DSS-induced colitis
DSS damaged the mucosal barrier of the colon, leading to gut inflammation, weight loss, and other clinical symptoms. Mice in the DSS and DSS+LL-control groups showed significantly greater weight loss than the control group on days 5–8, whereas weight loss of the mice in the DSS+LL-LFCA group was significantly relieved (Fig. 3a). The DAI score, which is an indicator of the severity of colitis, is based on weight loss, fecal blood, and fecal consistency (Kasinathan et al. 2018). As shown in Fig. 3b, the DAI scores for the DSS and DSS+LL-control groups were significantly higher than those of the control group (p < 0.01). However, the DAI scores for the DSS+LL-LFCA group were significantly decreased (p < 0.01). The DSS+LL-LFCA group exhibited remarkably less colon shortening than the DSS group (p < 0.01). LL-control–treated mice also showed slightly less colon shortening than DSS mice. However, when compared with the DSS group, the difference in colon shortening failed to reach statistical significance (Fig. 3c, d).
LL-LFCA treatment improves DSS-induced colitis in mice. a Body weight changes in each group (n = 12, per group) after induction of colitis. b Disease activity index (DAI). c Macroscopic appearance and d colon length were measured in each group of mice. Data are presented as the means ± SD (n = 12, per group). **p < 0.01 vs. the DSS-treated group on the same day; #p < 0.05 and ##p < 0.01 vs. the LL-LFCA group
The severity of colonic inflammation and ulceration was further evaluated by histopathological analysis using hematoxylin and eosin staining (Fig. 4a). LL-LFCA–treated mice exhibited less epithelium cell layer destruction, mucosa inflammation, and cell infiltration, which resulted in significantly lower histological scores than the DSS group (p < 0.01; Fig. 4b). LL-control–treated mice displayed slightly less structural lesions than DSS mice. However, when compared with the DSS group, the difference in histological scores failed to reach statistical significance. LL-LFCA treatment also significantly attenuated DSS-induced hyperactivation of MPO (p < 0.01; Fig. 4c), whereas there was no significant difference between the DSS and DSS+LL-control groups, which agreed with the histological findings. To determine the anti-inflammatory effect of LL-LFCA on DSS-induced colitis, the expression levels of the inflammatory markers TNF-α, IL-6, IL-1β, and IL-12 in serum and colon tissues were evaluated. The mRNA expression levels of TNF-α, IL-6, IL-1β, and IL-12 were lower in the DSS+LL-LFCA group than in the DSS and DSS+LL-control groups (p < 0.01; Fig. 5a). The same trend was observed in serum (Fig. 5b).
LL-LFCA treatment protects against DSS-induced colon damage in mice. a Serial sections of colon tissues stained with hematoxylin and eosin (H&E). b Histopathology scores and c MPO activities in the colonic tissues. Data are presented as means ± SD. **p < 0.01 vs. the DSS group; #p < 0.05 and ##p < 0.01 vs. the LL-LFCA group
LL-LFCA inhibits the production of proinflammatory cytokines in colon tissues and serum from DSS-colitis mice. a Real-time PCR analysis of the mRNA expression levels of the inflammation-related cytokines TNF-α, IL-6, IL-1β, and IL-12 in the colon tissues of mice with DSS-induced colitis. b Protein levels of TNF-α, IL-6, IL-1β, and IL-12 in serum measured by ELISA in triplicate. Data are presented as mean ± SD. **p < 0.01 vs. the DSS group; #p < 0.05 and ##p < 0.01 vs. the LL-LFCA group
LL-LFCA ameliorates DSS-induced disruption of TJ structure and intestinal permeability
Intestinal epithelial TJs play a key role in protecting against inflammation. Therefore, the expression levels of the TJ-associated proteins ZO-1, E-cadherin, and claudin-2 in the colon were assessed and compared between groups using real-time PCR and western blotting. As shown in Fig. 6a, administration of LL-LFCA significantly enhanced the mRNA expression levels of these three factors compared with DSS-treated mice (p < 0.01). The mRNA expression levels of E-cadherin and claudin-2, but not ZO-1, in the DSS+LL-control group were higher than those in the DSS group (p < 0.05 and p < 0.01, respectively). The mRNA expression levels of ZO-1, E-cadherin, and claudin-2 were prominently upregulated in the DSS+LL-LFCA group as compared with the levels in the DSS+LL-control group. To further evaluate the protective effect of LL-LFCA against the disruption of TJs induced by DSS, we assessed the protein levels of ZO-1, E-cadherin, and claudin-2 by western blotting. As shown in Fig. 6b, the same trends were observed.
Effects of LL-LFCA on tight junction proteins in DSS-induced mice. a Real-time PCR analysis of the mRNA levels of ZO-1, E-cadherin, and claudin-2 in colon tissues. b ZO-1, E-cadherin, and claudin-2 protein levels in colon tissues detected by western blotting. c Effect of LL-LFCA on serum DAO activity and LPS levels in mice with DSS-induced colitis. Data are presented as the mean ± SD. Different letters indicate statistically significant differences, *p < 0.05 and **p < 0.01 vs. the DSS group; #p < 0.05 and ##p < 0.01 vs. the LL-LFCA group
Intestinal permeability was assessed by evaluating the changes in plasma DAO and LPS, which are two useful markers for assessing intestinal injury and monitoring increases in intestinal permeability following severe injury (Liu et al. 2016b; Niu et al. 2018). As shown in Fig. 6c, the concentrations of DAO and LPS were significantly higher in the DSS group than in the control group (p < 0.01). LL-LFCA treatment significantly lowered the levels of plasma DAO and LPS compared to those in the DSS and DSS+LL-control groups (p < 0.01).
LL-LFCA enhances expression of ZO-1, E-cadherin, and claudin-2 in Caco-2 cells
Immunofluorescence staining and real-time PCR were performed to examine the localization of TJ proteins in Caco-2 monolayers. As shown in Fig. 7a–f, both recombinant LFCA and sLFCA treatments significantly improved the expression of ZO-1, E-cadherin, and claudin-2 compared to cells treated with DSS alone (p < 0.01). The expression levels of E-cadherin and claudin-2, but not ZO-1, in the DSS+LL-control group were higher than those in the DSS group (p < 0.05). Although the expression levels of E-cadherin and claudin-2 in DSS+LL-control–treated cells were higher than those in DSS-treated cells, the effect was not as strong as that observed in DSS+LL-LFCA–treated cells.
Effects of LL-LFCA on the expression and localization of tight junction (TJ) proteins in vitro. Caco-2 cells were grown in a 24-well plate for 3 days until the TEER was > 300 Ω/cm2. The cells were treated with cell lysates (containing 10 μg of protein) from LL-LFCA for 24 h and then cotreated with 3% DSS for 24 h. The subcellular localization of ZO-1 (a, red), E-cadherin (b, red), and claudin-2 (c, red) was determined by immunofluorescence staining. Cell nuclei are stained with DAPI (blue). Scale bar, 100 μM. The mRNA expression of TJ proteins assessed by real-time PCR (d–f). The results (mean ± SD; n = 4) from three independent experiments are shown. *p < 0.05 and **p < 0.01 vs. the DSS group; ##p < 0.01 vs. the LL-LFCA group
LL-LFCA ameliorates LPS-induced inflammation and downregulates the NF-κB pathway in RAW264.7 cells
TNF-α, IL-6, IL-1β, and IL-12 are key factors in inflammation-related diseases. As described above, LL-LFCA inhibited the secretion of TNF-α, IL-6, IL-1β, and IL-12 in vivo (Fig. 5a, b). Subsequently, we confirmed the anti-inflammatory effects of LL-LFCA in vitro. As expected, the mRNA levels of TNF-α, IL-6, IL-1β, and IL-12 in LPS-induced RAW264.7 cells were remarkably suppressed by LL-LFCA (p < 0.01) (Fig. 8a). To extend our observations, we examined the effect of LL-LFCA on the activation of NF-κB in vitro. LL-LFCA markedly suppressed LPS-induced phosphorylation of p65 and IκBα, and the nuclear translocation of NF-κB, and similar effects were observed for sLFCA (Fig. 8b). Based on these results, we propose that LL-LFCA exerts its anti-inflammatory effects by inhibiting NF-κB.
Lysates of LL-LFCA cells inhibit NF-κB activation under inflammatory conditions and reduce the production of proinflammatory cytokines in RAW264.7 macrophages. a RAW264.7 macrophages were pretreated with lysates from LL-LFCA cells (10 μg) for 12 h and then stimulated with LPS (1 μg/mL) for 1 h. Real-time PCR analysis was performed to determine the mRNA levels of the proinflammatory cytokines TNF-α, IL-1β, IL-6, and IL-12. b Western blot analysis of p65, p-p65, IκB-α, p-IκB-α, lamin B, and β-actin. Data are means ± SD. *p < 0.05 and **p < 0.01 vs. the LPS group; #p < 0.05 and ##p < 0.01 vs. the LL-LFCA group
LL-LFCA improves DSS-induced dysbiosis of the gut microbiota
A 16S rRNA–based phylogenetic approach was used to investigate the effect of LL-LFCA treatment on the cecal microbial population. Alpha diversity was analyzed by calculating the Chao1 and Shannon indices (Fig. 9a, b). The Chao1 and Shannon index values were not significantly different among the groups. Principal component analysis (PCA) showed that the microbial communities in the four experimental groups were distinct (Fig. 9c). The box-and-whisker plot of microbial communities in the CON, DSS, DSS+LL-control, and DSS+LL-LFCA groups based on the weighted 16S UniFrac distances clearly showed that the microbial β-diversity in the DSS+LL-LFCA group was greater than that in the other groups, and the difference was statistically significant (p < 0.01, Fig. 9d).
Effects of LL-LFCA on the cecal microbiota in DSS-induced mice. Cecal contents from control (CON), DSS, DSS-LL-control, and DSS-LL-LFCA mice were collected, and the intestinal microbiota were examined by 16S rRNA sequencing. Alpha diversity was analyzed by Chao1 richness (a) and Shannon diversity (b) indices. Data are presented as box plots. c Principal component analysis (PCA) of the operational taxonomic units in the colonic microbiomes of control (blue), DSS (red), LL-control (green), and LL-LFCA (purple) mice plotted on the first two principal components accounted for approximately 42% of the observed variation. d β-Diversity between groups was analyzed by weighted UniFrac distance. Data are presented as mean ± SD. **p < 0.01, DSS vs. CON; ##p < 0.01, DSS-LL-LFCA vs. CON. e Phylum-level microbiota composition
The cartogram of the microbiota at the phylum level is shown in Fig. 9e. DSS treatment increased the proportions of Proteobacteria, Actinobacteria, Cyanobacteria, and Deferribacteres and decreased the proportion of Firmicutes compared with those in the CON group. The presence of LL-LFCA reversed these changes. The relative proportion of Bacteroidetes was nearly the same among the groups. At the genus level, administration of LL-LFCA selectively blunted the expansion of the Escherichia–Shigella population and increased the relative proportion of Lactobacillus, whereas the other major families were only marginally affected when compared with the DSS group (Fig. 10a). Administration of LL-control also enhanced the relative proportion of Lactobacillus but did not decrease the relative proportion of the Escherichia–Shigella population when compared with the DSS group (Fig. 10a). Collectively, these results showed that LL-LFCA improved the DSS-induced dysbiosis in the gut microbiota of mice.
Administration of LL-LFCA decreases the Escherichia–Shigella population and the effect of LL-LFCA on the KEGG pathways in gut microbiota. Effect of LL-LFCA on the microbiota classification and abundance detected by 16S rRNA gene sequencing analysis of cecal contents from the mice with DSS-induced LL-LFCA at the genus level (a). The effect of LL-LFCA on the KEGG pathways of the gut microbiota in mice with DSS-induced colitis (b, c)
The metabolic alterations were analyzed to determine the relationship between the relative transcript abundance of genes in Kyoto Encyclopedia of Genes and Genomes database metabolic pathways and immunotoxicity (Fig. 10b, c). The Tax4Fun analysis showed that the intestinal microbiota of mice in the DSS+LL-LFCA and DSS+LL-control groups were not the same, and their functional genes seemed to be different. After treatment with DSS, most of the metabolism was slowed or reduced. In contrast, after treatment with LL-LFCA, almost all the characteristic indices recovered to or were nearly to the CON group.
Discussion
Inflammatory bowel disease (IBD) affects more than 3.5 million individuals worldwide (Miyoshi and Chang 2017). The existing treatments for IBD are impeded by high costs, severe side effects, and drug resistance associated with long-term treatment (Hanson et al. 2014). Several studies have shown that the use of LAB as vectors allow for the cost-effective and direct delivery of therapeutics to the site of inflammation, avoiding the risk of systemic side effects (Saha et al. 2017). Therefore, in this study, we developed a strategy for localized delivery of LFCA (Lfcin B and Lfampin joined with a flexible linker), which is synthesized in situ by L. lactis Δalr strain AMJ1543 (LL-LFCA) and tested its ability to reduce colitis in a mouse model.
We first investigated the bioactivity of LL-LFCA–derived LFCA by assessing its antimicrobial properties. LL-LFCA cell lysates could statistically inhibit both E. coli and S. aureus in vitro, implying that LL-LFCA–derived LFCA is bioactive. Then, we tried to evaluate the effect of LFCA in DSS-induced colitis mice. Bacterial dysbiosis in IBD is mainly characterized by an increased abundance in the Escherichia–Shigella genus (Xu et al. 2018). The observed bactericidal effect of LFCA derived from LL-LFCA may be exploited to decrease the abundance of Escherichia–Shigella and improve the intestinal microbiota.
We investigated the ability of LL-LFCA localization in vivo. After 24 h of administration, colonization of LL-LFCA in the colon was more than that in the jejunum and ileum, indicating that LL-LFCA has better colonization ability in the colon after administration. Furthermore, LFCA was expressed by LL-LFCA in the colon.
To confirm the intestinal injury-relieving effects of LL-LFCA in vivo, a mouse model of DSS-induced experimental colitis was used to mimic the symptoms of IBD patients (Deng et al. 2018b; Wirtz et al. 2007; Yu et al. 2018). The DSS-induced model displayed acute inflammation in the colon. LL-LFCA effectively ameliorated the symptoms of colonic inflammation induced by DSS, which included poor stool consistency, bloody stool, and body weight loss, thereby reducing the DAI score. Additionally, administration of LL-LFCA relieved the signs of inflammation and mucosal injury, as indicated by improved colon length and lower histological scores. Colon shortening was associated with the severity of DSS-induced colitis (Han et al. 2015). L. lactis was reported to alleviate colitis (Jones and Foxx-Orenstein 2007). Here, L. lactis AMJ1543 was used as a vehicle to express LFCA. The use of the LL-control proved that LFCA derived from LL-LFCA did alleviate DSS-induced colitis. Both LL-LFCA and LL-control treatments alleviated colon shortening, but LL-LFCA was more efficacious than the LL-control, indicating that LFCA expressed by L. lactis AMJ1543 was influential in ameliorating the signs and symptoms of inflammation, consistent with the physiological and pathological observations.
MPO activity is proportional to the concentration of neutrophils and is an index of neutrophil infiltration and inflammation (Rachmilewitz et al. 1989). Here, we observed that LL-LFCA decreased MPO activity and inflammatory cell infiltration into colon tissues. These results suggest that LL-LFCA has ameliorating effects on inflammatory cell infiltration.
Although the precise etiopathogenesis of UC remains unknown, there is accumulating evidence that excessive proinflammatory cytokines can damage the colon mucosa and affect intestinal homeostasis (Han et al. 2015; Salim and Soderholm 2011; Sun et al. 2015b) and proinflammatory cytokines, including TNF-α, IL-6, IL-1β, and IL-12, have been implicated in the pathophysiology of IBD (Chi et al. 2018). Thus, we evaluated the levels of TNF-α, IL-1β, IL-6, and IL-12 in colon tissues and serum and found that administration of LL-LFCA was beneficial on DSS-induced intestinal inflammation by inhibiting the expression of the proinflammatory cytokines. These results suggest that LL-LFCA has ameliorating effects on colonic inflammation, but LL-control does not. In an inflammatory state, the expression of some inflammatory factors, such as IL-1β and TNF-α, is upregulated mainly through the NF-κB signaling pathway (Doyle and O’Neill 2006). Therefore, we speculated that the anti-inflammatory effect of LFCA might be related to the NF-κB signaling pathway, as previous studies reported that DSS-induced colitis was ameliorated by NF-κB inhibitors (Kang et al. 2017; Takada et al. 2010). Thus, LPS-stimulated RAW264.7 cells were used to examine the mechanisms underlying the observed beneficial effects of LL-LFCA on DSS-induced experimental colitis. The expression levels of TNF-α, IL-1β, IL-6, and IL-12 were markedly increased in LPS-treated RAW264.7 and that pre-treatment with a lysate of LL-LFCA cells reduced this effect. To evaluate the mechanism of the anti-inflammatory activity, we assessed the effect of lysates from LL-LFCA cells on the NF-κB pathway. The findings revealed that the anti-inflammatory effects of LL-LFCA may be related to the inhibition of the nuclear transfer of p65 NF-κB in DSS-induced colitis in mice (Han et al. 2015). Western blot analysis showed that the phosphorylation of p65 and IκBα, which are initiated by the activation of NF-κB in RAW264.7 cells, was significantly increased by LPS and decreased by treatment with lysate from LL-LFCA cells, but not from LL-control cells. The data suggest that LFCA expressed by LL-LFCA may reduce the production of proinflammatory factors by suppressing NF-κB–mediated transcriptional activation of inflammatory gene expression.
In addition to the upregulation of proinflammatory mediators, disruption of epithelial barrier function is another well-known pathophysiology aspect of IBD (Chi et al. 2018; Vancamelbeke and Vermeire 2017). Proinflammatory cytokines induce disruption of the intestinal mucosa, and an impaired intestinal barrier increases the permeability of the intestinal mucosa to harmful bacteria, which augments gut inflammation, leading to a vicious circle (Han et al. 2015; Shen et al. 2018). The intestinal epithelial barrier is maintained and regulated mainly by TJs (Carvalho et al. 2017). A previous study demonstrated the decreased expression of TJ-related proteins in the intestinal mucosa of IBD patients, including the intracytoplasmic proteins ZO-1 and ZO-2 and the transmembrane proteins E-cadherin and Claudin (Carvajal et al. 2017; Yin et al. 2018). Presently, we observed significant upregulation of ZO-1, E-cadherin, and claudin-2 mRNA and protein expression in the colon of mice after administration of LL-LFCA, following reduced expression induced by DSS challenge. Administration of LL-control also increased the expression of E-cadherin and claudin-2 in the colon. However, it was not as effective as LL-LFCA. The expression of TJ proteins was then measured in an in vitro model of the intestinal epithelial barrier using Caco-2 cells by immunofluorescence, which revealed that DSS induced a significant change in the distribution of TJs and that LFCA derived from LL-LFCA cells attenuated this destructive effect. DAO and LPS contents are recognized as sensitive markers to monitor intestinal barrier permeability (Niu et al. 2018). When the intestinal permeability is abnormally increased, luminal DAO and LPS enter the blood, resulting in elevated serum DAO and LPS levels. Presently, the concentrations of DAO and LPS in serum were sharply increased in DSS-treated mice and were significantly inhibited by LL-LFCA treatment, whereas there was no statistically significant difference between the DSS-treated and DSS+LL-control–treated groups. These results indicated that the ameliorative effect of LL-LFCA against DSS-induced colitis might be tightly associated with protection of the intestinal mucosal barrier by maintaining the expression of TJ proteins and reducing the permeability of the intestinal barrier. Although administration of LL-control increased the expression of TJ proteins, it did not improve intestinal epithelial barrier permeability. This might be explained by the fact that LL-control does not reduce proinflammatory factor expression, and these proinflammatory factors may impair mucosal barrier function and increase intestinal permeability.
Once the mucosal barrier is breached, luminal antigens and microorganisms can enter the submucosa, leading to the intestinal dysbacteriosis. Bacterial dysbiosis in IBD is characterized by a reduction in bacterial diversity, a decrease in the Firmicutes phylum, and an increase in the Proteobacteria phylum (Xu et al. 2018). The present results of the Chao1 richness and Shannon indices indicated no difference in richness of species among groups. However, the results of PCA analysis and the intercommunity β-diversity determined by weighted 16S UniFrac distances indicated a significant difference among the gut microbial composition of the four groups. Our analyses also showed that administration of LL-LFCA decreased the percentage of Proteobacteria and increased the percentage of Firmicutes. After LL-LFCA treatment, the abundance of Escherichia–Shigella was significantly reduced, and the relative proportion of Lactobacillus was enhanced. LL-control treatment also enhanced the relative proportion of Lactobacillus, but LL-control treatment did not reduce the abundance of Escherichia–Shigella. These data indicate that LFCA expressed by LL-LFCA promotes the proliferation of beneficial bacteria and reduces the proliferation of harmful bacteria, thus regulating the microbial barrier function to ameliorate IBD. Metabolic pathway analysis using data from the Kyoto Encyclopedia of Genes and Genomes database indicated that LL-LFCA has balancing effects on the metabolic activities of the gut microbiota to maintain immunity.
In this study, we demonstrated that administration of LL-LFCA prevents DSS-induced colitis in mice and that this protective effect may be due to its effects on the maintenance of intestinal mucosal barrier integrity and modulation of the composition of the gut microbiota. These effects are mediated by modulation of TJ protein expression in the colonic tissue, inhibition of inflammatory mediator production (including IL-1β, IL-12, TNF-α, and IL-6), and inhibition of NF-κB signal transduction pathways. Taken together, these findings suggest that LL-LFCA has potential as a new therapeutic preparation for UC.
References
Bermudez-Humaran LG, Motta JP, Aubry C, Kharrat P, Rous-Martin L (2015) Serine protease inhibitors protect better than IL-10 and TGF-β anti-inflammatory cytokines against mouse colitis when delivered by recombinant lactococci. Microb Cell Factories 14:2–11. https://doi.org/10.1186/s12934-015-0198-4
Bolscher J, Nazmi K, van Marle J, van’t Hof W, Veerman E (2012) Chimerization of lactoferricin and lactoferrampin peptides strongly potentiates the killing activity against Candida albicans. Biochem Cell Biol 90:378–388. https://doi.org/10.1139/o11-085
Carvajal AE, Serrano-Morales JM, Vazquez-Carretero MD, Garcia-Miranda P, Calonge ML, Peral MJ, Ilundain AA (2017) Reelin protects from colon pathology by maintaining the intestinal barrier integrity and repressing tumorigenic genes. Biochim Biophys Acta 1863:2126–2134. https://doi.org/10.1016/j.bbadis.2017.05.026
Carvalho RD, Breyner N, Menezes-Garcia Z, Rodrigues NM, Lemos L, Maioli TU, da Gloria Souza D, Carmona D, de Faria AM, Langella P, Chatel JM, Bermudez-Humaran LG, Figueiredo HC, Azevedo V, de Azevedo MS (2017) Secretion of biologically active pancreatitis-associated protein I (PAP) by genetically modified dairy Lactococcus lactis NZ9000 in the prevention of intestinal mucositis. Microb Cell Factories 16:27–38. https://doi.org/10.1186/s12934-017-0624-x
Chi JH, Kim YH, Sohn DH, Seo GS, Lee SH (2018) Ameliorative effect of alnus japonica ethanol extract on colitis through the inhibition of inflammatory responses and attenuation of intestinal barrier disruption in vivo and in vitro. Biomed Pharmacother 108:1767–1774. https://doi.org/10.1016/j.biopha.2018.10.050
Chon H, Choi B, Jeong G, Lee E, Lee S (2010) Suppression of proinflammatory cytokine production by specific metabolites of Lactobacillus plantarum 10hk2 via inhibiting NF-κB and p38 MAPK expressions. Comp Immunol Microbiol Infect Dis 33:41–49. https://doi.org/10.1016/j.cimid.2009.11.002
Deng F, Peng L, Li Z, Tan G, Liang E, Chen S, Zhao X, Zhi F (2018a) Yap triggers the Wnt/beta-catenin signalling pathway and promotes enterocyte self-renewal, regeneration and tumorigenesis after DSS-induced injury. Cell Death Dis 9:153–165
Deng J, Zeng L, Lai X, Li J, Liu L, Lin Q, Chen Y (2018b) Metformin protects against intestinal barrier dysfunction via AMPKα1-dependent inhibition of JNK signalling activation. J Cell Mol Med 22:546–557. https://doi.org/10.1111/jcmm.13342
Doyle SL, O’Neill LA (2006) Toll-like receptors: from the discovery of NF-κB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol 72:1102–1113. https://doi.org/10.1016/j.bcp.2006.07.010
Flores-Villasenor H, Canizalez-Roman A, Reyes-Lopez M, Nazmi K, Garza M, Zazueta-Beltran J, Leon-Sicairos N, Bolscher JG (2010) Bactericidal effect of bovine lactoferrin, lfcin, lfampin and lfchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals. 23:569–578. https://doi.org/10.1007/s10534-010-9306-4
Gao K, Wang C, Liu L, Dou X, Liu J, Yuan L, Zhang W, Wang H (2017) Immunomodulation and signaling mechanism of Lactobacillus rhamnosus GG and its components on porcine intestinal epithelial cells stimulated by lipopolysaccharide. J Microbiol Immunol Infect 50:700–713. https://doi.org/10.1016/j.jmii.2015.05.002
Gifford JL, Hunter HN, Vogel HJ (2005) Lactoferricin: a lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell Mol Life Sci 62:2588–2598. https://doi.org/10.1007/s00018-005-5373-z
Han F, Zhang H, Xia X, Xiong H, Song D, Zong X, Wang Y (2015) Porcine beta-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J Immunol 194:1882–1893. https://doi.org/10.4049/jimmunol.1402300
Hanson ML, Hixon JA, Li W, Felber BK, Anver MR, Stewart CA, Janelsins BM, Datta SK, Shen W, McLean MH, Durum SK (2014) Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroentero. 146:210–221. https://doi.org/10.1053/j.gastro.2013.09.060
Jones JL, Foxx-Orenstein AE (2007) The role of probiotics in inflammatory bowel disease. Dgest Dis Sci 52:607–611. https://doi.org/10.1007/s10620-006-9225-y
Kang JH, Choi S, Jang JE, Ramalingam P, Ko YT, Kim SY, Oh SH (2017) Wasabia japonica is a potential functional food to prevent colitis via inhibiting the NF-κB signaling pathway. Food Funct 8:2865–2874. https://doi.org/10.1039/c7fo00576h
Kasinathan NK, Subramaniya B, Sivasithamparam ND (2018) NF-κB/twist mediated regulation of colonic inflammation by lupeol in abating dextran sodium sulfate induced colitis in mice. J Funct Foods 41:240–249. https://doi.org/10.1016/j.jff.2017.12.048
Li Y, Liu T, Yan C, Xie R, Guo Z, Wang S, Zhang Y, Li Z, Wang B, Cao H (2018) Diammonium glycyrrhizinate protects against nonalcoholic fatty liver disease in mice through modulation of gut microbiota and restoration of intestinal barrier. Mol Pharm 15:3860–3870. https://doi.org/10.1021/acs.molpharmaceut.8b00347
Liu DQ, Ge JW, Qiao XY, Jiang YP, Liu SM, Li YJ (2012) High-level mucosal and systemic immune responses induced by oral administration with Lactobacillus-expressed porcine epidemic diarrhea virus S1 region combined with Lactobacillus-expressed N protein. Appl Microbiol Biotechnol 93:2437–2446. https://doi.org/10.1007/s00253-011-3734-0
Liu S, Li Y, Deng B, Xu Z (2016a) Recombinant Lactococcus lactis expressing porcine insulin-like growth factor I ameliorates DSS-induced colitis in mice. Biotechnol. 16:25–35. https://doi.org/10.1186/s12896-016-0255-z
Liu M, Wu Q, Wang M, Fu Y, Wang J (2016b) Lactobacillus rhamnosus GR-1 limits Escherichia coli-induced inflammatory responses via attenuating MyD88-dependent and MyD88-independent pathway activation in bovine endometrial epithelial cells. Inflammation. 39:1483–1494. https://doi.org/10.1007/s10753-016-0382-7
Liu W, Zhang Y, Qiu B, Fan SJ, Ding HF, Liu ZH (2018) Quinoa whole grain diet compromises the changes of gut microbiota and colonic colitis induced by dextran sulfate sodium in C57BL/6 mice. Sci Rep 8:14916. https://doi.org/10.1038/s41598-018-33092-9
Martin R, Chain F, Miquel S, Natividad JM, Sokol H, Verdu EF, Langella P, Bermudez-Humaran LG (2014) Effects in the use of a genetically engineered strain of Lactococcus lactis delivering in situ IL-10 as a therapy to treat low-grade colon inflammation. Hum Vaccin Immunother 10:1611–1621. https://doi.org/10.4161/hv.28549
Miyoshi J, Chang EB (2017) The gut microbiota and inflammatory bowel diseases. Transl Res 179:38–48. https://doi.org/10.1016/j.trsl.2016.06.002
Nandakumar MP, Shen J, Raman B, Marten MR (2003) Solubilization of trichloroacetic acid (TCA) precipitated microbial proteins via naoh for two-dimensional electrophoresis. J Proteome Res 2:11–24. https://doi.org/10.1021/pr025541x
Niu GC, Liu L, Zheng L, Zhang H, Shih DQ, Zhang X (2018) Mesenchymal stem cell transplantation improves chronic colitis-associated complications through inhibiting the activity of toll-like receptor-4 in mice. Gastroenterol. 18:127–135. https://doi.org/10.1186/s12876-018-0850-7
O’Connor EB, O’Sullivan O, Stanton C (2007) pEOC01: a plasmid from Pediococcus acidilactici which encodes an identical streptomycin resistance (aadE) gene to that found in Campylobacter jejuni. Plasmid. 58:115–126. https://doi.org/10.1016/j.plasmid.2007.02.002
Patel S, Akhtar N (2017) Antimicrobial peptides (AMPs): the quintessential ‘offense and defense’ molecules are more than antimicrobials. Biomed Pharmacother 95:1276–1283. https://doi.org/10.1016/j.biopha.2017.09.042
Price KL, Totty HR, Lee HB, Utt MD, Fitzner GE, Yoon I, Ponder MA, Escobar J (2010) Use of saccharomyces cerevisiae fermentation product on growth performance and microbiota of weaned pigs during Salmonella infection. J Anim Sci 88:3896–3908. https://doi.org/10.2527/jas.2009-2728
Rachmilewitz D, Simon PL, Schwartz LW, Griswold DE, Fondacaro JD, Wasserman MA (1989) Inflammatory mediators of experimental colitis in rats. Gastroenterology. 97:326–337
Rebeca M, Chain F, Sylvie M, Jane MN, Harry S (2014) Effects in the use of a genetically engineered strain of Lactococcus lactis delivering in situ IL-10 as a therapy to treat low-grade colon inflammation. Hum Vaccin 10:1611–1621. https://doi.org/10.4161/hv.28549
Ren Y, Geng Y, Du Y, Li W, Lu ZM, Xu HY, Xu GH, Shi JS, Xu ZH (2018) Polysaccharide of hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J Nutr Biochem 57:67–76. https://doi.org/10.1016/j.jnutbio.2018.03.005
Ruangcharoen S, Suwannarong W, Lachica M, Bolscher JGM, Nazmi K, Khunkitti W, Taweechaisupapong S (2017) Killing activity of lfchimera on periodontopathic bacteria and multispecies oral biofilm formation in vitro. World J Microbiol Biotechnol 33:167–178. https://doi.org/10.1007/s11274-017-2334-2
Saha P, Chassaing B, Yeoh BS, Viennois E, Xiao X, Kennett MJ, Singh V, Vijay-Kumar M (2017) Ectopic expression of innate immune protein, lipocalin-2, in Lactococcus lactis protects against gut and environmental stressors. Inflamm Bowel Dis 23:1120–1132. https://doi.org/10.1097/MIB.0000000000001134
Salim SY, Soderholm JD (2011) Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis 17:362–381. https://doi.org/10.1002/ibd.21403
Shen P, Zhang Z, He Y, Gu C, Zhu K, Li S, Li Y, Lu X, Liu J, Zhang N, Cao Y (2018) Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci 196:69–76. https://doi.org/10.1016/j.lfs.2018.01.016
Shigemori S, Watanabe T, Kudoh K, Ihara M, Nigar S, Yamamoto Y, Suda Y, Sato T, Kitazawa H, Shimosato T (2015) Oral delivery of Lactococcus lactis that secretes bioactive heme oxygenase-1 alleviates development of acute colitis in mice. Microb Cell Factories 14:189–200. https://doi.org/10.1186/s12934-015-0378-2
Sinha M, Kaushik S, Kaur P, Sharma S, Singh TP (2013) Antimicrobial lactoferrin peptides: the hidden players in the protective function of a multifunctional protein. Int J Pept 2013:230–242. https://doi.org/10.1155/2013/390230
Silva RT, Sampaio BAA, Cremasco DT, Sakai AO, Lucio AF (2014) Identification and adhesion profile of Lactobacillus spp. strains isolated from poultry. Braz J Microbiol 45:1065-1073. https://doi.org/10.1590/S1517-83822014000300040
Sun T, Gao GZ, Li RF, Li X, Li DW, Wu SS (2015a) Bone marrow-derived mesenchymal stem cell transplantation ameliorates oxidative stress and restores intestinal mucosal permeability in chemically induced colitis in mice. Am J Transl Res 7:891–901
Sun Y, Zhao Y, Yao J, Zhao L, Wu Z, Wang Y, Pan D, Miao H, Guo Q, Lu N (2015b) Wogonoside protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB and NLRP3 inflammasome activation. Biochem Pharmacol 94:142–154. https://doi.org/10.1016/j.bcp.2015.02.002
Takada Y, Ray N, Ikeda E, Kawaguchi T, Kuwahara M, Wagner EF, Matsuo K (2010) Fos proteins suppress dextran sulfate sodium-induced colitis through inhibition of NF-κB. J Immunol 184:1014–1021. https://doi.org/10.4049/jimmunol.0901196
Tang Z, Yin Y, Zhang Y, Huang R, Sun Z, Li T, Chu W, Kong X, Li L, Geng M, Tu Q (2009) Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin-lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br J Nutr 101:998–1005. https://doi.org/10.1017/s0007114508055633
Tang SX, Andrew A, Yin YL, Tang ZR, Wang ZP, Liu ZQ (2012) Dietary supplementation with recombinant lactoferrampin-lactoferricin improves growth performance and affects serum parameters in piglets. J Anim Vet Adv 14:2548–2555
Toomey N, Bolton D, Fanning S (2010) Characterisation and transferability of antibiotic resistance genes from lactic acid bacteria isolated from Irish pork and beef abattoirs. Res Microbiol 161:127–135. https://doi.org/10.1016/j.resmic.2009.12.010
Van der Kraan MI, Groenink J, Nazmi K, Veerman EC, Bolscher JG, Nieuw Amerongen AV (2004) Lactoferrampin: a novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 25:177–183. https://doi.org/10.1016/j.peptides.2003.12.006
Vancamelbeke M, Vermeire S (2017) The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroent 11:821–834. https://doi.org/10.1080/17474124.2017.1343143
Wang M, Gao Z, Zhang Y, Pan L (2016) Lactic acid bacteria as mucosal delivery vehicles: a realistic therapeutic option. Appl Microbiol Biotechnol 100:5691–5701. https://doi.org/10.1007/s00253-016-7557-x
Wirtz S, Neufert C, Weigmann B, Neurath MF (2007) Chemically induced mouse models of intestinal inflammation. Nat Protoc 2:541–546. https://doi.org/10.1038/nprot.2007.41
Wong CC, Zhang L, Wu WK, Shen J, Chan RL, Lu L, Hu W, Li MX, Li LF, Ren SX, Li YF, Li J, Cho CH (2017) Cathelicidin-encoding Lactococcus lactis promotes mucosal repair in murine experimental colitis. J Gastroenterol Hepatol 32:609–619. https://doi.org/10.1111/jgh.13499
Xu J, Chen N, Wu Z, Song Y, Zhang Y, Wu N, Zhang F, Ren X, Liu Y (2018) 5-Aminosalicylic acid alters the gut bacterial microbiota in patients with ulcerative colitis. Front Microbiol 9:1274. https://doi.org/10.3389/fmicb.2018.01274
Yin M, Yan X, Weng W, Yang Y, Gao R, Liu M, Pan C, Zhu Q, Li H, Wei Q, Shen T, Ma Y, Qin H (2018) Micro integral membrane protein (MIMP), a newly discovered anti-inflammatory protein of Lactobacillus plantarum, enhances the gut barrier and modulates microbiota and inflammatory cytokines. Cell Physiol Biochem 45:474–490. https://doi.org/10.1159/000487027
Yu XT, Xu YF, Huang YF, Qu C, Xu LQ, Su ZR, Zeng HF, Zheng L, Yi TG, Li HL, Chen JP, Zhang XJ (2018) Berberrubine attenuates mucosal lesions and inflammation in dextran sodium sulfate-induced colitis in mice. PLoS One 13:e0194069. https://doi.org/10.1371/journal.pone.0194069
Zhou H, Li X, Wang Z, Yin J, Tan H, Wang L, Qiao X, Jiang Y, Cui W, Liu M, Li Y, Xu Y, Tang LJ (2018) Construction and characterization of thymidine auxotrophic (ΔthyA) recombinant Lactobacillus casei expressing bovine lactoferricin. Vet Res 14:206–217. https://doi.org/10.1186/s12917-018-1516-y
Funding
This work was supported by Grant No. 2018YFD0500600 from the National Key Research and Development Plan, the National Natural Science Foundation of China (Grant No. 31672461), and Technology Innovation Foundation of Harbin (Grant No. 2014RFXXJ084).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The animal experiment was approved by the Animal Care and Use Committee of Northeast Agricultural University (China) according to the Chinese Council on Animal Care guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 343 kb)
Rights and permissions
About this article
Cite this article
Song, L., Xie, W., Liu, Z. et al. Oral delivery of a Lactococcus lactis strain secreting bovine lactoferricin–lactoferrampin alleviates the development of acute colitis in mice. Appl Microbiol Biotechnol 103, 6169–6186 (2019). https://doi.org/10.1007/s00253-019-09898-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09898-6