Abstract
In the past century, yeasts from the genus Saccharomyces represented the only option in fermentation industries, such as winemaking, to produce wine, beer, and other fermented products. However, other genera are currently emerging to solve challenges in modern enology. Schizosaccharomyces pombe is showing promising results in solving specific challenges in northern, cool viticulture regions with highly acidic wines by deacidifying these wines through its malic acid metabolism. In addition, this microorganism is considered beneficial in warm growing regions with challenges such as the control of wine food safety problems such as the presence of biogenic amines, ochratoxin A, or ethyl carbamate. Indeed, the genus Schizosaccharomyces positively influences other important wine quality parameters, such as color and polysaccharide content. However, the main challenge of using this genus remains the selection of proper strains that alleviate problems such as the production of high acetate concentrations. Industries other than wine production such as ginger fermentation, apple wine, Kei-apple fermentation, plum wine, sparkling wine, and bilberry fermentation industries have also started to study Schizosaccharomyces species as an alternative tool for solving specific related problems. The review discusses the influence of Schizosaccharomyces on different fermentation quality parameters and its main applications in different industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The latest studies of modern enology techniques show the important role that non-Saccharomyces yeasts play in improving wine quality (Jolly et al. 2014; Padilla et al. 2016; Varela 2016; Petruzzi et al. 2017). As research in the field is evolving day by day, it is becoming easier to compare studies related to non-Saccharomyces yeasts, similar to the comparisons performed in previous years regarding the role of Saccharomyces cerevisiae strains in wine microbiology. Specific reviews have also been recently published for some of the most commercialized non-Saccharomyces species, such as Torulaspora delbrueckii (Benito 2018a) or Lachancea thermotolerans (Benito 2018b; Porter et al. 2019).
Among all non-Saccharomyces, Schizosaccharomyces shows a unique ability to deacidify wines during alcoholic fermentation (AF) (Su et al. 2014; Benito et al. 2018). The main option to deacidify wines, from a microbiological point of view, is the application of lactic acid bacteria such as Oenococcus oeni (Sumby et al. 2014, 2019), which are able to degrade l-malic acid into l-lactic acid. Malolactic fermentation (MLF) is still a complex process with several risks of deviation and is an important preoccupation for most red wine makers in warm viticultural regions (Sumby et al. 2019). High concentrations of ethanol can inhibit lactic acid bacterium performance (Bonomo et al. 2018), and high pH can cause the production of high levels of biogenic amines (Cinquanta et al. 2018). In addition, high SO2 content can inhibit the start or progress of MLF (Wang et al. 2018). The lack of nutrients after AF can also threaten the proper development of MLF, while nutrient addition can lead to the development of undesirable microorganisms (Sumby et al. 2019). Very low levels of malic acid can also make it difficult for lactic bacteria to complete fermentation at the right time (Sumby et al. 2019). Research on the interaction between yeasts and lactic bacteria is currently limited; thus, further studies are needed (Tristezza et al. 2016). MLF is a slow process with a risk of the development of spoilage microorganisms, such as Brettanomyces/Dekkera yeast, during the end of AF and the beginning of MLF when the wine does not contain high levels of SO2 (Lasik-Kurdyś et al. 2017). A reduction in color is also a common observation in red wines during MLF (Benito et al. 2019). In these specific situations, the use of Schizosaccharomyces can be an interesting option and worth considering.
Previous research regarding the genus Schizosaccharomyces focused mainly on Schizosaccharomyces pombe. Recent studies have begun examining combinations of S. pombe with S. cerevisiae (Benito et al. 2013) in the wine industry. In addition, other studies started testing combinations with other non-Saccharomyces species such as Lachancea thermotolerans (Benito et al. 2015) or Torulaspora delbrueckii (Liu et al. 2018). The latest studies reported in the field also focused on other species such as Schizosaccharomyces japonicus (Domizio et al. 2018).
Past studies of Schizosaccharomyces focused on the basic quality parameters of wine, with a further emphasis on malic acid degradation (Silva et al. 2003). Later, studies started to focus on other parameters, and the genus Schizosaccharomyces also showed promise in reducing levels of hazardous compounds for human health, such as ochratoxin A (Cecchini et al. 2006), biogenic amines, and ethyl carbamate (Benito et al. 2016a). In addition, other recent studies started to apply these organisms in wines with a low content of higher alcohols that could increase the aroma perception of some grape varieties or in wines with polysaccharides that improve the sensory perception in the mouth (Domizio et al. 2018; Benito et al. 2019). Other studies focused on wine color improvements shown when the MLF was avoided and on increasing the production of highly stable anthocyanins such as vitisins (Benito et al. 2017).
Although Schizosaccharomyces species possess many properties that can improve wine quality, some disadvantages have also been reported, including mainly high acetic acid production (Minnaar et al. (2017; Miljić et al. 2017) and slow kinetic fermentation (Du Plessis et al. 2017a). Several researchers tried to overcome these undesirable effects by performing combined fermentations with S. cerevisiae or other moderately fermentative species, applying selection programs, adding supplementation of nutrients such as magnesium (Hu et al. 2003) and using fed-batch technology (Roca-Domènech et al. 2018). Due to the difficulty of isolation, only the commercial strain ProMalic is currently available in the market (http://www.scottlabsltd.com/product/promalic-encapsulated-yeast/), and only a few studies managed to perform proper strain selection.
Although just a few previous studies on Schizosaccharomyces focused on industries different from the grape wine industry, such as the beer (Van Der Walt 1956), palm wine (Shamala and Sreekantiah 1988; Chilaka et al. 2010) and mango wine (Obisanya et al. 1987) industries. During recent years, other fermentative industries have started to use Schizosaccharomyces to solve specific problems similar to those encountered in the wine industry, such as high malic acid content. Most studies are applicable to fermentative industries producing products for human consumption. Some examples are apple wine (Satora et al. 2018), Kei-apple fermentation (Minnaar et al. (2017), sparkling wine (Ivit et al. 2018), bilberry fermentation (Liu et al. 2019), and plum wine (Miljić et al. 2017) industries. In addition, industries other than the food industry have also started to use Schizosaccharomyces species for specific purposes. For instance, the pharmacy industry uses S. pombe for ginger fermentation (Huh et al. 2018) to produce pharmaceutical active principles in the fight against some neurodegenerative diseases such as Alzheimer’s disease. Other applications for a better environment are also possible since Schizosaccharomyces allows for obtaining bioethanol from row molasses (Bakhiet and Mahmoud 2015) and removes heavy metals such as copper from water (Subhashini et al. 2011).
The number of studies related to Schizosaccharomyces in fermentative industries has increased extensively during the past 5 years in the same way that many studies were performed on S. cerevisiae at the beginning of wine microbiology research. This paper reviews the most recent scientific data on Schizosaccharomyces species applications in different industries. It focuses on comparing results reported in different studies in the field and on the analysis of their possible causes. This review will help industries understand possible applications of this specific genus and to inspire new insights into better uses of Schizosaccharomyces in the future.
Taxonomy, morphology, and physiology of Schizosaccharomyces
According to the most recent taxonomical classification, there are three main species in the Schizosaccharomyces genus: Schizosaccharomyces pombe, Schizosaccharomyces japonicus, and Schizosaccharomyces octosporus (Vaughan-Martini and Martini 2011). Currently, the most common species used in fermentation industries is S. pombe. However, S. japonicus applications are also starting to show promising results. The taxonomical classification is essentially based on the capacity for fermenting sucrose and raffinose; assimilation of sucrose, raffinose, and d-gluconate; growth at 32 °C and 37 °C; and the number of spores produced per ascus. S. pombe and S. japonicus ferment and assimilate sucrose and raffinose, while S. octosporus does not. S. pombe is the only species able to assimilate d-gluconate. S. japonicus and S. octosporus grow at temperatures over 37 °C, while S. pombe grows at temperatures up to 33 °C. S. japonicus and S. octosporus sporulate between six and eight spores per ascus, while S. pombe only generates four.
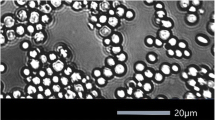
Schizosaccharomyces possesses a unique morphology among the yeast genera, and it is very easy to identify microscopically. Schizosaccharomyces is more rectangular than most other yeasts, which are mainly spherical or elliptical (Fig. 1). The short side of the rectangle varies from 3 to 5 μm in length, while the long side varies from 5 to 15 μm. The vegetative reproduction of Schizosaccharomyces is also unique among yeasts and is called binary fission (Fig. 2) or schizogony, which is very different from the behavior of common budding yeasts. It consists of cell division by a wall or septum located exactly in the center of the cell. S. pombe and S. japonicus also sometimes produce pseudomycelia. S. japonicus is also considered the only species in the genus Schizosaccharomyces that can produce true hyphae, doing so under certain conditions.
The main characteristic making Schizosaccharomyces different from other fermentative yeast genera is its ability to metabolize malic acid into ethanol and CO2 (Fig. 3). In addition, the genus cannot assimilate nitrates, does not exhibit β-glucosidase activity, and enzymatically breaks down arbutin. Other characteristics related to this genus are its specific urease enzymatic activity and its ability to be dyed by diazonium blue. In addition, it contains polysaccharides and sugar products in its cellular structure that are unique among the yeasts, such as α-galactomannose and β-(1 → 3) glucans (Benito et al. 2019).
Clonal diversity within S. pombe species
Most studies related to S. pombe have shown high strain variability in several metabolic parameters. Benito et al. (2012) observed differences in five strain parameters, including acetic acid production (variation from 0.8 to 1.1 g/L), malic acid degradation (variation from 50 to 100%), pyruvic acid production (variation from 200 to 400 mg/L), and color intensity (variation from 7.5 to 8.5). Another study, performed with more than 100 strains isolated from Spain (Benito et al. 2014), showed differences in parameters such as residual sugars, malic acid degradation, and acetic acid after the AF of a diluted concentrated must with 220 g/L initial sugar and enriched to 4 g/L malic acid. The concentration of residual sugars varied from 0 to 6.5 g/L, while 66% of the studied population had between 0 and 0.5 g/L. The malic acid degradation varied from 68 to 100%, with 66% of the population exhibiting between 88 and 100%. The acetic acid production varied from 0.3 to 1.5 g/L, and 66% of the population produced between 0.65 and 0.9 g/L. Further selection over 75 strains from England and China (Benito et al. 2016b) showed additional differences of approximately 50% in acetaldehyde production or approximately 70% in ethyl acetate production. Studies that report a high genomic phenotypic diversity of S. pombe (Jeffares et al. 2015) similar to that previously described for S. cerevisiae support the high strain variability observed in several fermentation parameters. Table 1 summarizes the results of several studies that compare the fermentation performance of several Schizosaccharomyces strains.
Isolation and detection
Schizosaccharomyces species are rarely isolated from fruit, grape juice, wine, beer, or other fermented beverages. According to Deák (2008), the genus Schizosaccharomyces does not belong to the 20 yeast genera frequently present in food or derivate products. The estimated probability of isolating one strain of Schizosaccharomyces is below 1% (Fig. 4). Its relatively low presence in nature is due to the presence of competitor microorganisms that are able to colonize the different media faster, such as S. cerevisiae or other non-Saccharomyces yeasts, bacteria, or fungi. For that reason, it is almost impossible to isolate Schizosaccharomyces strains following conventional microbiological procedures based on generic culture media. This difficulty explains the lack of commercial strains in the microbiological market, although the International Organization of Wine and Vine has recommended its use to deacidify wines since 1997 (Resolution OENO/MICRO/97/75/Stage seven). Nevertheless, its incidence is slightly higher in matrixes with high osmotic pressure such as dried fruit, honey, or concentrated grape juices, where Schizosaccharomyces shows a competitive advantage due to its high resistance to osmotic pressure, than in other matrixes.
Simplified model of the frequencies (%) of Schizosaccharomyces yeast genera found in foods by Deák (2008). a All foods. b Fruits, beverages, wine, and beer. c Low-water activity (aw) products. The incidence of the genus Schizosaccharomyces is shown with respect to that of the other most frequent genera. Sacch, Saccharomyces; Zyg, Zygosaccharomyces; Lach, Lachancea; Scdes, Saccharomycodes; Rho, Rhodotorula; Tor, Torulaspora; Hsp, Hanseniaspora; Dek, Dekkera; Cry, Cryptococcus; Pich, Pichia; Cand, Candida
Recent studies show that it is possible to increase the probability of isolating Schizosaccharomyces strains by up to 60% using specific selective media (Benito et al. 2018). These media are based on the resistance of Schizosaccharomyces strains to the antibiotic cycloheximide at concentrations over 100 mg/L, which is tolerated by only five yeast genera (Benito et al. 2018). Additional selective factors used to inhibit the development of the yeast genera resistant to cycloheximide other than Schizosaccharomyces are benzoic acid (over 200 mg/L) and sugar (over 400 g/L). The selective media are also composed of some differential factors that allow us to distinguish Schizosaccharomyces strains among possible false positives. The main differential parameters are bromocresol blue and malic acid. Bromocresol blue stains Schizosaccharomyces colonies a different specific color than other yeasts. Malic acid degradation easily indicates changes in the pH that allow easy detection of the presence of Schizosaccharomyces. The authors also recommend the use of chloramphenicol to inhibit the presence of bacteria and working in liquid media to avoid fungal contamination. Through isolation methodologies based on these selection media, large collections of Schizosaccharomyces strains have been isolated in different countries in recent years (Benito et al. 2014, 2016b). These methodologies allow the isolation of great numbers of Schizosaccharomyces strains that are used in selection for the development of future commercial strains.
Impact of S. pombe on different wine fermentation parameters
Malic acid, titrable acidity, and pH
The main industrial application of Schizosaccharomyces is its unique ability to metabolize malic acid into ethanol, which reduces the titrable acidity of wine and other alcoholic beverages with high contents of malic acid (Benito et al. 2013; Minnaar et al. (2017). Each molecule of malic acid is transformed into one molecule of alcohol and two molecules of CO2 (Fig. 3). First, malic acid is decarboxylated into pyruvic acid through the action of the enzyme malic acid decarboxylase and Mn2+/Mn3+ ions. Later, pyruvic acid follows the AF pathway, being decarboxylated to acetaldehyde and later reduced to ethanol. Under anaerobic conditions, the degradation of 2.33 g/L malic acid generates 0.1% v/v alcohol (Taillandier and Strehaiano 1991). Although the most preferred microorganisms in the fermentation industry that metabolize malic acid are lactic bacterial strains, the Schizosaccharomyces deacidification effect is more powerful than the action of these organisms since the lactic acid metabolite is not the final product. The main technological use of this effect was reducing the acidity of grape juices from cool viticulture regions, where high malic acid concentrations (over 5 g/L) are responsible for the production of unbalanced wines that are usually considered too acidic for average consumers. Some authors report malic acid reductions of approximately 5 g/L in grape white wine fermented by pure and combined fermentations using S. pombe and S. cerevisiae (Benito et al. 2013). The total conversion of malic acid into ethanol decreases total titrable acidity by approximately 4 g/L and increases the final pH by approximately 0.4 (Benito et al. 2013). Wines containing malic acid were considered smoother and less acidic in the sensorial evaluation after this treatment. Nevertheless, in recent years, the use of Schizosaccharomyces has become popular in warm viticulture areas, where the levels of malic acid are low, and this yeast is used to avoid performing MLFs with high risks of undesirable deviations, such as increases in acetic acid or biogenic amine content. The use of this deacidification ability is also common in other fermentation industries, with raw materials containing higher concentrations of malic acid than grapes. Some of these raw materials are Malus pumila fruit (Satora et al. 2018), Dovyalis caffra fruit (Minnaar et al. (2017), and Vaccinium myrtillus fruit (Liu et al. 2018, 2019).
Gluconic acid
Gluconic acid is a metabolite produced from glucose oxidation through the action of the oxidase enzyme laccase during the metabolism of spoilage fungi such as Botrytis cinerea or acetic acid bacteria from the genera Acetobacter and Gluconacetobacter on fresh grapes before harvest (Peinado et al. 2004, 2007). Gluconic acid is a common objective indicator of the degree of rottenness in grapes. Concentrations of gluconic acid over 1 g/L indicate the presence of Botrytis cinerea, while concentrations over 2 g/L can be mainly the result of additional acetic acid bacterial growth (Couto et al. 2003; Peinado et al. 2004). Grape juices with an initial content over 0.5 g/L are usually disqualified for making quality wines (Peinado et al. 2009). Gluconic acid may negatively influence wine stability. From a microbiological point of view, microorganisms such as lactic bacteria can easily metabolize gluconic acid to highly unusual and undesirable concentrations of lactic acid and volatile acidity (Peinado et al. 2007). From a chemical point of view, gluconic acid binds SO2 very easily and reduces its positive effects against oxidization and undesirable microbial growth (Peinado et al. 2007). Specific strains and clones of Schizosaccharomyces can reduce approximately 91% of the gluconic acid from an initial concentration of 2.5 g/L, avoiding the negative effects from that molecule on wine (Peinado et al. 2007). This biotechnology is a very promising resource for wet regions, where undesirable fungal attacks are common. It can also be very helpful in the production of noble rot wines, where gluconic character is common. Gluconic acid decreases the quality of final products in specific fermentation industries such as that producing “Palo Cortado” wine. In such industries, the use of specific strains to remove undesirable gluconic acid would be a remarkable solution (Palacios et al. 2018).
Acetic acid
The main disadvantage of using Schizosaccharomyces in fermentative industries such as winemaking is that its strains tend to generate high levels of acetic acid. When the concentration of that undesirable molecule is over 0.8 g/L, vinegar character dominates the flavor of the wine, generating one of the most serious faults in wine sensory perception (Lambrechts and Pretorius 2000). Mixed and sequential inoculations with selected commercial S. cerevisiae strains were the first solutions for that problem (Benito et al. 2013). These methodologies achieved the main objective, malic acid deacidification, while avoiding increases in acetic acid concentration over the fault threshold. In those scenarios, S. pombe consumed malic acid during the first stage of AF, while S. cerevisiae completed AF, moderating the production of acetic acid. Recent studies started to combine Schizosaccharomyces yeasts with other non-Saccharomyces yeasts that produce low levels of acetic acid, such as L. thermotolerans or T. delbrueckii, with similar results (Benito et al. 2015; Liu et al. 2018). Another initial option for the first generation of commercialized S. pombe strains was the use of S. pombe inside alginate cells, which allows its removal once malic acid deacidification was achieved (Silva et al. 2003).
Later, research trends were based on selecting Schizosaccharomyces yeast strains that produce moderate to low levels of acetic acid (Benito et al. 2014, 2016b). The most recent studies regarding Schizosaccharomyces and acetic acid production reported final acetic acid concentrations that varied from 0.07 g/L (Du Plessis et al. 2017a) to over 1 g/L (Mylona et al. 2016; Miljić et al. 2017). According to the selection processes, approximately 5% of isolated S. pombe strains can produce wines with final acetic acid concentrations below 0.3 g/L (Benito et al. 2014, 2016b). Although most of the Schizosaccharomyces strains tend to produce levels over 0.65 g/L, it is common to isolate strains that produce concentrations over 1 g/L. These levels are not acceptable for quality young wines. These results agree with the high variability of parameters such as volatile acidity, SO2 resistance, H2S production, fermentation power, polysaccharide release, higher alcohols, and ester production in other non-Saccharomyces species such as Torulaspora delbrueckii or Lachancea thermotolerans (Comitini et al. 2011; Du Plessis et al. 2017a; Escribano et al. 2018). These results indicate how important it is to select the most appropriate Schizosaccharomyces strains before their use on an industrial scale.
The application of a very promising new technology named fed-batch fermentation with S. pombe (Roca-Domènech et al. 2018) appears to be a reliable option to reduce the acetic acid production for any nonselected strain of S. pombe. The technique consists of reducing the sugar-induced osmotic stress that S. pombe withstands during AF (Roca-Domènech et al. 2018). This technique allows the production of wines with no detectable acetic acid, while the corresponding regular control exhibits a final concentration of 0.66 g/L after AF. The acetic acid reduction is close to 100%. The total consumption of sugar and malic acid is not affected, while acetaldehyde and glycerol production is additionally reduced by approximately 50% and approximately 13%, respectively (Roca-Domènech et al. 2018).
Ethanol
Although several non-Saccharomyces yeasts show positive effects on wine quality, the main drawback is that most of them are not able to ferment at ethanol concentrations greater than 4% (v/v). Only the genera Torulaspora and Lachancea can ferment at ethanol concentrations up to 9% (v/v). Nevertheless, Schizosaccharomyces can ferment at concentrations similar to those reported for Saccharomyces: 15% (Suárez-Lepe et al. 2012), 11.47% (v/v) (Roca-Domènech et al. 2018), 10.13% (v/v) (Du Plessis et al. 2017a), 13.88% (v/v) (Del Fresno et al. 2017), 12.7% (v/v) (Chen et al. 2018), 11.42% (v/v) (Liu et al. 2018; Liu et al. 2019), and 13.55% (v/v) (Benito et al. 2019). All these studies show final residual sugar concentrations close to 0 g/L. This fact makes Schizosaccharomyces the only non-Saccharomyces yeast that can ferment fully by itself, without being combined with S. cerevisiae as in most wine industries. However, most studies report that Schizosaccharomyces fermentations sometimes have slower kinetics than the S. cerevisiae controls, but we must also take into account that in the case of red wine, the slower, second fermentation, MLF, is not needed, and the global industrial process is faster.
Magnesium supplementation is closely related to the ability of S. pombe to tolerate ethanol. The presence of Mg2+ at concentrations over 3.5 mM magnifies the decrease in the plasma membrane permeability of cells subjected to ethanol stress (Hu et al. 2003). With that supplementation, S. pombe strains can tolerate up to 20% (v/v) ethanol at 30 °C. This ability is very interesting since there is still a lack of knowledge regarding nutrient optimization for the Schizosaccharomyces. Thus, the optimization of this nutrient supplementation could make it easier for Schizosaccharomyces strains to ferment in grape juices derived from warm viticulture areas with high initial sugar content.
Pyruvic acid
Pyruvic acid is a metabolite produced during the production of ethanol and glycerol. Its maximum concentration is reached between 48 and 96 h of fermentation (Benito et al. 2012, 2013). This molecule is considered very important in red wine production since it results in the formation of vitisin A (Benito et al. 2017), one of the most stable anthocyanins responsible for color stability in aged wines, where the levels of most grape anthocyanins start to decrease. Schizosaccharomyces strains usually produce approximately five times more pyruvic acid than the most common fermentative genus, Saccharomyces. Nevertheless, a great variability of approximately 60% was reported at the strain level, with pyruvic acid concentrations reaching a maximum of approximately 0.5 g/L (Benito et al. 2012, 2014, 2016b). Schizosaccharomyces pombe and Schizosaccharomyces japonicus are the highest fermentative yeast producers of pyruvic acid during an AF process. Domizio et al. (2017) reported that S. pombe produces six times the maximum concentrations of pyruvic acid as a S. cerevisiae control and showed a great strain variability for S. pombe pyruvic acid production (maximum of 0.1 g/L to 0.43 g/L).
Glycerol
High-quality wines usually contain high glycerol concentrations that positively influence the sensorial mouth-feel properties, especially those related to smoothness. Several studies report Schizosaccharomyces as being a high glycerol producer, reaching glycerol concentrations over 10 g/L. Domizio et al. (2017) reported that S. japonicus produced concentrations ranging from 8.3 to 10.5 g/L, while S. pombe production varied from 9 to 11.4 g/L for the same initial fermentation trial. These results suggest a large strain variability of approximately 20% to consider in selection processes. These results and previous pyruvic acid studies suggest that Schizosaccharomyces possesses a more active glycerol-pyruvic pathway than other yeast species. Although another non-Saccharomyces species, named Candida stellata, is the highest producer of glycerol in winemaking (up to 14 g/L) (Jolly et al. 2014), the use of S. pombe remains a moderate alternative to increase the final glycerol concentrations in wine.
Acetaldehyde
Yeasts naturally produce acetaldehyde during AF, where it reaches a maximum concentration during the tumultuous phase. Acetaldehyde concentrations over 125 mg/L can negatively influence the aroma of wine, especially in the case of white wines (Mateos et al. 2006). At those levels, acetaldehyde can produce undesirable aromas such as those of green apples and fresh-cut grass especially in white wines (Aguera et al. 2018). In contrast, in red winemaking, moderately high levels below the fault aroma threshold contribute to increased production of stable colored anthocyanins such as vitisin B (Osborne et al. 2000).
Schizosaccharomyces usually produces higher concentrations of acetaldehyde than S. cerevisiae. Benito et al. (2013) described one strain of S. pombe that produces 40% more acetaldehyde in white wine than a S. cerevisiae control, although the maximum concentration was approximately 27 mg/L, which is considered below the threshold. Nevertheless, Mylona et al. (2016) observed strain-dependent variability, with concentrations ranging from 29 to 76 mg/L in red wines. Chen et al. (2018) reported acetaldehyde levels that were not significant and different from those of a S. cerevisiae control, depending on the specific S. pombe strain that fermented. In contrast, if we compare most red wines on the market with wines fermented by Schizosaccharomyces, the regular red wines always show lower levels of acetaldehyde produced during the fermentation process, mainly due to the decrease that takes place during the MLF (Benito et al. 2017). Most of the acetaldehyde produced by yeasts during the AF is consequently metabolized to ethanol and acetic acid by lactic bacteria during the MLF (Osborne et al. 2000). For this reason, studies working with controls that performed MLF showed final acetaldehyde concentrations for MLF repetitions varied from 1.79 to 2.58 mg/L (Benito et al. 2015, 2016a, 2019). Another method is the use of fed-batch technology that can reduce the final concentration of acetaldehyde by approximately 50% when using S. pombe (Roca-Domènech et al. 2018). This approach could be of great interest since it allows the deacidification of white musts from northern regions without increasing the levels of acetaldehyde.
Aroma compounds
Most studies report that S. pombe strains produce lower amounts of higher alcohols than their S. cerevisiae controls; however, some studies also showed large variations among the studied strains (Benito et al. 2013, 2016b; Mylona et al. 2016; Del Fresno et al. 2017; Chen et al. 2018). Benito et al. (2013) observed less production of higher alcohols such as isobutanol, 2-methyl-butanol, 3-methyl-butanol, and 2-phenyl-ethanol (45%, 30%, 25%, and 40%, respectively) in S. pombe strains than in their S. cerevisiae controls.
This effect is positive in the fermentation of grape varieties with strong varietal character based on terpenes or thiols (Ruiz et al. 2018). In those cases, higher alcohols do not mask the varietal aroma compounds. Benito et al. (2016b) observed that six studied S. pombe strains produced lower concentrations of higher alcohols than the S. cerevisiae control: approximately 30% to 60% less for 1-propanol, approximately 25% to 50% for isobutanol, approximately 25% to 50% for 2-methyl-butanol, approximately 35% to 60% for 3-methyl-butanol, and approximately 50% for hexanol. The effect did not take place for some strains for 1-butanol. In contrast, all S. cerevisiae controls produced higher concentrations of 2-phenyl-ethanol (approximately 5 mg/L) than S. pombe strains. Mylona et al. (2016) also observed a decrease for the main higher alcohols 2-methyl-butanol, 3-methyl-butanol, and isobutanol but with much higher differences (from 57 to 89%, from 53 to 77%, and from 75 to 93%, respectively) depending on the S. pombe strain. The level of higher alcohols other than isobutanol slightly increased during the MLF for wines fermented by S. cerevisiae and O. oeni. One strain of S. pombe showed 2-butanol production, while the others did not. Del Fresno et al. (2017) observed lower production in total higher alcohols in S. pombe, approximately 66% of the amount produced by the S. cerevisiae control. Chen et al. (2018) observed lower higher alcohol concentrations in S. pombe fermentations than in their S. cerevisiae controls: approximately 45% less in 1-propanol, between 25 and 50% less in isobutanol, 75% less in 3-methyl-butanol, and 50% less in 3-methyl-butanol.
A similar effect occurs for esters; most studies show that Schizosaccharomyces tends to produce lower concentrations than S. cerevisiae controls. The exception is ethyl acetate, where in several cases, its concentration is higher with S. pombe than in the S. cerevisiae controls, with large differences between the different S. pombe strains. This effect depends on the ability of each strain to produce acetic acid. Ethyl lactate is always present in higher concentrations in most regular red wines than the corresponding S. pombe wines, as the former usually perform MLF before the bottling process, increasing lactic acid concentrations (Benito et al. 2016a). Benito et al. (2013) observed decreased production for isoamyl acetate (approximately 20% and 15% less) in the case of phenyl-ethyl acetate for S. pombe compared to S. cerevisiae. Benito et al. (2016b) observed lower concentrations for isoamyl acetate and 2-phenyl-ethyl acetate (approximately 36% and 15% less, respectively) in S. pombe fermentations than in S. cerevisiae fermentations. The largest differences took place for ethyl acetate, where some S. pombe strains did not differ from the S. cerevisiae controls, while other strains produced approximately 75% more ethyl acetate than the controls. Mylona et al. (2016) reported similar ethyl acetate levels between S. pombe and S. cerevisiae controls after AF. Nevertheless, the level increased approximately 66% in the S. cerevisiae controls during MLF. Ethyl butyrate appeared in only S. cerevisiae controls after AF, at concentrations of approximately 2.2 mg/L, and disappeared after MLF. The ethyl lactate concentration was approximately seven times higher in the repetitions that performed MLF than in those without MLF. Del Fresno et al. (2017) observed lower total ester production in S. pombe strains (approximately 33% less) than in a S. cerevisiae control. In that work, S. pombe strains produced 0 mg/L isoamyl acetate, while the S. cerevisiae controls produced between 4.61 and 5.29 mg/L. Chen et al. (2018) observed the same effect.
S. pombe fermentations showed higher acetoin concentrations than the S. cerevisiae controls, varying from 10 to 167 mg/L depending on the studied strains (Del Fresno et al. 2017). Chen et al. (2018) observed 50% higher concentrations (approximately 8 mg/L higher) in S. pombe fermentations than in the S. cerevisiae control, but not so large differences between strains occurred.
Del Fresno et al. (2017) observed lower production of total volatiles (approximately 30% less) in S. pombe fermentations than in their S. cerevisiae control. This difference can be disadvantageous when fermenting neutral grape varieties, where the wine typicality is given by the fermentative aromas, in contrast to other varieties, such as Sauvignon blanc, Verdejo, Gewürztraminer, or Muscat, in which this characteristic can be considered very positive since the varietal aromas are the main quality factor and are not masked by other compounds.
Anthocyanins
Some studies regarding deacidification in white wines by Schizosaccharomyces reported increases in pyruvic acid concentrations, probably due to it having a more developed glycerol-pyruvic pathway than S. cerevisiae (Benito et al. 2013). Another study showed that four strains of S. pombe that produced between three and seven times more pyruvic acid than the S. cerevisiae control showed increases in red wine color intensity of approximately 12.5% to 18% after AF (Benito et al. 2012). These results suggested that S. pombe is able to generate highly stable anthocyanin that contains pyruvic acid in its composition, such as vitisin A. Later, specific chemical analyses of vitisin A indicated that S. pombe yeasts are able to generate two to four more times vitisin A than the S. cerevisiae controls, depending on the S. pombe strain and its pyruvic acid metabolism (Benito et al. 2017). Nevertheless, the most important influence of S. pombe on red wine color is related to the absence of MLF. Lactic bacterial activity degrades and absorbs some anthocyanins during the MLF (Benito et al. 2014, 2015; Mylona et al. 2016). Benito et al. (2015) reported a difference in color intensity of approximately 10% between the fermentation of S. pombe, whose color was more intense, and the S. cerevisiae control after AF. The difference increased to 23% when the S. cerevisiae control performed MLF. Mylona et al. (2016) observed important decreases in grape anthocyanin levels after MLF in the S. cerevisiae control; e.g., delphinidin-3-O-glucoside decreased approximately 60%, cyanidin-3-O-glucoside decreased approximately 100%, petunidin-3-O-glucoside decreased approximately 66%, peonidin-3-O-glucoside decreased approximately 75%, and malvidin-3-O-glucoside decreased approximately 66%, explaining the higher content of total anthocyanin in the S. pombe controls. Strickland et al. (2016) reported a lightened color due to a decrease in polymeric pigments by 10% to 30% after MLF. Burns and Osborne (2015) attributed this effect to lactic bacterial glycosidase enzymes. The decrease in acetaldehyde during MLF also reduces the formation of stable colored compounds such as vitisin B (Wang et al. 2018).
Amino acids and nitrogen metabolism
To date, only two scientific articles have studied amino acid release by S. pombe during AF (Benito et al. 2016a, 2016b). Benito et al. (2016b) studied the amino acid profiles of six different S. pombe strains and two S. cerevisiae controls after AF. S. pombe strains released more of each amino acid than the S. cerevisiae controls, with some exceptions, such as ornithine (in all cases) and alanine (depending on the studied strain). Previous research described S. pombe as less demanding in nitrogen nutrients since the final value of 20 mg/L in primary amino nitrogen in S. pombe is higher than the value in the S. cerevisiae controls after AF (Benito et al. 2012). Later, studies reported positive protease activity for S. pombe and negative protease activity for the S. cerevisiae controls (Du Plessis et al. 2017a), which justifies a greater amount of amino acid release during AF for the former species. Schizosaccharomyces fermentations showed higher histidine (from 11 to 23 mg/L), tyrosine (from 5 to 7 mg/L), and lysine (from 6 to 18 mg/L) levels than the S. cerevisiae controls (Benito et al. 2016a). These amino acids are the precursors of the corresponding biogenic amines (histamine, tyrosine, and cadaverine). Although there is no direct correlation between those precursors and the corresponding biogenic amines, lactic bacterial activity must take place to metabolize them. After S. pombe ferments, no sugars and no malic acid remain in the media. Consequently, lactic bacteria cannot use malic acid as a nutrient, and its development in wine becomes very difficult.
S. pombe fermentations showed higher final concentrations of threonine (50% higher), valine (two to three times higher), isoleucine (five times higher), and leucine (four to five times higher) than the S. cerevisiae controls. These differences explain the lower reported levels in some higher alcohols in S. pombe, such as 1-propanol, isobutanol, 2-methyl-butanol, or 3-methyl-butanol, which are produced from yeast metabolism through their corresponding amino acid precursors (Benito et al. 2016b; Ivit et al. 2018).
Biogenic amines
Biogenic amines are currently the main concern regarding food safety in wine industries. Histamine is the most dangerous biogenic amine in wine and produces symptoms such as headaches, respiratory distress, blushing, heart palpitation, hypertension or hypotension, tachycardia, itching, skin irritation, vomiting, and several allergenic disorders in consumers (Ladero et al. 2010; Martuscelli et al. 2013). Biogenic amines can be a great risk for people suffering from histamine intolerance, since even small amounts in combination with ethanol can cause serious health problems. Several lactic bacterial species produce biogenic amines. Oenococcus oeni is considered the most common that performs MLF for wine. Most red wines are obtained via MLF to achieve microbial stabilization to avoid undesirable bottle refermentations, which could generate turbidity or deterioration of the bottle cap. For that reason, the presence of histamine is common in red wines. The traditional methodology to avoid this food safety hazard is to use selected lactic bacterial strains that do not possess the decarboxylase enzymatic activity to metabolize histidine into histamine (Coton et al. 2010).
Although S. pombe does not produce higher amounts of biogenic amines in wines than S. cerevisiae, S. pombe has the important advantage of being able to remove all the sugar and malic acid in wine during the AF (Benito et al. 2015). Therefore, wines fermented by Schizosaccharomyces no longer contain any nutrients that could be used by any lactic bacteria to colonize the wine or to generate biogenic amines. Therefore, the use of Schizosaccharomyces strains appears to be an interesting alternative method for producing wines free of histamine for specific consumers and markets. The use of S. pombe biotechnology is starting to be considered an interesting alternative for the control of biogenic amines in wines from warm viticulture areas, where the high pH and sugar levels increase the capacity of lactic bacteria to produce biogenic amines.
Ochratoxin A
Ochratoxin A is a toxic compound of fungal origin (Petruzzi et al. 2014). It is considered hazardous to human health because of its nephrotoxic, neurotoxic, immunotoxic, mutagenic, and teratogenic properties (Petzinger and Ziegler 2000). The International Agency for Research of Cancer considers ochratoxin A a carcinogenic compound (http://www.inchem.org/documents/iarc/vol56/13-ochra.html). Ochratoxin A is mainly produced in winemaking due to the metabolism of fungal species such as Aspergillus carbonarius, Aspergillus fumigatus, Aspergillus niger, Aspergillus tubingensis, Aspergillus japonicus, or Penicillium tubingensis on grapes in the vineyards. The European Union set a legal limit of 2 μg/kg for this compound (Martínez-Rodríguez and Carrascosa 2009). The average value of ochratoxin A in European wines is approximately 0.19 μg/L (Martínez-Rodríguez and Carrascosa 2009). According to some data in the field, 1% of Spanish wines are over the legal limit (Gil-Serna et al. 2018). Cecchini et al. (2006) reported S. pombe as the most efficient yeast species among eight different studied species in the removal of ochratoxin A during a red must fermentation process. The studied S. pombe strain removed 70% of the initial ochratoxin A content. The initial level of ochratoxin A in the grape juice was over the European legal limit, and the final concentration was below.
There are some corrective measures to reduce ochratoxin A (Petruzzi et al. 2014), such as fining with activated carbon or tangential filtrations at 0.45 μm, whose reduction effectiveness are 32% and 80%, respectively. S. pombe fermentation is an interesting preventive measure that can avoid the secondary effects of those corrective measures for the aroma and color profile of wines.
Ethyl carbamate precursors
Ethyl carbamate, along with biogenic amines and ochratoxin A, is considered one of the main food safety issues in the wine industry. This molecule is carcinogenic and has a legal limit in several countries and food products. It is the most dangerous compound than can be naturally produced in wine. The compound can be produced by lactic bacterial metabolism (Mira de Orduña et al. 2000) or through a chemical reaction between urea and ethanol (Monteiro et al. 1989). Currently, its control measures during wine production are mainly based on two strategies: the first is based on avoiding MLF. The second is based on using commercial synthetized urease enzymes (http://www.oiv.int/public/medias/3542/e-code-ii-3411.pdf) that remove the ethyl carbamate precursor (urea) from the wine. The main problem of that strategy is that the cost of the commercial urease increases the final value of the wine. The three Schizosaccharomyces species possess natural urease enzymatic activity (Deák 2008). That enzymatic activity is observed in only 18 other yeast species, which belong to the genera Bulleromyces, Cryptococcus, Filobasidium, Leucosporidium, Moniliella, Rhodotorula, Trichosporon, and Cystofilobasidium (Deák 2008). However, none of those genera is able to complete AF. This phenomenon justifies why several studies report low final levels of urea (approximately 0.1 mg/L) in wines fermented by S. pombe, while the S. cerevisiae controls show final levels of several milligrams/liter to up to 30 times higher (Benito et al. 2015). S. pombe is a cheap alternative for producing wines stabilized against ethyl carbamate formation to be exported to countries with legal limits for ethyl carbamate.
Polysaccharides and galactomannoproteins
Polysaccharides and mannoproteins influence the sensory perception of wines by increasing quality characteristics related to the wine structure (Domizio et al. 2014). They reduce the wine astringency, improving the global balance in the mouth. Traditionally, selected S. cerevisiae strains were used to increase the concentrations of polysaccharides and mannoproteins. Schizosaccharomyces possesses higher contents of α-galactomannose and β-glucans in its cells than S. cerevisiae. Recent studies have proven that S. pombe and S. japonicus can be successfully used to increase the polysaccharide content in wines (Domizio et al. 2017, 2018; Benito et al. 2019). Domizio et al. (2018) reported that S. japonicus produced five times more polysaccharide than its S. cerevisiae control. Benito et al. (2019) reported that approximately 2.5 more hydrolyzed mannose was released from polysaccharides in wines fermented by S. pombe than in wines fermented by S. cerevisiae controls.
Sensory influence in wine
According to most studies, the main influence of Schizosaccharomyces yeasts, compared to the Saccharomyces controls, on sensory analysis is the strong reduction in the acidity sensory descriptor (Fig. 5) (Benito et al. 2013). Most of those studies explain this influence from a chemical point of view due to the disappearance of several grams of malic acid, which increases the pH by up to 0.5 unit. This phenomenon seems beneficial when this biotechnology is applied in the fermentation of musts from cold areas, where malic acid levels are extremely high and result in regular consumers commonly considering the wine too acidic. Some authors observed this effect in grape juices with an initial malic acid concentration of 6 g/L (Benito et al. 2013). However, other studies have also investigated the application of these microorganisms in warm areas with different objectives than deacidification, such as generating wines free of biogenic amines or ethyl carbamate. Some studies describe the lack of acidity as an undesirable effect produced by Schizosaccharomyces fermentations in grape juices from warm viticulture areas (Benito et al. 2015). The main negative property described in most Schizosaccharomyces fermentations is a strong vinegar character (Benito et al. 2013). That sensory perception is chemically explained by the high acetic acid levels (over 0.8 g/L) observed in several studies related to Schizosaccharomyces fermentations (Mylona et al. 2016; Miljić et al. 2017; Minnaar et al. (2017). In these cases, the Schizosaccharomyces fermentations are generally considered worse than the Saccharomyces controls. Nevertheless, in fermentations combining Schizosaccharomyces with S. cerevisiae (Benito et al. 2013) or L. thermotolerans (Benito et al. 2015), this effect has not been observed, and on several occasions, the produced wine was the preferred wine by panelists. The undesirable effect can also be avoided using selected S. pombe strains with a lower capacity for producing acetic acid (Benito et al. 2016b). Figure 6 summarizes the influences of S. pombe on the sensory perception of fermented products.
S. pombe and L. thermotolerans combined use
The application of Schizosaccharomyces in Atlantic cold viticulture regions is very useful for the deacidification of wines with malic acid contents over 5 g/L, which are usually considered sharp in regular markets. The purpose of this application in warm viticulture regions is to avoid MLF collateral effects such as high levels of acetic acid, biogenic amines, or ethyl carbonate in wines with a high pH (close to 4). Most of the time, the main inconvenience of fermentations in such specific cases is their lack of acidity (Fig. 5). In this case, although the Schizosaccharomyces-produced wines are safer from a food safety point of view, regular consumers consider them unbalanced.
Thus, to avoid this undesirable effect, one alternative is to combine S. pombe with L. thermotolerans to compensate for the side effect of lost acidity. L. thermotolerans acidifies through the production of lactic acid during AF (Benito 2018b; Porter et al. 2019). This modern biotechnology (Benito et al. 2015, 2019) has some advantages over MLF in warm viticulture areas, whose samples have high sugar content and a high pH and the risk of deviation during AF and MLF is thus high. The main advantages are making final wines with a moderate pH (approximately 3.5 to 3.6) due to the ability of L. thermotolerans to generate lactic acid from initial grape juices, which have a pH close to 4.00 (Benito et al. 2015; Porter et al. 2019). Moreover, microbiologically speaking, to directly obtain stabilized wines after the AF, due to the complete consumption of sugar and malic acid nutrients by Schizosaccharomyces, lactic bacteria cannot survive. Moreover, this approach can also be used to obtain wines free of biogenic amines and ethyl carbamate and with low levels in ochratoxin A. Although the combined fermentation with L. thermotolerans and S. pombe is slower than the fermentation of the controls using commercial S. cerevisiae for 2 days to 4 days (Benito et al. 2015, 2016a). The global process of microbial stabilization after the sequential use of S. cerevisiae and Oenococcus oeni is much slower (approximately 21 additional days in the best scenario) under industrial conditions than that with the new biotechnology. The aroma profile seems to improve due to the decreased production of higher alcohols from the selected S. pombe strains and L. thermotolerans, while L. thermotolerans usually increases the level of fruity esters (Benito et al. 2016a). The color intensity is improved by S. cerevisiae and O. oeni compared to controls due to the production of highly stable colored compounds such as vitisin A from S. pombe and due to the decrease in pH by L. thermotolerans. This low pH increases the color intensity of anthocyanins such as flavylium ions (Benito et al. 2017). The problems of managing sequential fermentations under industry conditions have oriented researchers to produce hybrids between S. pombe and L. thermotolerans (Benito 2018b) to facilitate the industrial use of a single strain without any need for a sequential protocol. For this specific case, the hybridization process seems easy due to the genetic proximity of those two species (Malpertuy et al. 2000). More recent studies also reported improvements in color intensity changes due to increases in levels of polymeric anthocyanins, although there was no control trial that performed MLF, after which the differences could have been even larger (Escott et al. 2018). The main increases in color or anthocyanin contents appear to be related to avoiding MLF (Benito et al. 2017). Benito et al. (2019) also observed improvements in polysaccharide concentrations with respect to the S. cerevisiae and O. oeni sequential control, although the fermentation performed by S. pombe alone showed higher final anthocyanin concentrations than the sequential fermentation using S. pombe and L. thermotolerans. The high production of biogenic amine precursors released by S. pombe such as histidine is reduced to levels similar to those of the S. cerevisiae control with the combination of S. pombe and L. thermotolerans (Benito et al. 2016a).
S. japonicus applications
Most of the studies regarding the application of Schizosaccharomyces to fermentation industries used mostly S. pombe. However, recent studies have also used other species such as Schizosaccharomyces japonicus (Domizio et al. 2018). For instance, in a recent study, the reduction in malic acidity during maloalcoholic fermentation varied from 71% in free fermentation to 82% for immobilized fermentation in alginate cells. The species seemed to ferment slower than S. cerevisiae since after 14 days of AF, S. japonicus still showed residual sugar concentrations over 10 g/L from an initial concentration of 24% (w/v) reducing sugars (Domizio et al. 2018). Nevertheless, the combined fermentation with S. cerevisiae completely fermented all the sugars in a similar time as S. cerevisiae alone. However, the combined fermentations deacidified less malic acid than the pure S. japonicus controls (approximately 50%). The results implied that the species possesses a preference for glucose instead of fructose. Fermentations involving S. japonicus showed approximately 50% higher levels in glycerol than the S. cerevisiae control. The main negative effect for basic enological parameters was a volatile acidity of 0.76 g/L, which is a similar problem to that previously described for S. pombe. Nevertheless, fermentation in alginate-immobilized cells reduced the undesirable effect by approximately 65%. The immobilized system showed other important advantages, such as reducing the final ethanol concentration to values from 2.38 to 3.13% (v/v), depending on using the combined strategy with S. cerevisiae and the immobilized or free fermentation option. This effect could be of great interest in reducing the ethanol level in musts with high initial sugar content (Contreras et al. 2014, 2015; Varela et al. 2015). S. japonicus shows a similar effect in the production of higher alcohols as S. pombe, as the former species produced approximately 45% to 60% lower higher alcohol concentrations than the S. cerevisiae control, depending on the type of fermentation (Domizio et al. 2018). Nevertheless, the studied strain produced slightly higher 2-phenyl-ethanol concentrations than the S. cerevisiae control, approximately 5 mg/L. No previous studies have reported such an effect for any S. pombe strain. S. japonicus produces from 55 to 66 times more 3-hydroxy-2-butanone than the S. cerevisiae control. S. japonicus produced higher amounts of ethyl acetate than the S. cerevisiae control, and this effect is similar to that reported for S. pombe, which depends on acetic acid production. Nevertheless, S. japonicus produced higher concentrations of other esters, such as isoamyl acetate, hexyl acetate, and 2-phenyl-ethyl acetate, which are not produced by S. pombe. Regarding fatty acids, S. japonicus produces approximately three times higher amounts of short-chain fatty acids, such as isobutyric and isovaleric acids, than the S. cerevisiae control for free fermentation and approximately two times higher for immobilized fermentation. Nevertheless, regarding medium-chain fatty acids, S. japonicus produced approximately three times less octanoic acid than the S. cerevisiae control and similar levels of decanoic acid. According to Domizio et al. (2018), the main advantages of S. japonicus in comparison with S. cerevisiae are the increase in polysaccharide release (up to five times greater amounts) and the protection against protein haze due to the protective polysaccharide effect.
S. pombe compared to other non-Saccharomyces species
During recent years, there have been many marketing campaigns worldwide to promote the benefits of non-Saccharomyces species in enological applications. This fact made it very difficult to select the most appropriate species related to each specific case and application. Therefore, today, comparing Schizosaccharomyces properties to the properties of not only the Saccharomyces genus but also other non-Saccharomyces species that are becoming popular in the winemaking industry is essential. Several scientific studies have compared the effects of different non-Saccharomyces microorganisms, but very few studies have compared them to the effects of Schizosaccharomyces species.
Du Plessis et al. (2017a) performed the most extensive comparison between Schizosaccharomyces pombe, other non-Saccharomyces species (Candida stellata, Candida zemplinina, Hanseniaspora uvarum, Lachancea thermotolerans, Metschnikowia pulcherrima, and Torulaspora delbrueckii), and Saccharomyces controls. According to those results, S. pombe degrades the most malic acid (approximately 80%), followed by C. zemplinina, which showed a maximum degradation of approximately 50%. T. delbrueckii showed a maximum of approximately 31%, while the other species showed lower malic acid degradation levels that varied from 7 to 25%. These results show that the genus Schizosaccharomyces remains the most reliable option among yeasts to deacidify products with high contents of malic acid.
Regarding extracellular enzymes not produced by the S. cerevisiae controls, such as protease, pectinase, and β-glucosidase, S. pombe showed positive protease activity and negative pectinase and β-glucosidase activities. C. stellata showed the same enzymatic activity as S. pombe. H. uvarum and M. pulcherrima showed β-glucosidase activity. C. zemplinina, L. thermotolerans, and T. delbrueckii were negative for all the studied enzymatic activities, as were the S. cerevisiae controls. The same study (Du Plessis et al. 2017a) also tested several AFs in which different parameters were compared between the different studied species. S. pombe showed slightly slower kinetic fermentation than S. cerevisiae, L. thermotolerans, and T. delbrueckii, although S. pombe reached the highest final ethanol concentration of 10.13%, which indicated a higher yield in ethanol production. The species C. stellata, C. zemplinina, H. uvarum, and M. pulcherrima required fermentation times up to 180 days, which made their application at the industrial level impossible without being combined with a more fermentative yeast species. S. pombe, T. delbrueckii, and L. thermotolerans showed the lowest final volatile acidity (approximately 0.1 g/L). C. stellata, C. zemplinina, S. cerevisiae, and M. pulcherrima showed final volatile acidity that varied from 0.3 to 0.5 g/L, while H. uvarum showed the highest values (up to 0.8 g/L). S. pombe showed the lowest total acidity (1.82 g/L), due to malic acid degradation. C. zemplinina, T. delbrueckii, and L. thermotolerans showed total acidities from 3 to 3.3 g/L, while S. cerevisiae, C. stellata, H. uvarum, and M. pulcherrima showed higher values that varied from 3.3 to 3.8 g/L.
Chen et al. (2018) compared one selected Schizosaccharomyces strain and one nonselected strain to a S. cerevisiae control and to sequential fermentations with Saccharomyces and commercial strains of M. pulcherrima, T. delbrueckii, and L. thermotolerans. S. pombe showed the lowest total acidity, varying from 3.9 to 4.3 g/L; M. pulcherrima and T. delbrueckii showed 5.3 g/L; and L. thermotolerans showed the highest value (6.4 g/L) due to its acidification activity. Those total acidity concentrations generated changes in the pH, with pH values ranging from 3.8 for S. pombe to 3.4 for L. thermotolerans and intermediate values for M. pulcherrima and L. thermotolerans. Final concentrations of glucose and fructose did not show a significant difference between the fermentations of Schizosaccharomyces strains and the sequential fermentations involving the other non-Saccharomyces strains. T. delbrueckii sequential fermentation produced the lowest acetic acid concentration (0.06 g/L), while the fermentations with the selected S. pombe strain, the S. cerevisiae control, and L. thermotolerans and M. pulcherrima did not show significant differences in acetic acid levels, with final concentrations of approximately 0.17 g/L. However, the nonselected S. pombe strain produced the highest acetic acid concentration (0.7 g/L). Regarding the final total polyphenol index, S. pombe fermentations showed the highest final concentrations (by approximately 12 units). However, sequential fermentations involving M. pulcherrima and L. thermotolerans showed approximately 40 mg/L higher final total anthocyanin concentrations than fermentations with S. pombe, while T. delbrueckii showed similar levels to one of the S. pombe strains. The two strains of S. pombe differed in their final anthocyanin concentration by 20 mg/L. S. pombe fermentations showed final color intensity values approximately 25% higher than the other non-Saccharomyces sequential fermentations. It is also worth considering that the other non-Saccharomyces sequential fermentations did not perform MLF, which is needed to microbiologically stabilize the wine, after which the differences in color intensity could have increased. These fermentations exhibited final malic acid concentrations of approximately 1.7 g/L, while S. pombe strains consumed all the malic acid. M. pulcherrima fermentations showed the lowest ethanol index, and L. thermotolerans and T. delbrueckii showed an ethanol index approximately twice as large, while one strain of S. pombe had an ethanol index five times greater, and the other, ten times greater. This fact indicates a higher level of polysaccharide release by S. pombe than by the other species during the AF. These results show important differences in some parameters not only among the studied species but also between the different S. pombe strains. Table 2 summarizes and compares the primary fermentation parameters of S. pombe and the other non-Saccharomyces species in winemaking.
S. pombe compared to lactic acid bacteria
Although many studies have been performed on the effects of Schizosaccharomyces during AF, only very few of them performed MLF as controls (Benito et al. 2015, 2016a, 2017, 2019; 2016). The vast majority of commercial red wines undergo MLF before bottling to avoid future uncontrolled refermentations inside the bottle, making it very difficult to establish comparisons with S. cerevisiae controls that performed only AF.
The first study that compared an S. pombe fermentation and a sequential fermentation between S. cerevisiae and O. oeni (Benito et al. 2015) reported no significant differences in final pH, although the initial malic acid concentration was 0.96 g/L and was completely consumed in both cases. The O. oeni control generated 0.54 g/L lactic acid by the end of MLF. Citric acid disappeared during MLF due to consumption by O. oeni, and an increase in acetic acid was consequently observed. The main differences took place in color intensity, which decreased during the MLF (by 12%), and the final difference between the color intensities in samples with S. pombe fermentation and those with the sequential fermentation between S. cerevisiae and O. oeni was 16%. In the same study, although all the trials showed lower biogenic amine levels than recommended, the repetitions that performed MLF showed higher levels in histamine and tyramine (approximately 1 mg/L and 0.1 mg/L, respectively). The AF of S. pombe required 12 days, while that of S. cerevisiae required 10 days, and the later process, MLF, required 14 additional days at 18 °C.
Mylona et al. (2016) reported that acetic acid increased by the end of the MLF by 0.09 g/L and that the citric acid consumption in this case was only approximately 50%. Two strains of S. pombe showed higher acetic acid concentrations than the MLF control (approximately 0.4 g/L), while two selected strains showed lower concentrations, approximately 0.2 g/L. All S. pombe fermentations showed a pH approximately 0.2 unit higher than the other fermentations. The initial level of malic acid was 3.76 g/L, and after MLF, the concentration of lactic acid was 1.79 g/L. The study reported a great decrease in pyruvic acid and acetaldehyde content (approximately 84% and 96%, respectively) after MLF. The S. pombe fermentations showed higher pyruvic acid levels (from 155 to 316 mg/L, depending on the strain) than the other microorganisms. S. pombe controls showed higher vitisin A and B concentrations than other microorganisms, with levels varying from 50 to 125% higher depending on the S. pombe strain. The S. pombe strains also showed higher acetaldehyde levels that varied from 24 to 67 mg/L. S. pombe fermentation resulted in a 25% higher color intensity. In this study, the total anthocyanin concentration decreased more than 50% during the MLF.
Benito et al. (2016a) observed an increase in the urea concentration of approximately 50% during MLF. The final urea concentration was 95% higher in the MLF trial than in a S. pombe fermentation. The largest differences in volatile compounds between MLF and S. pombe fermentations occurred for ethyl lactate and diacetyl, whose concentrations were approximately 20 mg/L and 10 mg/L higher in the former case, respectively. During the MLF, slight increases in asparagine, serine, glycine, threonine, and ornithine contents occurred, while isoleucine and leucine concentrations slightly decreased. Significant increases in alanine of approximately 25% and 50% in tryptophan took place during the MLF. Histidine and arginine concentrations decreased 14% and 18%, respectively. Nevertheless, the final histidine and arginine contents were 29% and 15% higher in the S. pombe fermentations than in the MLF. The main differences that occurred over the course of the S. pombe fermentations were the production of 50% higher contents of threonine, tyrosine, phenylalanine, and lysine; approximately 25% more alanine; four times as much valine and tryptophan; and three times as much isoleucine and leucine. Ornithine and alanine levels decreased approximately 50% and 25%, respectively. The ethanol index, which indicates the level of polymerization between tannins and polysaccharides, was 30% lower in the case of MLF than in the S. pombe fermentations.
Mylona et al. (2016) observed that MLF trials showed approximately 66% higher diacetyl concentrations and approximately seven times higher ethyl acetate concentrations than the S. pombe fermentations. Those were the most significant differences after the MLF. During the MLF, slight increases occurred in the concentrations of higher alcohols, such as 2-methyl-1-butanol, 3-methyl-1-butanol, 2,3-butanediol, and 2-phenyl-ethanol, while 1-butanol and isobutanol levels slightly decreased. Nevertheless, the results showed slightly higher alcohol concentrations for S. pombe. During the MLF, the acetoin concentration increased by approximately 50%. However, all S. pombe fermentations showed higher levels of acetoin than the MLF control, varying from two to four times higher depending on the S. pombe strain. The S. pombe fermentation length ranged from 10 to 14 days depending on the strain, while the S. cerevisiae control performed AF in 7 days, and the MLF required 21 additional days. Table 3 summarizes the main differences between S. pombe and O. oeni fermentations.
When performing a S. cerevisiae trial after MLF, Benito et al. (2017) observed decreases of approximately 30% in the total anthocyanins and approximately 22% in the color intensity. In addition, the Schizosaccharomyces trial after AF showed a 35% increase in total anthocyanins compared with the MLF control and an approximately 30% higher color intensity. The Schizosaccharomyces fermentations showed much higher concentrations of the grape anthocyanins (4 mg/L, 0.36 mg/L, 5 mg/L, 3 mg/L, and 28 mg/L higher for delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, petunidin-3-O-glucoside, peonidin-3-O-glucoside, and malvidin-3-O-glucoside, respectively) than the control performing MLF. This difference was explained by the glycosidase activity of lactic acid bacteria (Burns and Osborne 2015; Strickland et al. 2016) and their cellular absorption. S. pombe fermentations showed 3 mg/L and 1 mg/L higher concentrations of vitisin A and vitisin B than the MLF control, respectively, caused by the S. pombe producing higher levels of pyruvic acid and acetaldehyde than the S. cerevisiae and O. oeni species, while the acetaldehyde decrease during MLF did not occur in the case of S. pombe AF. S. pombe fermentation also resulted in higher concentrations of acetylated anthocyanins, with levels reaching up to 10 mg/L in the case of malvidin-3-O-(6″-acetylglucoside).
S. pombe applications in other industries
Ginger
Schizosaccharomyces is effective in health matters, such as decreasing the presence of hazardous chemical compounds such as biogenic amines, ochratoxin A, or ethyl carbamate. The most important advance related to Schizosaccharomyces and human health is the production of 6-paradol, mainly because this molecule is a powerful neuroprotective agent (Choi et al. 2018) that can be applied in treatments against neurodegenerative diseases such as Alzheimer’s disease. This advance facilitates the synthetization of that medical compound, making its production in pharmaceutical industries possible and reducing production costs relative to other industrial methods of synthesis (Choi et al. 2017). Currently, 6-paradol is being produced by ginger fermentation (Huh et al. 2018), although other potentially fermentative sources have to be studied due to the importance of the application of Schizosaccharomyces from an industry point of view and its potential role in improving the quality of life of many patients with Alzheimer’s disease and other neurodegenerative diseases.
Apple wine
Malus domestica wines are popularly consumed in countries that lack vineyards and where apples are the main fruit produced (Satora et al. 2018). The most common yeast species used for making these wines is S. cerevisiae (Satora et al. 2009). Nevertheless, as apple fruits usually contain high levels of malic acid that mainly vary from 6 to 16 g/L depending on the apple variety, the use of Schizosaccharomyces species is an interesting option for obtaining smoother fruit wines (Satora et al. 2018). Satora et al. (2018) reported deacidification in apple wines of approximately 50% total acidity using S. pombe. However, they also reported undesirable effects, such as the production of acetic acid at levels (0.5 g/L) higher than those of the S. cerevisiae control. That effect is mitigated in combined fermentation using S. cerevisiae, Saccharomyces paradoxus, and S. pombe, with the samples reaching a final acetic acid concentration similar to those of the S. cerevisiae controls.
Kei-apple fermentation
Kei-apple is the fruit of Dovyalis caffra L., which is native to South Africa. This fruit contains approximately 16 °Brix and 45 g/L malic acid (Minnaar et al. (2017), while the average malic acid content of a ‘Granny Smith’ apple is approximately 12 g/L (Violeta et al. 2010). Due to these contents, the kei-apples possess a very sour taste that is difficult for regular customers to appreciate, although they also contain high antioxidative activity due to the presence of several phenolic compounds (Minnaar et al. (2017). This fact makes the fruit the perfect substrate to be fermented by a strong malic acid deacidifier yeast such as Schizosaccharomyces. Minnaar et al. (2017) reported total deacidification of a kei-apple juice with an initial malic acid concentration of 45 g/L for a S. pombe control, while the corresponding S. cerevisiae control deacidified the initial juice by approximately 13%. The pH of the final fermentation was approximately 1 unit higher for the S. pombe fermentation than for the S. cerevisiae control, and the total tritable acidity was 70% less in the case of S. pombe. However, the final products exhibited some off-odors. These results are very promising, as if the biotechnology is optimized, a high antioxidative beverage free of off-odors and excessive sourness could become available in the market, finding possibilities for the kei-apple fruit from South Africa.
Sparkling wine
The main microbiological problem in sparkling beverage industries is related to the difficulty of the yeast performing the second fermentation inside the bottle. The second fermentation must take place with an initial alcohol content of approximately 11%, high pressures (over 6 bar), a lack of nutrients, a low pH due to high malic acid concentrations, and SO2 in the media. Although S. cerevisiae and Saccharomyces bayanus are the most common yeast species used for this purpose, S. pombe has been reported to show better adaptation to these specific circumstances (Ivit et al. 2018). S. pombe is an osmophilic yeast that can withstand higher pressures than S. cerevisiae, and its fermentation power is similar to that of S. cerevisiae and S. bayanus (up to 15% alcohol content). It possesses high malic dehydrogenase activity to reduce the acidity of early harvest musts and can tolerate SO2 concentrations up to 120 mg/L (Benito et al. 2016b). Additionally, its relatively high autolysis activity can increase the polysaccharide release (Domizio et al. 2017; Benito et al. 2019) and reduce the time of aging in bottles, resulting in smoother sparkling wine. Indeed, the higher production of stable anthocyanins such as vitisin A by S. pombe makes it ideal for the production of red sparkling wines (Ivit et al. 2018). Ivit et al. (2018) observed that the sparkling wines fermented by S. pombe showed approximately 80% less malic acid, 50% more pyruvic acid, approximately 20% more vitisin A, 70% more vitisin B, and approximately 14% greater color intensity than sparkling wines made with other microorganisms.
Bioethanol
Bioethanol is currently considered the most commonly used biofuel due to its contribution to lowering crude oil consumption and environmental contamination. It is produced from various types of raw materials. The main limitations for yeasts that perform fermentation in bioethanol production are high ethanol concentrations, high temperature, the fermentation of pentoses, and high osmotic stress and bacterial contamination. Schizosaccharomyces species can be interesting in the production of bioethanol from specific raw materials, such as molasses, with elevated osmotic pressures and fermentation at high temperatures (Bakhiet and Mahmoud 2015). Under such circumstances, the optimum production of bioethanol (23.51 mL) takes place for row molasses with 85.55 g/solids and a pH between 6 and 5 at 33 °C.
Bilberry
Bilberry, or Vaccinium myrtillus, is a fruit with many benefits for the food industry due to its deep blue color, attractive taste, varietal aroma, and healthy properties (Liu et al. 2018, 2019). This fruit is mostly commercialized in its fresh form or as jam or juice. Recent studies reported making some fermentation products from bilberry. However, one of the main problems in fermenting bilberry juice is its low nitrogen concentration (Liu et al. 2019). An ideal solution to this challenge would be using S. pombe, since it is a low consumer of nitrogen nutrients (Benito et al. 2012). Moreover, the low production of fermentative esters by S. pombe (Benito et al. 2016b) did not mask the nice varietal bilberry fruit character and maintained its typicality.
Bilberry fruit contains malic acid in its acid composition (which totals approximately 1.5 g/L), similar to most red grape juices. The first study that reported the use of S. pombe on bilberry juice observed that the studied strains of S. pombe consumed all the malic acid, increasing the influence of citric acid and quinic acid as the main acids remaining in bilberry fermentation. Nevertheless, the best improvement of bilberry fermentation quality by S. pombe, compared to the S. cerevisiae control, was its influence on the anthocyanin content of bilberry (Liu et al. 2018). One of the studies on S. pombe strains showed that they produced similar total monomeric anthocyanin, total vitisin, total hydroxycinnamic acid derivative, and total anthocyanin levels as the S. cerevisiae control. A different strain study showed notable improvements of approximately 23% in total monomeric anthocyanins, approximately 25% in total anthocyanins, approximately 44% in total vitisins, and approximately 20% in total vinyl phenol derivative anthocyanins. These effects notably improved the color of bilberry fermentation samples and, most importantly, emphasized the importance of selecting the most appropriate S. pombe strain to reach the objective. Other studied parameters indicated that some strains of S. pombe produce lower levels of acetic acid, as the studied S. pombe strains produced approximately 23% less acetic acid than the S. cerevisiae control. A similar effect took place for ethyl acetate. The final concentrations of acetaldehyde were higher in the S. pombe samples than in the S. cerevisiae control, varying from 26 to 67 mg/L higher than that of the S. cerevisiae control. The amount of pyruvic acid produced was also higher in fermentations performed by S. pombe, varying from 25 to 75% higher than that of the S. cerevisiae control. These behaviors increased the production of vitisin A and vitisin B in the S. pombe fermentations compared with the S. cerevisiae control. In contrast, the final production of total higher alcohols was lower for S. pombe fermentations (approximately 24 mg/L to 100 mg/L). Another interesting result was the glycerol content being approximately 2 g/L greater than the S. cerevisiae control level. A recent study showed strain-dependent differences in several aroma parameters (Liu et al. 2019). Both studied strains showed higher levels of acetoin than the S. cerevisiae control. This negative effect was compensated for in sequential fermentations with S. cerevisiae.
Plum wine
The use of S. pombe is an option for fermenting plum juice (Prunus domestica L.). Miljić et al. (2017) introduced a sequential fermentation between S. pombe and S. cerevisiae as a control. The other controls were single fermentations performed by different strains of S. cerevisiae and S. bayanus. The S. pombe fermentation resulted in the second best taste and flavor qualities among seven options; its score was 11.5, while the best score was 11.6 out of 12, which was achieved by a S. cerevisiae strain. S. pombe fermentation produced slightly higher (by approximately 0.2% to 0.5%) ethanol contents than the S. cerevisiae and S. bayanus controls. S. pombe fermentations showed higher final pH values than the controls (by approximately 0.2 to 0.31, depending on the S. cerevisiae and S. bayanus controls). The fermentation lasted approximately 4 days later than the S. cerevisiae and S. bayanus control fermentations. The final volatile acidity was from 0.48 to 0.69 g/L higher than that of the Saccharomyces controls, depending on the strains. S. pombe fermentations showed approximately 50% higher acetaldehyde concentrations. S. pombe fermentations produced lower concentrations of most higher alcohols, such as isobutanol, 3-methyl-1-butanol, 1-heptanol, and 1-octanol, than most of the S. cerevisiae and S. bayanus strains. The main differences occurred for 1-hexanol, which was present at concentrations two to four times lower in S. pombe, depending on the Saccharomyces strain. 1-Octanol concentrations ranged from 25 to 30% lower for S. pombe. One peculiarity not reported in the literature was the production of furfural in all trials (varying from 0.4 to 1.5 mg/L) except for the S. pombe trial, where it was not detected. The same trend was found for ethyl isovalerate. The differences in esters and terpenes were not as evident and varied depending on the Saccharomyces strains.
Water purification
The accumulation of heavy metals is a serious environmental problem in both the water and the soil. Schizosaccharomyces pombe is a low-cost and efficient alternative approach for removing copper. Although copper can be present in small concentrations, it is considered highly toxic. The use of Schizosaccharomyces pombe allows an approximately 73% reduction in copper ions in aqueous solutions, as reported in batch and column studies (Subhashini et al. 2011).
Future specific selection criteria for S. pombe strains
The main use of the Schizosaccharomyces genus in fermentation industries is to deacidify juices with high malic acid contents. Some studies have reported malic acid degradation varying from 75 to 100% depending on the strain (Silva et al. 2003; Benito et al. 2016b). The reference value (100%) must be the highest malic acid degradation rate. Although most studies report total malic acid deacidification in different fermentative industries, Schizosaccharomyces fermentations commonly have undesirable effects. The main challenge is obtaining high acetic acid concentrations (over 0.8 g/L) (Mylona et al. 2016). Thus, the ability of Schizosaccharomyces strains to produce moderate concentrations of acetic acid is considered the second most important selection parameter after malic acid degradation. Deacidified wines with high levels of acetic acid are not desired in the market. The objective is mainly to obtain concentrations close to 0.1 g/L (Du-Plessis et al. 2017b), although concentrations below 0.3 g/L would also be acceptable. A proper selection based on that parameter would generate strains that would not require corrective techniques such as fed-batch technology (Roca-Domènech et al. 2018) or the strains’ combined use with other species that produce lower concentrations, such as S. cerevisiae or L. thermotolerans (Domizio et al. 2018; Benito et al. 2019). After achieving completely deacidified fermentations without faults in acetic acid levels, we should aim to complete all the fermentations without any residual sugars. As Schizosaccharomyces species are strong fermenters, the reference value should be the maximum concentration of alcohol reported, which is 15% (v/v) (Suárez-Lepe et al. 2012). The first selections for S. pombe were based on these three initial parameters (Benito et al. 2014, 2016b), similar to the S. cerevisiae selections performed previously. Nevertheless, after several years of research, many other secondary parameters, depending on the industrial objective, can be taken into account.
The most important discovery for S. pombe is its application in the production of compounds with positive neuroprotective effects against Alzheimer’s disease, such as 6-paradol. This feature should be considered even more crucial than the role of S. pombe in the AF industry due to its importance for human health. The 6-paradol content in the first ginger fermentation by S. pombe was 7.5 mg/g, which should be considered the reference value until new studies emerge that make comparison with the current reference value possible.
To produce smoother and more structured wines, improving the release of polysaccharides should aim to achieve the maximum concentration of 1085 mg/L reported during AF (Domizio et al. 2018), while the final concentration of glycerol should be approximately 11.5 g/L (Domizio et al. 2017).
In the production of red wines, strains should be selected to produce amounts similar to the maximum reported pyruvic acid formation, approximately 500 mg/L (Benito et al. 2016b; Domizio et al. 2017), to generate the most-stable colored molecules as possible to preserve the red color longer in wines.
In the case of acetaldehyde, if the strains are selected to produce red wines, the objective should be to select strains that produce moderately high acetaldehyde concentrations below the fault threshold of 125 mg/L to generate high amounts of stable colored molecules such as vitisin B while avoiding possible aroma faults related to this compound. The reference value should be approximately 75 mg/L, which is a concentration of acetaldehyde produced by some S. pombe strains that improve the color of red wines (Mylona et al. 2016; Benito et al. 2017). In the case of white wine production, the strains should produce levels close to the minimum reported for S. pombe, which is approximately 12 mg/L, to minimize any possible influence on the aroma (Chen et al. 2018).
To reduce the negative impact of rotten grapes on the final wine, the strains should be selected to reduce gluconic acid by approximately 91%, which is the maximum reduction reported in the literature (Peinado et al. 2007). Indeed, the ochratoxin A concentration should be reduced from its ochratoxin A reference value by at least 70% (Cecchini et al. 2006).
As S. pombe produces less higher alcohol and ester content (Chen et al. 2018) than other species, in the vinification of varietal wines, Schizosaccharomyces strains should be selected to produce a low concentration as possible of higher alcohols and esters in order to not mask the varietal character. Acetoin varies from 21 to 177 mg/L depending on the studied strain (Del Fresno et al. 2017). As acetoin content can be a fault, the reference value should be the lowest value, 21 mg/L.
However, there is no direct correlation between the presence of histidine and the final concentration of histamine. It would be prudent to select Schizosaccharomyces strains with a low production of the histamine precursor as possible, as S. pombe produces high levels of histidine (Benito et al. 2016a).
Parameters other than the enological quality parameters, such as the viability enhancement of cells during desiccation stress, should also be taken into account. Most non-Saccharomyces species have difficulties under desiccation stress. Roca-Domènech et al. (2016) reported strain variabilities that can be enhanced up to 70% when media are supplemented with trehalose and MgSO4. Their study optimized the first protocol to obtain Schizosaccharomyces as an active dry yeast. Other behavior, such as urease activity, which does not seem to show strain variability, should only be verified after the strain selection.
Figure 4 summarizes the main parameters proposed for examination in the future selection of S. pombe strains.
Conclusions
Contrary to the previous era, when the industrial interest in Schizosaccharomyces was focused on the deacidification of malic acid in winemaking, especially in Northern European regions, Schizosaccharomyces is currently widely recognized and considered a microbiological option in fermentation industries, similar to other non-Saccharomyces microorganisms, such as Torulaspora delbrueckii or Lachancea thermotolerans. In fact, the genus offers solutions to modern winemaking challenges, mostly in warm viticulture areas, where performing MLFs in grape juices with high sugar levels and high pH values can cause undesirable deviations. To date, many studies have reported improvements in wine color related to the formation of highly stable anthocyanins and in aroma composition due to the production of lower higher alcohol content, and several fermentation industries, such as the kei-apple, apple, sparkling wine, and bilberry industries, are starting to use Schizosaccharomyces. Moreover, the use of Schizosaccharomyces is emerging in industries other than the food beverage industry, among which the pharmacy and bioethanol industries and even environmental applications such as the removal of heavy metals are the main examples. In addition, Schizosaccharomyces is an important genus due to its great help in solving food safety problems resulting from ochratoxin A, biogenic amines, or ethyl carbamate. Several other studies also show undesirable collateral effects, such as high production of acetic acid or acetaldehyde. Nevertheless, other works show the use of sequential fermentations, strain selection processes, feed-batch technology, or Mg2+ supplementation as possible solutions.
References
Aguera E, Sire Y, Mouret J, Sablayrolles J, Farines V (2018) Comprehensive study of the evolution of gas-liquid partitioning of acetaldehyde during wine alcoholic fermentation. J Agric Food Chem 66:6170–6178. https://doi.org/10.1021/acs.jafc.8b01855
Bakhiet SE, Mahmoud MA (2015) Production of bio-ethanol from molasses by Schizosaccharomyces species. Annu Res Rev Biol 7:45–53. https://doi.org/10.9734/ARRB/2015/15918
Benito S (2018a) The impact of Torulaspora delbrueckii yeast in winemaking. Appl Microbiol Biotechnol 102:3081–3094. https://doi.org/10.1007/s00253-018-8849-0
Benito S (2018b) The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl Microbiol Biotechnol 102:1–16. https://doi.org/10.1007/s00253-018-9117-z
Benito S, Palomero F, Morata A, Calderón F, Suárez-Lepe JA (2012) New applications for Schizosaccharomyces pombe in the alcoholic fermentation of red wines. Int J Food Sci Technol 47(10):2101–2108. https://doi.org/10.1111/j.1365-2621.2012.03076.x
Benito S, Palomero F, Morata A, Calderon F, Palmero D, Suárez-Lepe JA (2013) Physiological features of Schizosaccharomyces pombe of interest in making of white wines. Eur Food Res Technol 236(1):29–36. https://doi.org/10.1007/s00217-012-1836-2
Benito S, Palomero F, Calderón F, Palmero D, Suárez-Lepe JA (2014) Selection of appropriate Schizosaccharomyces strains for winemaking. Food Microbiol 42:218–224. https://doi.org/10.1016/j.fm.2014.03.014
Benito Á, Calderón F, Palomero F, Benito S (2015) Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules 20(6):9510–9523. https://doi.org/10.3390/molecules20069510
Benito Á, Calderón F, Benito S (2016a) Combined use of S. pombe and L. thermotolerans in winemaking. Beneficial effects determined through the study of wines’ analytical characteristics. Molecules 21:1744. https://doi.org/10.3390/molecules21121744
Benito Á, Jeffares D, Palomero F, Calderón F, Bai F, Bähler J, Benito S (2016b) Selected Schizosaccharomyces pombe strains have characteristics that are beneficial for winemaking. PLoS One 11:e0151102. https://doi.org/10.1371/journal.pone.0151102
Benito Á, Calderón F, Benito S (2017) The combined use of Schizosaccharomyces pombe and Lachancea thermotolerans—effect on the anthocyanin wine composition. Molecules 22:739. https://doi.org/10.3390/molecules22050739
Benito A, Calderon F, Benito S (2018) Schizosaccharomyces pombe isolation protocol. In: Singleton TL (ed) Methods molecular biology. Springer, Berlin Chapter 20. ISBN 978-1-4939-7545-7365-370
Benito Á, Calderón F, Benito S (2019) Mixed alcoholic fermentation of Schizosaccharomyces pombe and Lachancea thermotolerans and its influence on mannose-containing polysaccharides wine composition. AMB Express 9(1):17. https://doi.org/10.1186/s13568-019-0738-0
Bonomo M, Di Tomaso K, Calabrone L, Salzano G (2018) Ethanol stress in Oenococcus oeni: transcriptional response and complex physiological mechanisms. J Appl Microbiol 125:2–15. https://doi.org/10.1111/jam.13711
Burns TR, Osborne JP (2015) Loss of Pinot noir wine color and polymeric pigment after malolactic fermentation and potential causes. Am J Enol Vitic 66:130–137. https://doi.org/10.5344/ajev.2014.14061
Cecchini F, Morassut M, Moruno EG, Di Stefano R (2006) Influence of yeast strain on ochratoxin A content during fermentation of white and red must. Food Microbiol 23:411–417. https://doi.org/10.1016/j.fm.2005.08.003
Chen K, Escott C, Loira I, del Fresno JM, Morata A, Tesfaye W, Calderon F, Suárez-Lepe JA, Han S, Benito S (2018) Use of non-Saccharomyces yeasts and oenological tannin in red winemaking: influence on colour, aroma and sensorial properties of young wines. Food Microbiol 69:51–63. https://doi.org/10.1016/j.fm.2017.07.018
Chilaka C, Uchechukwu N, Obidiegwu J, Akpor O (2010) Evaluation of the efficiency of yeast isolates from palm wine in diverse fruit wine production. African J Food Sci 4:764–774 ISSN: 1996-0794
Choi JW, Park HY, Oh MS, Yoo HH, Lee SH, Ha SK (2017) Neuroprotective effect of 6-paradol enriched ginger extract by fermentation using Schizosaccharomyces pombe. J Funct Foods 31:304–310. https://doi.org/10.1016/j.jff.2017.02.010
Choi JG, Kim SY, Jeong M, Oh MS (2018) Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol Therapeut 182:56–69. https://doi.org/10.1016/j.pharmthera.2017.08.010
Cinquanta L, De Stefano G, Formato D, Niro S, Panfili G (2018) Effect of pH on malolactic fermentation in southern Italian wines. Eur Food Res Technol 244:1261–1268. https://doi.org/10.1007/s00217-018-3041-4
Comitini F, Gobbi M, Domizio P, Romani C, Lencioni L, Mannazzu I, Ciani M (2011) Selected non Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol 28:873–882. https://doi.org/10.1016/j.fm.2010.12.001
Contreras A, Hidalgo C, Henschke PA, Chambers PJ, Curtin C, Varela C (2014) Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl Environ Microbiol 80(5):11670–11678. https://doi.org/10.1128/AEM.03780-13
Contreras A, Hidalgo C, Schmidt S, Henschke PA, Curtin C, Varela C (2015) The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int J Food Microbiol 205:7–15. https://doi.org/10.1016/j.ijfoodmicro.2015.03.027
Coton M, Romano A, Spano G, Ziegler K, Vetrana C, Desmarais C, Lonvaud-Funel A, Lucas P, Coton E (2010) Occurrence of biogenic amine-forming lactic acid bacteria in wine and cider. Food Microbiol 27:1078–1085. https://doi.org/10.1016/j.fm.2010.07.012
Couto J, Graça A, Soares-Franco J, Hogg T (2003) The use of gluconic acid level as an indicator of the activity of acetic acid bacteria in grapes. VII Symposium International d’Oenologie, Bordeaux
Deák T (2008) Handbook of food spoilage yeasts, 2nd edn. CRC, Boca Raton
Del Fresno JM, Morata A, Loira I, Bañuelos MA, Escott C, Benito S, Chamorro CG, Suárez-Lepe JA (2017) Use of non-Saccharomyces in single-culture, mixed and sequential fermentation to improve red wine quality. Eur Food Res Technol 243:2175–2185. https://doi.org/10.1007/s00217-017-2920-4
Domizio P, Liu Y, Bisson LF, Barile D (2014) Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol 43:5–15. https://doi.org/10.1016/j.fm.2014.04.005
Domizio P, Liu Y, Bisson L, Barile D (2017) Cell wall polysaccharides released during the alcoholic fermentation by Schizosaccharomyces pombe and S. japonicus: quantification and characterization. Food Microbiol 61:136–149. https://doi.org/10.1016/j.fm.2016.08.010
Domizio P, Lencioni L, Calamai L, Portaro L, Bisson LF (2018) Evaluation of the yeast Schizosaccharomyces japonicus for use in wine production. Am J Enol Vitic 69:266–277. https://doi.org/10.5344/ajev.2018.18004
Du Plessis H, Du Toit M, Nieuwoudt H, Van Der Rijst M, Kidd M, Jolly N (2017a) Effect of Saccharomyces, non-Saccharomyces yeasts and malolactic fermentation strategies on fermentation kinetics and flavor of Shiraz wines. Fermentation 3:64. https://doi.org/10.3390/fermentation3040064
Du Plessis H, Du Toit M, Hoff J, Hart R, Ndimba B, Jolly N (2017b) Characterisation of non- Saccharomyces yeasts using different methodologies and evaluation of their compatibility with malolactic fermentation. South Afr J Enol Vitic 38:46–63. https://doi.org/10.21548/38-1-819
Escott C, Morata A, Ricardo-da-Silva J, Callejo M, González M, Suarez-Lepe J (2018) Effect of Lachancea thermotolerans on the formation of polymeric pigments during sequential fermentation with Schizosaccharosmyces pombe and Saccharomyces cerevisiae. Molecules 23(9):2353. https://doi.org/10.3390/molecules23092353
Escribano R, González-Arenzana L, Portu J, Garijo P, López-Alfaro I, López R, Santamaría P, Gutiérrez AR (2018) Aromatic compound production and fermentative behavior within different non-Saccharomyces species and clones. J Appl Microbiol 124:1521–1531. https://doi.org/10.1111/jam.13735
Gil-Serna J, García-Díaz M, González-Jaén MT, Vázquez C, Patiño B (2018) Description of an orthologous cluster of ochratoxin A biosynthetic genes in Aspergillus and Penicillium species. A comparative analysis. Int J Food Microbiol 268:35–43. https://doi.org/10.1016/j.ijfoodmicro.2017.12.028
Hu C, Bai F, An L (2003) Enhancing ethanol tolerance of a self-flocculating fusant of Schizosaccharomyces pombe and Saccharomyces cerevisiae by Mg2 via reduction in plasma membrane permeability. Biotechnol Lett 25:1191–1194. https://doi.org/10.1023/A:1024583503274
Huh E, Lim S, Kim HG, Ha SK, Park H, Huh Y, Oh MS (2018) Ginger fermented with Schizosaccharomyces pombe alleviates memory impairment via protecting hippocampal neuronal cells in amyloid beta 1–42 plaque injected mice. Food Funct 9:171–178. https://doi.org/10.1039/C7FO01149K
Ivit NN, Loira I, Morata A, Benito S, Palomero F, Suárez-Lepe JA (2018) Making natural sparkling wines with non-Saccharomyces yeasts. Eur Food Res Technol 244:925–935. https://doi.org/10.1007/s00217-017-3015-y
Jeffares DC, Rallis C, Rieux A, Speed D, Převorovský M, Mourier T, Marsellach FX, Iqbal Z, Lau W, Cheng TM (2015) The genomic and phenotypic diversity of Schizosaccharomyces pombe. Nat Genet 47:235–241. https://doi.org/10.1038/ng.3215
Jolly NP, Varela C, Pretorius IS (2014) Not your ordinary yeast: non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res 14:215–237. https://doi.org/10.1111/1567-1364.12111
Ladero V, Calles-Enríquez M, Fernández M, A Alvarez M (2010) Toxicological effects of dietary biogenic amines. Curr Nutr Food Sci 6: 145–156. https://doi.org/10.2174/157340110791233256
Lambrechts M, Pretorius I (2000) Yeast and its importance to wine aroma—a review. S Afr J Enol Vitic 21:97–129
Lasik-Kurdyś M, Gumienna M, Nowak J (2017) Influence of malolactic bacteria inoculation scenarios on the efficiency of the vinification process and the quality of grape wine from the Central European region. Eur Food Res Technol 243:2163–2173. https://doi.org/10.1007/s00217-017-2919-x
Liu S, Laaksonen O, Kortesniemi M, Kalpio M, Yang B (2018) Chemical composition of bilberry wine fermented with non-Saccharomyces yeasts (Torulaspora delbrueckii and Schizosaccharomyces pombe) and Saccharomyces cerevisiae in pure, sequential and mixed fermentations. Food Chem 266:262–274. https://doi.org/10.1016/j.foodchem.2018.06.003
Liu S, Laaksonen O, Yang B (2019) Volatile composition of bilberry wines fermented with non-Saccharomyces and Saccharomyces yeasts in pure, sequential and simultaneous inoculations. Food Microbiol 80:25–39. https://doi.org/10.1016/j.fm.2018.12.015
Malpertuy A, Tekaia F, Casarégola S, Aigle M, Artiguenave F, Blandin G, Bolotin-Fukuhara M, Bon E, Brottier P, de Montigny J (2000) Genomic exploration of the hemiascomycetous yeasts: 19. Ascomycetes-specific genes. FEBS Lett 487:113–121. https://doi.org/10.1016/S0014-5793(00)02290-0
Martínez-Rodríguez JA, Carrascosa AV (2009) HACCP to control microbial safety hazards during winemaking: ochratoxin A. Food Control 20:469–475. https://doi.org/10.1016/j.foodcont.2008.07.015
Martuscelli M, Arfelli G, Manetta AC, Suzzi G (2013) Biogenic amines content as a measure of the quality of wines of Abruzzo (Italy). Food Chem 140:590–597. https://doi.org/10.1016/j.foodchem.2013.01.008
Mateos JR, Pérez-Nevado F, Fernández MR (2006) Influence of Saccharomyces cerevisiae yeast strain on the major volatile compounds of wine. Enzym Microb Technol 40(1):151–157. https://doi.org/10.1016/j.enzmictec.2005.10.048
Miljić U, Puškaš V, Vučurović V, Muzalevski A (2017) Fermentation characteristics and aromatic profile of plum wines produced with indigenous microbiota and pure cultures of selected yeast. J Food Sci 82:1443–1450. https://doi.org/10.1111/1750-3841.13736
Minnaar P, Jolly N, Paulsen V, Du Plessis H, Van Der Rijst M (2017) Schizosaccharomyces pombe and Saccharomyces cerevisiae yeasts in sequential fermentations: effect on phenolic acids of fermented Kei-apple (Dovyalis caffra L.) juice. Int J Food Microbiol 257:232–237. https://doi.org/10.1016/j.ijfoodmicro.2017.07.004
Mira de Orduña R, Liu S, Patchett M, Pilone G (2000) Ethyl carbamate precursor citrulline formation from arginine degradation by malolactic wine lactic acid bacteria. FEMS Microbiol Lett 183:31–35. https://doi.org/10.1111/j.1574-6968.2000.tb08929.x
Monteiro FF, Trousdale EK, Bisson LF (1989) Ethyl carbamate formation in wine: use of radioactively labeled precursors to demonstrate the involvement of urea. Am J Enol Vitic 40:1–8
Mylona A, Del Fresno J, Palomero F, Loira I, Bañuelos M, Morata A, Calderón F, Benito S, Suárez-Lepe JA (2016) Use of Schizosaccharomyces strains for wine fermentation—effect on the wine composition and food safety. Int J Food Microbiol 232:63–72. https://doi.org/10.1016/j.ijfoodmicro.2016.05.023
Obisanya M, Aina J, Oguntimein G (1987) Production of wine from mango (Magnifera indica L.) using Saccharomyces and Schizosaccharomyces species isolated from palm wine. J Appl Bacteriol 63:191–196. https://doi.org/10.1111/j.1365-2672.1987.tb04935.x
Osborne J, Mira de Orduna R, Pilone G, Liu S (2000) Acetaldehyde metabolism by wine lactic acid bacteria. FEMS Microbiol Lett 191:51–55. https://doi.org/10.1111/j.1574-6968.2000.tb09318.x
Padilla B, Gil JV, Manzanares P (2016) Past and future of non-Saccharomyces yeasts: from spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front Microbiol 7:411. https://doi.org/10.3389/fmicb.2016.00411
Palacios V, Roldán A, Jiménez-Cantizano A, Amores-Arrocha A (2018) Physicochemical and microbiological characterization of the sensory deviation responsible for the origin of the special sherry wines “Palo Cortado” type. PLoS One 13:e0208330. https://doi.org/10.1371/journal.pone.0208330
Peinado R, Moreno J, Medina M, Mauricio J (2004) Changes in volatile compounds and aromatic series in sherry wine with high gluconic acid levels subjected to aging by submerged flor yeast cultures. Biotechnol Lett 26:757–762. https://doi.org/10.1023/B:BILE.0000024102.58987.de
Peinado RA, Moreno JJ, Maestre O, Mauricio JC (2007) Removing gluconic acid by using different treatments with a Schizosaccharomyces pombe mutant: effect on fermentation byproducts. Food Chem 104:457–465. https://doi.org/10.1016/j.foodchem.2006.11.070
Peinado RA, Maestre O, Mauricio JC, Moreno JJ (2009) Use of a Schizosaccharomyces pombe mutant to reduce the content in gluconic acid of must obtained from rotten grapes. J Agric Food Chem 57:2368–2377. https://doi.org/10.1021/jf803479r
Petruzzi L, Sinigaglia M, Corbo MR, Campaniello D, Speranza B, Bevilacqua A (2014) Decontamination of ochratoxin A by yeasts: possible approaches and factors leading to toxin removal in wine. Appl Microbiol Biotechnol 98:6555–6567. https://doi.org/10.1007/s00253-014-5814-4
Petruzzi L, Capozzi V, Berbegal C, Corbo MR, Bevilacqua A, Spano G, Sinigaglia M (2017) Microbial resources and enological significance: opportunities and benefits. Front Microbiol 8:995. https://doi.org/10.3389/fmicb.2017.00995
Petzinger E, Ziegler K (2000) Ochratoxin A from a toxicological perspective. J Vet Pharmacol Ther 23:91–98. https://doi.org/10.1046/j.1365-2885.2000.00244.x
Porter TJ, Divol B, Setati ME (2019) Lachancea yeast species: origin, biochemical characteristics and oenological significance. Food Res Int 119:378–389. https://doi.org/10.1016/j.foodres.2019.02.003
Roca-Domènech G, López-Martínez G, Tirado V, Borrull A, Candelas O, Rozès N, Cordero-Otero R (2016) Viability enhancement of Schizosaccharomyces pombe cells during desiccation stress. J Microbiol Res 6:82–91. https://doi.org/10.5923/j.microbiology.20160604.03
Roca-Domènech G, Cordero-Otero R, Rozès N, Cléroux M, Pernet A, de Orduña RM (2018) Metabolism of Schizosaccharomyces pombe under reduced osmotic stress conditions afforded by fed-batch alcoholic fermentation of white grape must. Food Res Int 113:401–406. https://doi.org/10.1016/j.foodres.2018.07.003
Ruiz J, Belda I, Beisert B, Navascués E, Marquina D, Calderón F, Rauhut D, Benito S (2018) Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl Microbiol Biotechnol 102(19):8501–8509. https://doi.org/10.1007/s00253-018-9255-3
Satora P, Tarko T, Duda-Chodak A, Sroka P, Tuszynski T, Czepielik M (2009) Influence of prefermentative treatments and fermentation on the antioxidant and volatile profiles of apple wines. J Agric Food Chem 57(23):11209–11217. https://doi.org/10.1021/jf9025053
Satora P, Semik-Szczurak D, Tarko T, Bułdys A (2018) Influence of selected Saccharomyces and Schizosaccharomyces strains and their mixed cultures on chemical composition of apple wines. J Food Sci 83:424–431. https://doi.org/10.1111/1750-3841.14042
Shamala T, Sreekantiah K (1988) Microbiological and biochemical studies on traditional Indian palm wine fermentation. Food Microbiol 5:157–162. https://doi.org/10.1016/0740-0020(88)90014-7
Silva S, Ramón-Portugal F, Andrade P, Abreu S, de Fatima TM, Strehaiano P (2003) Malic acid consumption by dry immobilized cells of Schizosaccharomyces pombe. Am J Enol Vitic 54(1):50–55
Strickland MT, Schopp LM, Edwards CG, Osborne JP (2016) Impact of Pediococcus spp. on Pinot noir wine quality and growth of Brettanomyces. Am J Enol Vitic 67:188–198. https://doi.org/10.5344/ajev.2015.15011
Su J, Wang T, Wang Y, Li YY, Li H (2014) The use of lactic acid-producing, malic acid-producing, or malic acid-degrading yeast strains for acidity adjustment in the wine industry. Appl Microbiol Biotechnol 98(6):2395–2413. https://doi.org/10.1007/s00253-014-5508-y
Suárez-Lepe JA, Palomero F, Benito S, Calderón F, Morata A (2012) Oenological versatility of Schizosaccharomyces spp. Eur Food Res Technol 235(3):375–383. https://doi.org/10.1007/s00217-012-1785-9
Subhashini SS, Kaliappan S, Velan M (2011) Removal of heavy metal from aqueous solution using Schizosaccharomyces pombe in free and alginate immobilized cells. In: 2nd International Conference on Environmental Science and Technology. Singapore 6:108–111
Sumby KM, Grbin PR, Jiranek V (2014) Implications of new research and technologies for malolactic fermentation in wine. Appl Microbiol Biotechnol 98:8111–8132. https://doi.org/10.1007/s00253-014-5976-0
Sumby KM, Bartle L, Grbin PR, Jiranek V (2019) Measures to improve wine malolactic fermentation. Appl Microbiol Biotechnol:1–19. https://doi.org/10.1007/s00253-018-09608-8
Taillandier P, Strehaiano P (1991) The role of malic acid in the metabolism of Schizosaccharomyces pombe: substrate consumption and cell growth. Appl Microbiol Biotechnol 35:541–543. https://doi.org/10.1007/BF00169765
Tristezza M, di Feo L, Tufariello M, Grieco F, Capozzi V, Spano G, Mita G (2016) Simultaneous inoculation of yeasts and lactic acid bacteria: effects on fermentation dynamics and chemical composition of Negroamaro wine. LWT-Food Sci Technol 66:406–412. https://doi.org/10.1016/j.lwt.2015.10.064
Van Der Walt J (1956) Kaffircorn malting and brewing studies. II.—Studies on the microbiology of Kaffir beer. J Sci Food Agric 7:105–113. https://doi.org/10.1002/jsfa.2740070203
Varela C (2016) The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl Microbiol Biotechnol 100:9861–9874. https://doi.org/10.1007/s00253-016-7941-6
Varela C, Dry PR, Kutyna DR, Francis IL, Henschke PA, Curtin CD, Chambers PJ (2015) Strategies for reducing alcohol concentration in wine. Aust J Grape Wine Res 21:670–679. https://doi.org/10.1111/ajgw.12187
Vaughan-Martini A, Martini A (2011) Schizosaccharomyces Lindner (1893). In: Kurtzman C, Fell JW, Boekhout T (eds) The yeasts: a taxonomic study, 5th edn. Elsevier, Amsterdam, pp 779–784
Violeta N, Trandafir I, Ionica ME (2010) Compositional characteristics of fruits of several apple (Malus domestica Borkh.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 38(3):228–233. https://doi.org/10.15835/nbha3834762
Wang S, Li S, Zhao H, Gu P, Chen Y, Zhang B, Zhu B (2018) Acetaldehyde released by Lactobacillus plantarum enhances accumulation of pyranoanthocyanins in wine during malolactic fermentation. Food Res Int 108:254–263. https://doi.org/10.1016/j.foodres.2018.03.032
Funding
Funding for the research in this paper was provided by Ossian Vides y Vinos S. L under the framework of the project FPA1720300120 (Centre for the Development of Industrial Technology (CDTI), Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Benito, S. The impacts of Schizosaccharomyces on winemaking. Appl Microbiol Biotechnol 103, 4291–4312 (2019). https://doi.org/10.1007/s00253-019-09827-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09827-7