Abstract
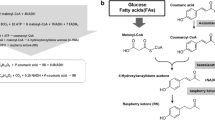
Raspberry ketone is an important ingredient in the flavor and fragrance industries. Due to its low content in fruits and vegetables, the production of natural raspberry ketone using heterologous synthesis in microbial strains is recently attracting increased attention. In this work, a heterologous pathway to produce raspberry ketone from p-coumaric acid, including 4-coumarate: CoA ligase (4CL), benzalacetone synthase (BAS), and raspberry ketone/zingerone synthase (RZS1) from plants, was successfully assembled in Escherichia coli. When the RZS1 gene was introduced into E. coli and co-expressed with two other genes, the intermediate 4-hydroxybenzylidene acetone in the pathway was almost completely transformed into a raspberry ketone. Substituting TB medium for M9 medium increased raspberry ketone titers by 3–4 times. Furthermore, the heterologous pathway was partitioned into two modules; module one produced p-coumaroyl-CoA from p-coumaric acid by 4CL, and module two produced raspberry ketone from coumaroyl-CoA by the action of BAS and RZS1. Optimizing the balanced expression of the two modules, it was shown that moderate expression of module one and high expression of module two was the best combination to enhance raspberry ketone production. The engineered strain CZ-8 reached 90.97 mg/l of raspberry ketone, which was 12 times higher than previously reported. In addition, the preferred approach of the heterologous pathway was related to the heterologous genes from different sources; for example, 4CL from Arabidopsis thaliana seemed to be more suitable for raspberry ketone production than that from Petroselinum crispum. This work paves an alternative way for future economic production of natural raspberry ketone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Raspberry ketone (RK) known as 4-(4-hydroxyphenyl)-butan-2-one is the only one compound responsible for natural raspberry fruit aroma (Lee 2016). It was originally discovered in 1903, and its chemical structure was first identified in 1951 from raspberries (Hugueny et al. 1995). RK not only has been applied in the food and cosmetic industries for its unique fragrance properties (Beekwilder et al. 2007), but also in recent years has drawn attention in the pharmaceutical industry for its medicinal value (Kim et al. 2016). In particular, RK has beneficial effects on lipid metabolism and can help to prevent obesity and lose weight (Morimoto et al. 2005; Wang et al. 2012). The odor threshold of RK is approximately 1–10 ppb (Larsen and Poll 1992), and when used as an additive in food or other products, its concentration ranges from 5 to 50 ppm. RK can be naturally found in a variety of fruits and vegetables, such as peaches, grapes, various berries, and rhubarb, and in the bark of trees, including yew, maple, and pine (Beekwilder et al. 2007). However, its content in fruits is typically very low, at levels of approximately 1–4 mg/kg (Larsen et al. 1991), therefore the cost of extracting RK from fruits is too high and the price of natural RK is approximately $3000/kg (Fischer et al. 2001a; Stabnikova et al. 2010).
Today, RK is the second most economical flavor, after vanillin, in the spice industry, with a total potential market value of between 7 and 12 million dollars (Hakkinen et al. 2015). There are many ways to chemically synthesize RK, and it is simpler and more abundant than the extraction of RK from plants. However, during the synthetic process, various toxic by-products can be formed and environmental pollution can result. Furthermore, the use of chemically synthesized RK has limited applications and it cannot be marketed as a “natural” flavor compound, according to The EC Flavor Directive (88/388/EEC) (Beekwilder et al. 2007). Therefore, if biosynthesis of RK could be achieved and it met the requirements of industrial production, it would have great economic value.
Previous reports as early as 2001 have shown that the biosynthetic pathway exists in the filamentous fungi Nidula niveo-tomentosa (Fischer et al. 2001b). In plants, the natural synthesis of RK begins with the phenylpropanoid pathway (Borejsza-Wysocki and Hrazdina 1994). In the first step, p-coumaric acid is activated to 4-coumaroyl-CoA by the action of 4-coumarate: CoA ligase (4CL) (Wu et al. 2013). This compound is subsequently condensed with one malonyl-CoA by the action of type III polyketide synthase, benzalacetone synthase (BAS) to 4-hydroxybenzylidene acetone (Abe et al. 2001; Abe et al. 2007). In the final step, the intermediate product, 4-hydroxybenzylidene acetone, is reduced to RK by benzalacetone reductase (BAR), which is defined as a NADPH-dependent reductase (Beekwilder et al. 2007). Chalcone synthase (CHS) has both BAS and CHS activity in vitro. The final RK production reached levels of 5 mg/l when RiCHS (from Rubus idaeus) was heterologously expressed in E. coli and this was the first time to produce RK in bacteria (Beekwilder et al. 2007). The de novo synthesis of RK was also achieved in S. cerevisiae (AWRI2975) by protein fusion, resulting in a yield of 2.8 mg/l of RK, and when p-coumaric acid was used as a substrate, the yield of RK reached 7.5 mg/l (Lee et al. 2016).
These pioneering efforts have demonstrated the potential of RK production using engineered microorganisms. However, the production of RK was not satisfactory in that final yields were no more than 10 mg/l. Moreover, not only RK was produced, but also other products such as naringenin in the E. coli expression system (Beekwilder et al. 2007; Lee et al. 2016). In this study, a heterologous metabolic pathway of RK production in E. coli was achieved. The heterologous raspberry ketone/zingerone synthase (RiRZS1) belong to NADPH-dependent BAR and TB medium used to improve RK production. Balance expression of heterologous genes was employed to optimize the multivariate modular metabolic pathway for enhancement of the RK titers from p-coumaric acid. With optimization efforts, further improvements in metabolic capabilities of the engineered E. coli strains resulted, increasing yields to a final titer of 90.97 mg/l RK.
Materials and methods
Strains, plasmids, and media
LB medium (composed of 5 g/l yeast extract, 10 g/l peptone, and 10 g/l NaCl) and TB medium (composed of 24 g/l yeast extract, 12 g/l peptone, 40 g/l glycerol, 17 mM KH2PO4, and 72 mM K2HPO4) were used. E. coli JM109 (Novagen) was used for plasmid propagation. E. coli BL21 (DE3) (Novagen) was used for plasmid cloning and recombinant molecule production. Various combinations of ampicillin (100 mg/ml), chloramphenicol (20 mg/ml), and streptomycin (40 mg/ml) were added to media for culturing plasmid-bearing E. coli strains. The expression vectors pETDuet-1, pCDFDuet-1, and pACYCDuet-1 were purchased from Novagen (Darmstadt, Germany). PrimeSTAR Max DNA polymerase, all restriction enzymes, and T4 DNA ligase were purchased from TaKaRa (Dalian, China). ClonExpress II (One Step Cloning Kit) was obtained from Vazyme Biotech Co., Ltd. (Nanjing, China). P-coumaric acid and 4-hydroxybenzylidene acetone were purchased from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). RK was obtained from Aladdin Biotech Co., Ltd. (Shanghai, China). All other chemicals were purchased from Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai, China). Cell growth was monitored by measuring the absorbance at 600 nm (OD600) with a UV/vis spectrophotometer (Jinghua instruments Co., Ltd., Shanghai, China).
Construction of plasmids and strains
The plasmids and strains constructed and used in this study are listed in Table 1. At4CL1 (GenBank ID: AAA82888.1) from Arabidopsis thaliana (Lee et al. 2016), Pc4CL2 (GenBank ID: CAA31697.1) from Petroselinum crispum (Lim et al. 2011), RpBAS (GenBank ID: AAK82824.1) from Rheum palmatum (Abe et al. 2007), and RiRZS1 (GenBank ID: JN166691) from Rubus idaeus (Koeduka et al. 2011) were codon-optimized for E. coli expression, synthesized, and inserted into cloning vector pUC57. The At4CL1 and Pc4CL2 genes were cloned into pACYCDuet-1, pCDFDuet-1, and pETDuet-1, creating plasmids pAC-At4CL1, pAC-Pc4CL2, pCD-At4CL1, pCD-Pc4CL2, pET-At4CL1, and pET-Pc4CL2, respectively. Likewise, the RpBAS and RiRZS1 genes were PCR-amplified and cloned into pACYCDuet-1, pCDFDuet-1, pETDuet-1, pAC-At4CL1, and pAC-Pc4CL2, resulting in plasmids PAC-RpBAS-RiRZS1, pCD-RpBAS-RiRZS1 and pET-RpBAS-RiRZS1, pAC-At4CL1-RpBAS, pAC-Pc4CL2-RpBAS, pAC-RpBAS, pCD-RiRZS1, pET-RpBAS, and pET- RiRZS1, respectively. The primers and specific structure of plasmids used in this study are listed in Supplementary Table and Fig. S1–15. And the GenBank ID of codon-optimized At4CL1, Pc4CL2, RpBAS, and RiRZS1 genes are MK035997, MK035998, MK035999, and MK036000, respectively.
Culture conditions
The wild type or engineered E. coli strains were first grown overnight in LB at 37 °C, a small portion of this culture was then diluted 2:100 in fresh LB medium and growth continued at 37 °C until OD600 = 0.6–0.8. Then, Isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM and induced at 20 °C for 9 h. Next, the cells were collected by centrifugation and re-suspended in M9 medium (10 g/l glucose) containing the necessary antibiotics, 100 mg/l p-coumaric acid and 1 mM IPTG. Cultures were subsequently conducted at 20 °C for the fermentation product RK. RK concentrations were measured after a total fermentation time of 72 h. When TB medium was used for RK production, overnight-grown seed cultures were inoculated into fresh TB medium (with 5 g/l glucose added) at 5% inoculation and 300 mg/l p-coumaric acid was added as a precursor.
HPLC and GC-MS analysis of RK
Both the standard and samples were analyzed using a Waters 2487 HPLC system (Waters, Milford, MA, USA), equipped with a reverse-phase Amethyst C18-H column (4.6 × 250 mm). The column was at 35 °C, and a flow rate of 1 ml/min was used. The fixed mobile phase was 80% water with 0.1% (vol/vol) phosphoric acid and 20% (vol/vol) acetonitrile. The p-coumaric acid, RK, and 4-hydroxybenzylidene acetone peaks were obtained at 9.5 min, 17.5 min, and 18.9 min, respectively.
The GC-MS system was equipped with an TG-5MS capillary column (30 m × 0.25 mm, 0.25 mm; Thermo Fisher Scientific, 81 Wyman Street, Waltham, MA, USA). For each sample, a splitless injection was used and the injection volume was 1 μl. Helium (99.999%) was the carrier gas employed at a flow rate of 1 ml/min. The temperature of the injection port was 280 °C. The column temperature program was at an initial temperature of 40 °C for 1 min, followed by an increase to 280 °C at a rate of 10 °C/min, and was maintained at 280 °C for 5 min. The MS conditions included a transmission line temperature of 280 °C, an ion source temperature of 230 °C, and a mass scan range of 29–350 amu.
Results
Co-expression of 4CL and BAS genes for RK production
It has been reported that the short pathway for RK production in tissue culture consists of two steps: (1) p-coumaroyl-CoA and malonyl-CoA synthesize p-hydroxybenzalacetone by the action of BAS and (2) reduction to RK by BAR (Hrazdina 2006). When p-coumaric acid was used as a precursor, the introduction of two heterologous enzymes 4-aminocoumaric acid 4CL and BAS produced RK in S. cerevisiae (Lee et al. 2016). Therefore, 4CL and BAS were likely to be the key enzymes involved in the synthetic pathway of RK from p-coumaric acid when the original systems were mimicked. The 4CL gene has been found in many plants, such as Nicotiana tabacum, Arabidopsis thaliana,and Petroselinum crispum (Beekwilder et al. 2007; Beekwilder et al. 2006; Lee et al. 2016; Lim et al. 2011), while the BAS gene has only been identified in Rheum palmatum (Abe et al. 2001). As the differences between the original and E. coli hosts were quite significant, and the effects of these differences on enzyme expression and activity were impossible to predict, the approaches of using combinations of 4CL gene homologs from different sources with the BAS gene were compared.
Firstly, the plasmids, pAC-At4CL1-RpBAS (At4CL1 from Arabidopsis thaliana) and pAC-Pc4CL2-RpBAS (Pc4CL2 from Rheum palmatum), were constructed with each gene’s own T7 promoter to facilitate strong expression within E. coli BL21, and the engineered strains CZ-1 and CZ-2 were obtained, respectively. The consumption of p-coumaric acid and the accumulation of 4-hydroxybenzylidene acetone and RK in the culture were monitored after fermentation. Results showed that strain CZ-1 consumed small amounts of p-coumaric acid (less than 10 mg/l) and there were non-detectable levels of 4-hydroxybenzylidene acetone or RK produced during 48 h of culturing (Fig. 2a). However, the consumption of p-coumaric acid and the production of 4-hydroxybenzylidene acetone and RK were detected in strain CZ-2. Furthermore, GC-MS analysis was performed to confirm the production of 4-hydroxybenzylidene acetone and RK during fermentation by strain CZ-2. The clear peaks observed in the GC-MS chromatogram, recorded at m/z 162 and 164, had the same retention times and mass spectra as 4-hydroxybenzylidene acetone and RK, respectively (Fig. 1). Results from HPLC analysis showed that CZ-2 produced 14.96 mg/l of 4-hydroxybenzylidene acetone and 4.68 mg/l of RK when 100 mg/l of p-coumaric acid was used as precursor substrate (Fig. 2b). Based on the above results, there are two possible explanations why strain CZ-1 did not produce detectable RK, one of which was that At4CL1 activity was too low and that this may present a major barrier for producing RK in CZ-1. Another possibility was that the At4CL1 and RpBAS genes expressed in the plasmid pACYCDuet-1 imposed some kinetic or regulatory barrier on RpBAS activity.
Fermentation time curves for engineered strains. Cultivation was conducted in M9 medium at 20 °C for 72 h, and 1 mM IPTG and 100 mg/l p-coumaric acid (precursor) were added during culture. a The engineered strain CZ-1. b The engineered strain CZ-2. c The engineered strain CZ-3. d The engineered strain CZ-4. The data represents average values ± standard deviations, calculated from three biological replicates
To determine whether the loss of activity could simply be related to transcription or translation of At4CL1 and RpBAS from the same plasmid, both At4CL1 and RpBAS were provided on a separate vector (pCDF-At4CL1 and pAC-RpBAS) and strain CZ-3 was generated. As expected, RK was detected in the fermentation broth of strain CZ-3 and 9.87 mg/l of 4-hydroxybenzylidene acetone and 7.43 mg/l of RK were produced on the addition of 100 mg/l of p-coumaric acid as a precursor (Fig. 2c). This result indicated that the enzyme expressed by the At4CL1 gene was active and a nonfunctional gene cluster was generated when the At4CL1 and RpBAS genes were co-expressed in one plasmid. Pc4CL2 and RpBAS genes were also provided on a separate vector (pCDF-Pc4CL2 and pAC-RpBAS) to construct the engineered strain CZ-4. When 100 mg/l of p-coumaric acid was added, the strain CZ-4 could produce 8.83 mg/l of 4-hydroxybenzylidene acetone and 4.89 mg/l of RK (Fig. 2d). Therefore, with the Pc4CL2 gene, RK was generated when expressing two genes on the same vector or expressing two genes on different vectors; however, in the case of the At4CL1 gene, RK was only generated when the At4CL1 and RpBAS genes were expressed on different vectors.
Expression of the RiRZS1 gene increased RK production in E. coli
E. coli contains endogenous NADPH-dependent BAR activity (Beekwilder et al. 2007). As can be seen in Fig. 2, the intermediate product of 4-hydroxybenzylidene acetone was continuously accumulating during fermentation; however, it could not be completely converted into the end product limiting RK production. This implied that the endogenous BAR of E. coli was unable to fully support RK production. It has been reported that the enzyme RiRZS1 (from Rubus idaeus) has a high specificity for substrates with the α-, β-unsaturated double bond in the butenyl side chain, unlike previously characterized phenylpropenal reductases (Koeduka et al. 2011). To assess the reductase activity of the enzyme, the RiRZS1 gene was assembled into plasmid pETDuet-1 and the engineered strain CZ-5 was obtained (SDS-PAGE of expression of RiRZS1 was in Supplementary Fig. S16). The CZ-5 strain was fermented in M9 medium containing 90 mg/l 4-hydroxybenzylidene acetone as a substrate, and E. coli BL21 harboring the empty pETDuet-1 was used as a control. Comparing the consumption of 4-hydroxybenzylidene acetone and RK production with that of the control (Fig. 3a), the substrate conversion rate increased from 40.82 to 70.17% with strain CZ-5. This showed that the RiRZS1 gene played a significant role in the conversion of 4-hydroxybenzylidene acetone to RK in E. coli.
Effect of overexpressed RiRZS1 gene on raspberry ketone titer. a Control. E. coli BL21 (DE3) harboring empty pETDuet-1, the strain CZ-5; E. coli BL21 (DE3) harboring plasmid pET-RiRZS1. Cultivation was conducted in M9 medium at 20 °C for 72 h, and 1 mM IPTG and 90 mg/l 4-hydroxybenzylidene acetone (precursor) were added during culture. b Comparative strains CZ-2 and CZ-6, CZ-3 and CZ-13, and CZ-4 and CZ-19; the difference was that the RiRZS1 gene was overexpressed in strains CZ-6, CZ-13, and CZ-19. Cultivation was conducted in M9 medium at 20 °C for 72 h, and 1 mM IPTG and 90 mg/l p-coumaric acid (precursor) were added during culture. The data represents average values ± standard deviations, calculated from three biological replicates
Furthermore, the RiRZS1 gene was introduced into the engineered strains CZ-2, CZ-3, and CZ-4, to construct engineered strains CZ-6, CZ-13, and CZ-19, respectively. As shown in Fig. 3b, the strains CZ-6, CZ-13, and CZ-19 produced 12.47 mg/l, 22.84 mg/l, and 10.88 mg/l RK, respectively. In comparison with strains CZ-2, CZ-3, and CZ-4, RK production had separately increased by 266.17%, 261.48%, and 222.55%, respectively, and the intermediate 4-hydroxybenzylidene acetone had almost disappeared in culture. This confirmed that the activity of intracellular BAR was improved by over-expressing the RiRZS1 gene in E. coli, and thus more 4-hydroxybenzylidene acetone was converted into RK.
The production of flavonoids generally uses two separate stages: the first step increases bacterial biomass using nutrient-rich media and the second step produces flavonoids in minimal media (Santos et al. 2011). M9 medium is a very nutrient-poor medium with only glucose as the carbon source, and therefore RK production was also relatively low. When TB medium was used instead of M9 (5 g/l glucose added), results showed that RK yields of strains CZ-6, CZ-13, and CZ-19 reached levels of 42.93 mg/l, 68.65 mg/l, and 31.77 mg/l and increased by 3.44, 3.54, and 2.92 times, respectively (Fig. 4). In addition, the above results seemed to indicate that introducing At4CL1 into the heterologous synthetic pathways (CZ-13) was better than introducing Pc4CL2 (CZ-6 and CZ-19).
Effects of different fermentation media (M9 or TB) on RK titer. The cultivation in M9 medium was conducted at 20 °C for 72 h, and 1 mM IPTG and 100 mg/l p-coumaric acid (precursor) were added during culture. The cultivation in TB (with 5 g/l glucose added) medium was conducted at 20 °C for 72 h, and 1 mM IPTG and 300 mg/l p-coumaric acid (precursor) were added during culture. Yellow bars: the RK titer of the engineered strain CZ-6 in M9 and TB media, respectively. Pink bars: the RK titer of engineered strain CZ-13 in M9 and TB media, respectively. Red bars: the RK titer of engineered strain CZ-19 in M9 and TB media, respectively. The data represents average values ± standard deviations, calculated from three biological replicates
Balancing the expression of three genes to optimize the multivariate modular pathway
A multivariate modular approach to metabolic pathway engineering succeeded in increasing levels of taxadiene in an engineered E. coli strain (Ajikumar et al. 2010). Therefore, the strategy of a multivariate-module pathway optimally balanced to maximize RK production from p-coumaric acid was employed. According to the results shown above (Fig. 2a), a nonfunctional gene cluster was formed when the At4CL1 and RpBAS genes were expressed on the one plasmid but RK was detected when the At4CL1 and RpBAS genes were provided on separate plasmids. Furthermore, malonyl-CoA was required as a precursor to generate the intermediate metabolite, 4-hydroxybenzylidene acetone, catalyzed by BAS after 4CL action and RiRZS1also played a reducing reaction. Therefore, the heterologous expression pathways from p-coumaric acid to RK in E. coli were partitioned into two modules as illustrated in Fig. 5a (approach A), whereby module one contained At4CL1 or Pc4CL2 genes for production of p-coumaroyl-CoA from p-coumaric acid and module two contained RpBAS and RiRZS1 genes for RK production from coumaroyl-CoA. The balanced expression of the three genes was optimized for RK production, and the expression of modules was calculated by modifying copy numbers of plasmids, where plasmid copy numbers of pACYCDuet-1 (p15A origin), pCDFDuet-1 (CDF origin), and pETDuet-1 (pBR322 origin) were 10, 20, and 40, respectively (Wu et al. 2013).
Schematic diagram of the two different approaches (A and B) At4CL1: 4-coumarate: CoA ligase came from Arabidopsis thaliana; Pc4CL2: 4-coumarate: CoA ligase came from Petroselinum crispum; RpBAR: benzalacetone synthase came from Rheum palmatum; RiRZS1: NADPH-dependent raspberry ketone/zingerone synthase,came from Rubus idaeus. a Approach A: module one (At4CL1), module two (RpBAR, RiRZS1). b Approach B: module one (Pc4CL2, RpBAR), module two (RiRZS1)
Regarding the heterologous expression pathway containing At4CL1 gene (approach A), a two-module and three-level expression test was conducted. When the expression of module one was constant, RK production improved by increasing the expression of module two (Fig. 6a). For example, when the copy number of module one was constant at 40, and the copy number of module two was increased from 10 to 20, the yield of RK improved from 20.17 to 57.88 mg/l (CZ-12 and CZ-10). In addition, the yield of RK improved from 68.65 to 90.97 mg/l when module one was constant at 20 and module two was increased from 10 to 40 (CZ-13 and CZ-8). Similar results were revealed when the copy number of module one was constant at 10 and module two was increased from 20 to 40 (CZ-11 37.28 mg/l and CZ-9 49.96 mg/l). However, if the expression of module one was too low (copy number 10), the consumption of p-coumaric acid was limited and this resulted in larger amounts of p-coumaric acid residues. When the expression of module two was constant, RK production improved as an expression of module one increased, except when the number of expressions of module two was 10. Interestingly, when module two was constant at 10, the RK levels decreased when module one was increased from 20 to 40 (CZ-13 68.65 mg/l and CZ-12 20.17 mg/l). This suggested that the yield of RK was not completely consistent with the expression level of the At4CL1 gene, rather it needed to match the expression level of the RpBAS and RiRZS1 genes in module two in order to maintain the balance between genes. The average yields of RK were 43.62 mg/l, 79.81 mg/l, and 49.03 mg/l, respectively, when the expression of module one was 10, 20, and 40, respectively. When the expression of module two was at 10, 20, and 40, the average yield of RK increased as the copy numbers of module two increased (44.45 mg/l, 47.58 mg/l, and 70.46 mg/l, respectively). This indicates that moderate expression of module one and high expression of module two was the best combination for RK production.
RK was generated by the engineered strains whether Pc4CL2 and RpBAS genes were on one vector, and the heterologous expression pathways containing the Pc4CL2 gene were partitioned into two types of approach, A and B (Fig. 5). When approach A was tested, the same results were obtained as those with the At4CL1 gene (Fig. 6b). For example, when the expression of module one was constant at 40, and the copy number of module two was increased from 10 to 20, the yield of RK improved from 14.67 to 25.9 mg/l (CZ-18 and CZ-16). The yield of RK also improved from 25.17 to 34.49 mg/l when module one was constant at 20 and module two was increased from 10 to 40 (CZ-19 and CZ-14). However, RK was not detected when the Pc4CL2 gene was expressed in the plasmid pACYCDuet-1 (copy number 10), and when the expression level of module one was too low this had an important impact on the consumption of p-coumaric acid. The same results occurred as with At4CL1, in that the titer of RK decreased from 25.17 mg/l to 14.67 mg/l (CZ-19 and CZ-18) when module two was constant at 10, and module one increased from 20 to 40. This further proved that it was necessary to match the expression levels of module one and module two in order to achieve better RK production.
The engineered strain CZ-14 containing the Pc4CL2 gene showed a high RK concentration (34.49 mg/l) after the balanced expression level was optimized. However, this result was not as good as results obtained with the Pc4CL2 and RpBAS genes on the same plasmid and with RiRZS1 gene on another plasmid (CZ-6 and CZ-7), which were levels of 49.59 mg/l and 73.18 mg/l, respectively (Fig. 6b). Approach B (Fig. 5b) containing the Pc4CL2 gene seemed to be more suitable for the heterologous expression pathway, where module one consisted of Pc4CL2 and RpBAS genes for the production of 4-hydroxybenzylidene acetone from p-coumaric acid, and module two consisted of the RiRZS1 gene for RK production from 4-hydroxybenzylidene acetone. This suggested that the heterologous expression pathway for PK production was related to genes from different sources.
Discussion
In this study, engineered E. coli strains were constructed with the aim of producing RK in supplementation with phenylpropanoic precursor, p-coumaric acid. The results showed that an engineered strain CZ-8 which co-overexpressed At4CL1, RpBAS, and RiRZS1 achieved levels of 90.97 mg/l of RK. The yield was significantly higher than had been previously reported (Beekwilder et al. 2007; Lee et al. 2016), and the introduction of RiRZS1 into the engineered strains was the main distinction. Although there was endogenous benzyl acetate reductase activity in E. coli (Beekwilder et al. 2006), a phenomenon was discovered whereby large amounts of an intermediate product (4-hydroxybenzylidene acetone) accumulated during the fermentation of the strains co-expressing the 4CL and BAS genes. After overexpression of RiRZS1, the intermediate was almost all converted into RK by BAR (Fig. 3). This suggested that the enzyme activity in the last step in the synthetic metabolic pathway was important for the formation of the end product RK.
Moreover, the strain’s biomass was also a factor affecting the yield of flavonoid product during fermentation by the engineered microbial strains (Katsuyama et al. 2007; Leonard et al. 2007; Leonard et al. 2008; Miyahisa et al. 2005). One alternative was to use a more nutrient-rich medium to increase the engineered strain’s biomass. When TB medium (with 5 g/l glucose added) was used as the fermentation medium, the RK titer was significantly enhanced, which might be due to an increase in cell concentration (OD600) (Fig. 4).
According to modular metabolic engineering, another strategy for improving RK production was based on balancing the expression of genes (At4CL1, Pc4CL2, RpBAS, and RiRZS1) by two types of approach, each with two models (Fig. 5). In approach A, the combination of moderate expression of module one and high expression of module two gave the best yields of RK production (CZ-8, CZ-14, respectively), indicating that matching the expression levels of module one and module two was necessary. Simultaneously, introducing the At4CL1 gene into the synthetic pathway gave better results than the Pc4CL2 gene, and the optimal recombinant strain CZ-8 achieved 90.97 mg/l RK titer (Fig. 6). However, the engineered strain with the Pc4CL2 gene introduced in approach B showed a higher yield of RK than that obtained in approach A. For example, strain CZ-7 produced 73.18 mg/l of RK, which was 2.12 times more than the yield of strain CZ-14 in approach A (Fig. 6b). Therefore, the preferred approach of the heterologous synthetic pathway was related to the heterologous genes from different sources.
As demonstrated in numerous reports, malonyl-CoA is generally considered to be a major barrier in the phenylpropanoid pathway, which is as a precursor for the synthesis of flavonoids (Cheng et al. 2016; Fowler and Koffas 2009; Leonard et al. 2007; Leonard et al. 2008; Lim et al. 2011; Miyahisa et al. 2005; Xu et al. 2011), and the levels of malonyl-CoA is found to be typically low within cells (Takamura and Nomura 1988). Several tools or approaches have been utilized to increase the concentration of intracellular malonyl-CoA and to find relative optimal expressions (Pfleger et al. 2006). Acetyl-CoA carboxylase (accBC and dtsR1) was cloned from C. glutamicum to increase the intracellular concentration of malonyl-CoA (Gande et al. 2007; Miyahisa et al. 2005). However, the expression of acetyl-CoA carboxylase did not significantly improve the yield of RK in our engineered strains (data not shown). Thus, re-balancing of entire metabolic pathways is needed when new heterologous genes are introduced.
References
Abe I, Takahashi Y, Morita H, Noguchi H (2001) Benzalacetone synthase. A novel polyketide synthase that plays a crucial role in the biosynthesis of phenylbutanones in Rheum palmatum. Eur J Biochem 268:3354–3359. https://doi.org/10.1046/j.1432-1327.2001.02255.x
Abe T, Morita H, Noma H, Kohno T, Noguchi H, Abe I (2007) Structure function analysis of benzalacetone synthase from Rheum palmatum. Bioorg Med Chem Lett 17(11):3161–3166. https://doi.org/10.1016/j.bmcl.2007.03.029
Ajikumar PK, Xiao WH, Tyo KE, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G (2010) Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 330(6000):70–74. https://doi.org/10.1126/science.1191652
Beekwilder J, Wolswinkel R, Jonker H, Hall R, de Vos CH, Bovy A (2006) Production of resveratrol in recombinant microorganisms. Appl Environ Microbiol 72(8):5670–5672. https://doi.org/10.1128/AEM.00609-06
Beekwilder J, van der Meer IM, Sibbesen O, Broekgaarden M, Qvist I, Mikkelsen JD, Hall RD (2007) Microbial production of natural raspberry ketone. J Biotechnol 2(10):1270–1279. https://doi.org/10.1002/biot.200700076
Borejsza-Wysocki W, Hrazdina G (1994) Biosynthesis of p-hydroxyphenylbutan-2-one in raspberry fruits and tissue cultures. Phytochem 35(3):623–628. https://doi.org/10.1016/S0031-9422(00)90575-2
Cheng Z, Jiang J, Wu H, Li Z, Ye Q (2016) Enhanced production of 3-hydroxypropionic acid from glucose via malonyl-CoA pathway by engineered Escherichia coli. Bioresour Technol 200:897–904. https://doi.org/10.1016/j.biortech.2015.10.107
Fischer M, Böker A, Berger RG (2001a) Fungal formation of raspberry ketone differs from the pathway in plant cell culture. Bioresour Technol 15(3):147–155. https://doi.org/10.1081/fbt-100107626
Fischer M, Böker A, Berger RG (2001b) Raspberry ketone from submerged cultured cells of the basidiomycete Nidula niveo-tomentosa. Biotechnol Prog 17:568–572
Fowler ZL, Koffas MA (2009) Biosynthesis and biotechnological production of flavanones: current state and perspectives. Appl Microbiol Biotechnol 83(5):799–808. https://doi.org/10.1007/s00253-009-2039-z
Gande R, Dover LG, Krumbach K, Besra GS, Sahm H, Oikawa T, Eggeling L (2007) The two carboxylases of Corynebacterium glutamicum essential for fatty acid and mycolic acid synthesis. J Bacteriol 189(14):5257–5264. https://doi.org/10.1128/JB.00254-07
Hakkinen ST, Seppanen-Laakso T, Oksman-Caldentey KM, Rischer H (2015) Bioconversion to raspberry ketone is achieved by several non-related plant cell cultures. Front Plant Sci 6:1035. https://doi.org/10.3389/fpls.2015.01035
Hrazdina G (2006) Aroma production by tissue cultures. J Agric Food Chem 54(4):1116–1123. https://doi.org/10.1021/jf053146w
Hugueny P, Dumont B, Ropert F, Belin JM (1995) The raspberry ketone, a biotechnological way for production. Colloq De Linra
Katsuyama Y, Funa N, Miyahisa I, Horinouchi S (2007) Synthesis of unnatural flavonoids and stilbenes by exploiting the plant biosynthetic pathway in Escherichia coli. Chem Biol 14(6):613–621. https://doi.org/10.1016/j.chembiol.2007.05.004
Kim M, Baek HS, Lee M, Park H, Shin SS, Choi DW, Lim KM (2016) Rhododenol and raspberry ketone impair the normal proliferation of melanocytes through reactive oxygen species-dependent activation of GADD45. Toxicol in Vitro 32:339–346. https://doi.org/10.1016/j.tiv.2016.02.003
Koeduka T, Watanabe B, Suzuki S, Hiratake J, Mano J, Yazaki K (2011) Characterization of raspberry ketone/zingerone synthase, catalyzing the alpha, beta-hydrogenation of phenylbutenones in raspberry fruits. Biochem Biophys Res Commun 412(1):104–108. https://doi.org/10.1016/j.bbrc.2011.07.052
Larsen M, Poll L (1992) Odour thresholds of some important aroma compounds in strawberries. Z Lebensm Unters Forsch 191:129–131. https://doi.org/10.1007/BF01201770
Larsen M, Poll L, Callesen O, Lewis M (1991) Relations between the content of aroma compounds and the sensory evaluation of 10 raspberry varieties (Rubus idaeusL). Acta Agric Scand 41(4):447–454. https://doi.org/10.1080/00015129109439927
Lee J (2016) Further research on the biological activities and the safety of raspberry ketone is needed. NFS J 2:15–18. https://doi.org/10.1016/j.nfs.2015.12.001
Lee D, Lloyd ND, Pretorius IS, Borneman AR (2016) Heterologous production of raspberry ketone in the wine yeast Saccharomyces cerevisiae via pathway engineering and synthetic enzyme fusion. Microb Cell Factories 15:49. https://doi.org/10.1186/s12934-016-0446-2
Leonard E, Lim KH, Saw PN, Koffas MA (2007) Engineering central metabolic pathways for high-level flavonoid production in Escherichia coli. Appl Environ Microbiol 73(12):3877–3886. https://doi.org/10.1128/AEM.00200-07
Leonard E, Yan Y, Fowler Z, Li Z, Lim C, Koffas MA (2008) Strain improvement of recombinant Escherichia coli for efficient production of plant flavonoids. Mol Pharm 5:257–265
Lim CG, Fowler ZL, Hueller T, Schaffer S, Koffas MA (2011) High-yield resveratrol production in engineered Escherichia coli. Appl Environ Microbiol 77(10):3451–3460. https://doi.org/10.1128/AEM.02186-10
Miyahisa I, Kaneko M, Funa N, Kawasaki H, Kojima H, Ohnishi Y, Horinouchi S (2005) Efficient production of (2S)-flavanones by Escherichia coli containing an artificial biosynthetic gene cluster. Appl Microbiol Biotechnol 68(4):498–504. https://doi.org/10.1007/s00253-005-1916-3
Morimoto C, Satoh Y, Hara M, Inoue S, Tsujita T, Okuda H (2005) Anti-obese action of raspberry ketone. Life Sci 77(2):194–204. https://doi.org/10.1016/j.lfs.2004.12.029
Pfleger BF, Pitera DJ, Smolke CD, Keasling JD (2006) Combinatorial engineering of intergenic regions in operons tunes expression of multiple genes. Nat Biotechnol 24:1027. https://doi.org/10.1038/nbt1226 https://www.nature.com/articles/nbt1226#supplementary-information
Santos CN, Koffas M, Stephanopoulos G (2011) Optimization of a heterologous pathway for the production of flavonoids from glucose. Metab Eng 13(4):392–400. https://doi.org/10.1016/j.ymben.2011.02.002
Stabnikova O, Wang JY, Ivanov V (2010) Value-added biotechnological products from organic wastes. Humana Press, New Jersey
Takamura Y, Nomura G (1988) Changes in the intracellular concentration of acetyl-CoA and malonyl-CoA in relation to the carbon and energy metabolism of Escherichia coli K12. J Gen Microbiol 134:2249–2253. https://doi.org/10.1099/00221287-134-8-2249
Wang L, Meng X, Zhang F (2012) Raspberry ketone protects rats fed high-fat diets against nonalcoholic steatohepatitis. J Med Food 15(5):495–503. https://doi.org/10.1089/jmf.2011.1717
Wu J, Liu P, Fan Y, Bao H, Du G, Zhou J, Chen J (2013) Multivariate modular metabolic engineering of Escherichia coli to produce resveratrol from L-tyrosine. J Biotechnol 167(4):404–411. https://doi.org/10.1016/j.jbiotec.2013.07.030
Xu P, Ranganathan S, Fowler ZL, Maranas CD, Koffas MA (2011) Genome-scale metabolic network modeling results in minimal interventions that cooperatively force carbon flux towards malonyl-CoA. Metab Eng 13(5):578–587. https://doi.org/10.1016/j.ymben.2011.06.00
Funding
The authors are grateful to the financial support from the National Natural Science Foundation of China (Grant No. 21604032), the National First-class Discipline Program of Light Industry Technology and Engineering (Grant No. LITE2018–04), and the Topnotch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 554 kb)
Rights and permissions
About this article
Cite this article
Wang, C., Zheng, P. & Chen, P. Construction of synthetic pathways for raspberry ketone production in engineered Escherichia coli. Appl Microbiol Biotechnol 103, 3715–3725 (2019). https://doi.org/10.1007/s00253-019-09748-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09748-5