Abstract
The exploration of nanoscale materials for their therapeutic potential against emerging and re-emerging infections has been increased in recent years. Silver nanoparticles (AgNPs) are known to possess antimicrobial activities against different pathogens including viruses and provide an excellent opportunity to develop new antivirals. The present study focused on biological synthesis of AgNPs from Andrographis paniculata, Phyllanthus niruri, and Tinospora cordifolia and evaluation of their antiviral properties against chikungunya virus. Synthesized plants AgNPs were characterized to assess their formation, morphology, and stability. The cytotoxicity assays in Vero cells revealed that A. paniculata AgNPs were most cytotoxic with maximum non-toxic dose (MNTD) value of 31.25 μg/mL followed by P. niruri (MNTD, 125 μg/mL) and T. cordifolia AgNPs (MNTD, 250 μg/mL). In vitro antiviral assay of AgNPs based on degree of inhibition of cytopathic effect (CPE) showed that A. paniculata AgNPs were most effective, followed by T. cordifolia and P. niruri AgNPs. The results of antiviral assay were confirmed by cell viability test using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) dye, which revealed that A. paniculata AgNPs inhibited the virus to a maximum extent. The cell viability of CHIKV-infected cells significantly increased from 25.69% to 80.76 and 66.8%, when treated with A. paniculata AgNPs at MNTD and ½MNTD, respectively. These results indicated that use of plants AgNPs as antiviral agents is feasible and could provide alternative treatment options against viral diseases which have no specific antiviral or vaccines available yet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanoparticles and their use for human healthcare and medicine has been an exciting area of nanotechnology. In recent years, metallic nanoparticles have been studied extensively for their medicinal potential as their dimensions are comparable to the biomolecules. Due to high surface–to-volume ratio, nanoparticles can interact with the pathogenic microbes efficiently and also play useful roles in drug delivery. Hence, more research has been focused on development and biological applications of ultrafine nanoparticles with sizes ranging from 1 to 100 nm (Salata 2004; Galdiero et al. 2011; Zhang et al. 2016). Among metallic nanoparticles, silver nanoparticles (AgNPs) are of particular medicinal interest because of antimicrobial properties of the silver metal. AgNPs have been used efficiently as antimicrobial agents against a wide range of bacteria and fungi. These properties of AgNPs are utilized in wound dressing, ointments, medical implants coatings, lining of food containers, and other applications (Kim et al. 2007). The broad-spectrum antimicrobial properties of AgNPs have been used successfully against drug-resistant strains of bacteria (Rai et al. 2012). In the recent decade, silver nanoparticles have been used as novel antiviral agents (Kim et al. 2007; Lara et al. 2010).
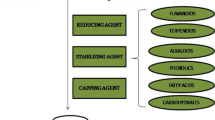
Green synthesis or phytosynthesis of AgNPs from medicinal plants is a cost-effective, eco-friendly, and non-toxic approach of nanoparticles generation than the physical or chemical approach. Medicinal plants have been very useful in traditional medicine since old times and are rich source of bioactive compounds having antiviral activities. Use of medicinal plants and their compounds is more effective and safer than synthetic drugs in treatment of certain diseases. Medicinal plants are a rich source of bioactive components including various secondary metabolites like alkaloids, flavonoids, phenolics, saponins, terpenoids, tannins, etc. Medicinal plants like Andrographis paniculata, Olea eurolaea, Pachyma hoelen, Phyllanthus niruri, Tinospora cordifolia, Swertia chirata, etc., have already shown antiviral properties against viruses like dengue, herpes, and human immunodeficiency virus (HIV) (Venkateswaran et al. 1987; Kalikar et al. 2008; Verma et al. 2008; Tang et al. 2012; Lee et al. 2013; Wintachai et al. 2015).
Chikungunya virus (CHIKV) is an enveloped arbovirus belonging to the genus Alphavirus of family Togaviridae and is responsible for the febrile illness called chikungunya fever (Robinson 1955; Griffin 2013). The disease has emerged as a global health threat due to high morbidity with maximum cases reported from Africa and Asia (Schwartz and Albert 2010; Halstead 2015; Rodrigues et al. 2016). CHIKV is transmitted in humans by female Aedes aegypti and Aedes albopictus mosquitoes in the domestic environment. These mosquitoes are found biting throughout daylight hours and symptoms of illness appears usually after 2–4 days of incubation period (Griffin 2013). Clinical manifestations include high fever, headaches, nausea, fatigue, rash, rashes, and severe joint pain which can be very debilitating. It is usually a self-limiting disease but in recent outbreaks, neurological complications as well as heart and gastrointestinal complications have been reported making it more severe (Schwartz and Albert 2010; Rodrigues et al. 2016). Moreover, the virus produces proinflammatory factors in the joint tissue which results in joint pain symptoms lasting for weeks up to years. Asymptomatic infections also occur occasionally and have been reported in around 1–15% of total cases (Schwartz and Albert 2010; Griffin 2013). There are no specific antivirals or vaccines available against chikungunya and the treatment is solely based on symptomatic and supportive care. So far, the disease control strategies rely on effective mosquito management programs only. There is a strong need to investigate and develop noble antiviral agents against chikungunya which will help in curbing the infection and controlling severe outbreaks.
Considering this, the present study aimed at synthesis of AgNPs from three medicinal plants viz., A. paniculata, P. niruri, and T. cordifolia and evaluation of their anti-chikungunya activities in Vero cells.
Materials and methods
Chemicals and reagents
Various chemicals and reagents used in the study include silver nitrate (AgNO3) which was procured from Sisco Research Laboratories (SRL), India; 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT); Dulbecco’s Modified Eagle’s Medium (DMEM); dimethyl sulfoxide (DMSO); fetal bovine serum (FBS); and other media supplements which were procured from HiMedia Laboratories Pvt. Ltd. India.
Preparation of plant extracts
All three plants, A. paniculata, P. niruri, and T. cordifolia were identified and samples were collected from the Herbal Garden of Maharshi Dayanand University, Rohtak, India. The stem of T. cordifolia and leaves of P. niruri and A. paniculata were selected for the preparation of plant extracts. The plant parts were cleaned twice with double-distilled water, diced into fine pieces, and dried at room temperature. Forty grams of stem of T. cordifolia and 10 g each of leaves of P. niruri and A. paniculata were added into separate 250-mL Erlenmeyer flasks along with 100 mL of sterile double-distilled water and heated for 20 min. The aqueous plant extracts were filtered through Whatman No.1 filter paper and were stored at 4 °C until used further.
Green synthesis of plant silver nanoparticles
Silver nanoparticles (AgNPs) were synthesized from respective plant extract by deploying green synthesis route. The plant extracts were treated with 1 mM aqueous solution of AgNO3 and were incubated at room temperature for 24 h. The incubation step was performed in dark to prevent photoactivation of silver nitrate. The color of each solution changed from pale yellow to yellowish brown within 10 min of mixing, showing reduction of AgNO3 to Ag+ ions. The aqueous solutions of AgNPs were centrifuged at 10,000×g for 20 min at room temperature followed by washing with sterile distilled water. The procedure was repeated three times to remove any unbound material from AgNPs, and final solution was lyophilized and stored as powdered form at 4 °C for further use.
Characterization of plant silver nanoparticles
Characterization of plant AgNPs was done by using ultraviolet-visible (UV-Vis) spectroscopy, Fourier transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), dynamic light scattering (DLS), and zeta potential measurements. UV-Vis absorbance spectral analysis of aqueous suspension of AgNPs was carried out using a spectrophotometer (UV-2450, Shimadzu Corp., Japan). The absorbance of each sample was taken at 300–600 nm with a resolution of ± 1 nm. The chemical compositions and potential biomolecules of the AgNPs were analyzed by FTIR spectrum, recorded by using an ALPHA FT-IR spectrometer (Bruker, Germany). The lyophilized AgNPs of each plant were subjected to FTIR using the potassium bromide (KBr) pellet technique and the measurements were taken in the region of 500–4000 cm−1. Morphology of plant AgNPs was studied through scanning electron micrograph of the aqueous suspension of AgNPs. The sample was prepared by mounting AgNPs suspension on specimen stubs and coated with gold by ion sputter coater (Hitachi Model-E1010, Japan). The SEM observations were performed by using EVO 18 special edition scanning electron microscope (Carl Zeiss Inc., Germany). The size distribution and stability of synthesized plant AgNPs was also analyzed by performing DLS and zeta potential measurements. These observations were made by using Malvern Zetasizer Nano ZS (Malvern Panalytical, UK).
Virus and Vero cells
The stocks of Vero cells were obtained from the Department of Microbiology, All India Institutes of Medical Sciences (AIIMS), New Delhi, India. The cells were grown in DMEM supplemented with 10% FBS by following standard tissue culture procedure (Ammerman et al. 2008). The cells were incubated at 37 °C in a humidified incubator with 5% CO2 atmosphere and were sub-cultured at 80–90% confluency. Chikungunya virus (CHIKV) of Indian Ocean lineage (IOL) genotype was used in the study and propagated in Vero cells. The virus was harvested after observation of cytopathic effect (CPE) or after 5 days postinfection. The virus stock was collected and stored at − 80 °C until used.
Computation of 50% tissue culture infectious dose
CHIKV stock was characterized for infectious titer in terms of 50% tissue culture infectious dose (TCID50/mL). Briefly, 5 × 103 Vero cells were seeded into each well of the 96-well plate and incubated under standard cell culture conditions for 24 h. The cells were infected with 100 μL of ten-fold serially diluted CHIKV. Ten replicates of each virus dilution were included and the plate was incubated for 5 days and observed for CPE. The wells showing CPE were marked as “+” and without CPE were marked as “−.” The highest dilution of the virus which demonstrated > 50% CPE was considered to calculate TCID50/mL of the virus stock by using the Reed-Muench method (Reed and Muench 1938).
Cytotoxicity assay
Cytotoxic activities of synthesized AgNPs from all three plants were evaluated on Vero cells and maximum non-toxic dose (MNTD) was determined for each plant AgNPs. Vero cells (1 × 104 cells per well) were seeded onto 96-well flat-bottom plate (Nunc, Thermo Fisher Scientific, USA) and incubated at 37 °C with 5% CO2 in a humidified incubator for 24 h until 80–90% confluency was reached. After 24 h, the old media was removed and replaced with 100 μL of new DMEM media (supplemented with 2% FBS) containing AgNPs concentrations ranging from 1000 to 7.81 μg/mL. Each concentration of AgNPs was set in triplicates along with negative control containing DMEM media without AgNPs. The plate was incubated at 37 °C with 5% CO2 in a humidified incubator for 72 h and cell viability was determined by using MTT assay (Denizot and Lang 1986). Briefly, 10 μL of MTT solution (5 mg/mL, prepared in PBS) was added into each well of the plate and incubated for 3–4 h in optimum conditions. All the contents were removed from each well and 100 μL of DMSO solution was added into each well to dissolve the formazan crystals. After incubating at room temperature for 30 min, the absorbance of the plate was taken at 595 nm using a microplate reader (Bio-Rad, USA). From absorbance values, percentage cell viability and toxicity were determined. Cell viability percentage was determined by using the following formula:
The cytotoxicity assay was repeated twice and triplicates were included each time.
In vitro antiviral assay
For evaluation of anti-CHIKV activity of the synthesized plant AgNPs, the Vero cells were treated with the calculated MNTD and half of the MNTD value of A. paniculata, P. niruri, and T. cordifolia AgNPs. The assay was initiated by incubating 96-well plate having a monolayer of Vero cells with 100 μL of each plant AgNPs at its MNTD and ½MNTD. After 1 h of incubation, 100 μL of CHIKV at 103 TCID50/mL was added into each well except in the wells marked as cell control. The plate setup included cell control (cells only), virus control (cells and virus only), and the AgNPs-treated cells. Each plant AgNPs concentration (MNTD and ½MNTD) was tried in triplicates. The plate was incubated for 5 days under standard culture conditions and observed for the presence of CPE thereafter. The results of CPE inhibition were analyzed by using the grading system method (Kudi and Myint 1999). The degree of CPE inhibition was marked as: ++++ (>7 5%), +++ (50–74%), ++ (25–49%), + (1–24%), and – (no inhibition of CPE). Cell viability percentages for cell control, virus control, and infected cells treated with plant AgNPs were determined by using MTT assay as described in the previous section. The antiviral assay was repeated twice and triplicates were included each time.
Statistical analysis
The experiment data were statistically evaluated using GraphPad Prism version 7.04 (GraphPad Software, CA, USA). One-way analysis of variance (ANOVA) was used to determine the difference in the mean values of treated and control samples. For all calculations, a p value of < 0.05 was considered to be significant.
Results
Synthesis and characterization of plant AgNPs
UV-Vis spectroscopy
The plant AgNPs were successfully synthesized from A. paniculata, P. niruri, and T. cordifolia. The color of each plant extracts changed from light yellow to yellowish brown after addition of silver nitrate solution (Fig. 1). This color change was observed due to reduction of Ag+ ions in the aqueous solution of AgNO3 into AgNPs. The UV-Vis absorption spectra of plant AgNPs were recorded in the range of 300–600 nm (Fig. 2). Synthesized plant AgNPs showed absorption maxima in the range of 420–455 nm due to the surface plasmon resonance (SPR) phenomenon. A broad SPR band was observed at 425 nm for AgNPs synthesized from A. paniculata. The SPR absorption maxima for AgNPs of P. niruri and T. cordifolia were seen at 424 and 452 nm, respectively. The broad absorption peaks were observed for synthesized plant AgNPs which showed the polydispersed nanoparticles in the solution.
FTIR spectroscopy
The FTIR analysis of synthesized plant AgNPs was carried out to identify the functional groups of the active biomolecules playing roles of reducing as well as capping agents in the synthesis of nanoparticles. The FTIR spectra of lyophilized plant AgNPs are shown in Fig. 3, in which spectrum of A. paniculata AgNPs showed prominent transmittance bands at 3240 cm−1 (-O-H- or -N-H-), 2929 cm−1 (-C-H-), 1391 cm−1 (-C-N-), 1575 cm−1 (-N-H-), and 1032 and 1075 cm−1 (-C-N-) (Fig. 3a). The spectrum of P. niruri showed distinct transmittance peaks at 3268 cm−1 (-O-H- or -N-H-), 2920 cm−1 (-C-H-), 1513 (-N-H-), 1633 cm−1 (-C=O), and 1000 and 1149 cm−1 (-C-N-) (Fig. 3b). The FTIR spectrum of T. cordifolia displayed sharp bands at 3319 cm−1 (-O-H- or -N-H-), 2831 and 2943 cm−1 (-C-H-), 1418 and 1449 cm−1 (-C-H-), and 1021 cm−1 (-C-N-) (Fig. 3c).
Scanning Electron microscopy
The plant AgNPs were also characterized for their size and morphology by SEM. Figure 4 shows the SEM images of the plant AgNPs along with their size. The average size of A. paniculata AgNPs were found to be in the range of 70–95 nm (Fig 4a). The AgNPs synthesized from P. niruri demonstrated a range of particle size from 70 to 120 nm (Fig. 4b) and AgNPs of T. cordifolia were found to be in the range of 50–70 nm (Fig. 4c).
Dynamic light scattering and zeta potential measurements
The average size distribution and stability of synthesized plant AgNPs were measured by DLS and zeta potential measurements as shown in Fig. 5. All synthesized plant AgNPs were of negative polarity and were stable at room temperature. The size measurements by DLS showed that all of the plant AgNPs were polydispersed and having a particle size less than 100 nm. The DLS of A. paniculata AgNPs showed the average size of 68.06 nm (Fig. 5a) and zeta potential measurement showed that particles carry a potential of − 21.4 mV (Fig. 5d). The size of P. niruri AgNPs was found to be in the range of 7 to72 nm with an average size of 28.38 nm (Fig. 5b) and these carry a potential of − 20 mV (Fig. 5e). Likewise, the size of T. cordifolia AgNPs was in the range of 4–54 nm with an average size of 37.10 nm (Fig. 5c) and zeta potential measurements showed that these AgNPs were of negative polarity with a potential of − 17.0 mV (Fig. 5f). The results of SEM and DLS were in agreement for the size measurement of synthesized plant AgNPs.
Cytotoxicity assays of plant AgNPs
The cytotoxicity of synthesized plant AgNPs were evaluated on the Vero cells before determining anti-CHIKV activity. The MNTD for synthesized plant AgNPs were determined by observing the cytopathic effect of ten-fold serial dilutions of plant AgNPs on Vero cells. As shown in Fig. 6, the MNTD value for AgNPs of T. cordifolia was found to be 250 μg/mL, which was highest among all three plant AgNPs (Fig. 6c). The MNTD values for AgNPs of A. paniculata and P. niruri were 31.25 and 125 μg/mL, respectively (Fig. 6a, b). From the cytotoxicity assay, we found that among all three plants, A. paniculata AgNPs were highly toxic while T. cordifolia AgNPs were least toxic to Vero cells. The calculated MNTD of these plant AgNPs were selected further for in vitro antiviral studies.
In vitro antiviral assay
The antiviral potential of synthesized plant AgNPs was evaluated against CHIKV using Vero cells. The antiviral activities of AgNPs were measured in terms of inhibition of CPE as well as increase of percentage cell viability. AgNPs of A. paniculata and T. cordifolia showed significant inhibition of CPE in comparison to virus control (Table 1). A. paniculata AgNPs at MNTD and ½MNTD brought about 75–100% and 25–49% inhibition of CPE, respectively. T. cordifolia AgNPs at both MNTD and ½MNTD showed 25–49% inhibition of CPE whereas P. niruri AgNPs did not show any significant inhibition of CPE in comparison to the virus control. These results were further compared with cell viability calculations using the MTT assay (Fig. 7). The wells marked as negative control (untreated cells) and virus control showed cell viability of 100% and 25.69% ± 1.53%, respectively. As shown in Fig. 7, MNTD and ½MNTD treatments of AgNPs of A. paniculata and T. cordifolia displayed significant increase in cell viability in comparison to virus control. A. paniculata AgNPs at MNTD and ½MNTD exhibited cell viability of 80.76% ± 1.5% and 66.8% ± 2.1%, respectively. Similarly, T. cordifolia AgNPs showed cell viability of 75.35% ± 3.4% and 55.98% ± 4.35% at MNTD and ½MNTD, respectively. However, treatment of infected cells with P. niruri AgNPs at MNTD and ½MNTD showed a small increase in cell viability as compared to the other two plant AgNPs. For MNTD and ½MNTD of P. niruri AgNPs, cell viability of 30.56% ± 1.1% and 26.47% ± 1.3% were recorded.
Discussion
The medicinal plants A. paniculata, P. niruri, and T. cordifolia are known to possess antiviral properties (Venkateswaran et al. 1987; Kalikar et al. 2008; Wintachai et al. 2015). In the present study, plant AgNPs were synthesized from aqueous extracts of A. paniculata, P. niruri, and T. cordifolia and their antiviral activities were evaluated against CHIKV in vitro using Vero cells. The formation of AgNPs was confirmed by visual inspection of change in color from light yellow to dark colloidal brown after 10 min of mixing of silver nitrate solution and aqueous extracts. This color change was observed because of excitation of surface plasmon vibrations which is a characteristic feature of silver nanoparticles in solution (Mulavney 1996; Chung et al. 2016). During the synthesis of AgNPs, silver ions are reduced to form metallic silver particles. The bioactive compounds present in the plant extract help in reduction of silver ions as well as stabilization of newly formed silver nanoparticles by preventing their aggregation (Chung et al. 2016). UV-Vis spectroscopy is used to monitor formation and stability of AgNPs in solution. The free electrons of metal nanoparticles absorb energy of the light waves and produce surface plasmon resonance (SPR) bands which are the characteristics of AgNPs. The position and intensity of these SPR bands rely on shape and size of AgNPs as well as dispersion medium used (Pileni 1998; Chung et al. 2016). The UV-Vis spectra of all three plant AgNPs showed the SPR absorption maxima within 400–500 nm. The broad peaks in UV-Vis spectra indicated that nanoparticles were polydispersed in nature due to slow reduction of silver ions. None of the AgNPs showed SPR maxima above 500 nm, indicating that the particles were similar in size and shape. In a related study involving green synthesis of AgNPs from A. paniculata, Sinha and Paul (2015) reported the absorption maxima at 432 nm (Sinha and Paul 2015). In another study on AgNPs from P. niruri, broad absorption peak was reported at 420 nm (Suresh et al. 2015). Similar observations have been made in the previous studies involving green synthesis of AgNPs (Banerjee et al. 2014; Singh et al. 2014). The FTIR analysis of synthesized AgNPs revealed the possible bioactive molecules which acted as reducing and capping agents during nanoparticle synthesis. The FTIR spectra of A. paniculata AgNPs exhibited broad transmittance peaks at 3240 cm−1 due to stretching vibrations of the -O-H- or –N-H- group and at 2929 cm−1 due to -C-H- stretching vibrations. These stretchings indicated the presence of alcoholic or amino and aldehydic groups associated with AgNPs. The presence transmittance peaks at 1391 and 1575 cm−1 confirmed the presence of aliphatic amine (-C-N-) and aromatic ring (-C=C-), respectively. The other two prominent peaks at 1032 and 1075 cm−1 were due to stretching vibrations of aliphatic amine or alcoholic/phenolic groups. Likewise, FTIR spectra of AgNPs of P. niruri and T. cordifolia indicated the presence of -OH, -NH, -CO,-C=C-, and -C-N- groups. These FTIR spectra denote the presence of bioactive compounds like polyols, flavonoids, polyphenols, and terpenoids, which are responsible for synthesis and stabilization of AgNPs (Banerjee et al. 2014; Singh et al. 2014; Sinha and Paul 2015; Chung et al. 2016). It has been reported that in phytosynthesis of AgNPs, flavonoids play a role in reduction of silver ions whereas phenolics, terpenoids, and amines act as capping agents (Iravani 2011). Further characterization of size and shape of plant AgNPs was done by SEM analysis. The plant AgNPs were primarily spherical in shape with an average size which ranged from 50 to 120 nm. Previous studies have reported that commonly nanoparticles are spherical in shape but for some plants, they may be cuboidal, flat, or triangular in appearance (Banerjee et al. 2014; Singh et al. 2014; Sinha and Paul 2015). The in vitro estimation of safe therapeutic dose of plant AgNPs is essential before checking their effect in vivo. The cytotoxicity of biosynthesized AgNPs were examined on the Vero cells in terms of cytopathic effect and MNTD of each plant AgNPs was determined. In our study, MNTD of A. paniculata AgNPs was found to be 31.25 μg/mL, which was lowest among all three plant AgNPs. The MNTD values of AgNPs of P. niruri and T. cordifolia were found to be 125 and 250 μg/mL, respectively. These results indicated that A. paniculata AgNPs were most toxic while T. cordifolia AgNPs were least toxic when tested in Vero cells. The cytotoxicity of AgNPs may be attributed to release of silver ions and generation of reactive oxygen species (Zhang et al. 2016). The level of cytotoxicity was also affected by the size of nanoparticles and smaller sized being more toxic than the larger ones (Liu et al. 2010).
The antiviral activities of synthesized plant AgNPs were determined by assessing the cell viability of Vero cells after treating with MNTD and ½MNTD of plant AgNPs and infecting with CHIKV. The results indicated that AgNPs synthesized from aqueous extract of A. paniculata has significant anti-chikungunya potential. The untreated infected cells showed about 25% cell viability which increased to about 81 and 67% after treatment with MNTD and ½MNTD of A. paniculata AgNPs, respectively. Andrographolide is the main phytoconstituent found in extract of A. paniculata and is known to have antiviral activity against a number of viruses (Lin et al. 2008; Gupta et al. 2017). In a previous study, Wintachai et al. (2015) have reported that andrographolide has potential to inhibit CHIKV genome replication in HepG2 cells (Wintachai et al. 2015). In our study, T. cordifolia AgNPs also showed anti-chikungunya activity and increased the viability of virus-infected Vero cells to about 75 and 56% when treated with AgNPs at MNTD and ½MNTD, respectively. T. cordifolia is known to possess immunomodulatory and immunostimulant properties due to the presence of different bioactive compounds including alkaloids, diterpenoid lactones, glycosides, and steroids (Kalikar et al. 2008). The crude extract of T. cordifolia has been reported to have anti-HIV potential and inhibited HIV reverse transcriptase activity (Estari et al. 2012). The extract of P. niruri has been reported to inhibit woodchuck hepatitis virus, hepatitis B virus, HIV, and dengue virus (Venkateswaran et al. 1987; Ogata et al. 1992; Lee et al. 2013). In our study, AgNPs of P. niruri were also inspected for anti-chikungunya activity and were found to inhibit CHIKV to a lesser extent than AgNPs of T. cordifolia and A. paniculata. The antiviral activities of AgNPs have been reported against a number of viruses such as herpes simplex virus, vaccinia virus, influenza virus, respiratory syncytial virus, monkey pox virus, human immunodeficiency virus, and hepatitis B virus (Rogers et al. 2008; Lara et al. 2010; Galdiero et al. 2011; Mori et al. 2013; Trefry and Wooley 2013; Hu et al. 2014; Yang et al. 2016; Shevtsov et al. 2017). AgNPs are known to interfere with the binding of virus particles to host cell and thereby preventing their entry but the exact mechanism of their antiviral action has not been well understood. Moreover, the interaction of AgNPs with different types of cells is a complex issue which requires further research in order to elucidate antiviral activities as well as cytotoxic potential of the nanoparticles.
In conclusion, AgNPs were successfully synthesized from aqueous extracts of A. paniculata, P. niruri, and T. cordifolia. The characterization of plant AgNPs showed the formation of stable nanoparticles having bioactive phytoconstituents linked to them. The AgNPs synthesized from A. paniculata and T. cordifolia showed excellent antiviral activity against CHIKV when tested on Vero cells. The biosynthesis of AgNPs using plant extract is simple, efficient, cost-effective, and an environment friendly alternative to develop broad-spectrum antiviral agents which could provide the alternative for treatment of viral diseases against which no specific antivirals or vaccines are available. The development of plant AgNPs with therapeutic potential could also combat with the problem of developing resistance as observed with conventional antivirals. However, before using plant AgNPs as therapeutic agents and safely design antiviral drugs, detailed in vivo studies are needed to understand their effects and mechanism of action at the molecular level.
References
Ammerman NC, Beier-Sexton M, Azad AF (2008) Growth and maintenance of Vero cell lines. Curr Protoc Microbiol 11(1):A.4E.1–A.4E.7. https://doi.org/10.1002/9780471729259.mca04es11
Banerjee P, Satapathy M, Mukhopahayay A, Das P (2014) Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour Bioprocess 1(3). https://doi.org/10.1186/s40643-014-0003-y
Chung IM, Park I, Seung-Hyun K, Thiruvengadam M, Rajakumar G (2016) Plant-mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale Res Lett 11(40):40. https://doi.org/10.1186/s11671-016-1257-4
Denizot F, Lang R (1986) Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89(2):271–277. https://doi.org/10.1016/0022-1759(86)90368-6
Estari M, Venkanna L, Reddy AS (2012) In vitro anti-HIV activity of crude extracts from Tinospora cordifolia. BMC Infect Dis 12(Suppl 1):P10. https://doi.org/10.1186/1471-2334-12-S1-P10
Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V, Galdiero M (2011) Silver nanoparticles as potential antiviral agents. Molecules 16(10):8894–8918. https://doi.org/10.3390/molecules16108894
Griffin DE (2013) Alphaviruses. In: Knipe DM, Howley PM (eds) Fields virology, vol 1, 6th edn. Lippincott Williams & Wilkins, Philadelphia, pp 651–686
Gupta S, Mishra KP, Ganju L (2017) Broad-spectrum antiviral properties of andrographolide. Arch Virol 162(3):611–623. https://doi.org/10.1007/s00705-016-3166-3
Halstead SB (2015) Reappearance of chikungunya, formerly called dengue, in the Americas. Emerg Infect Dis 21(4):561–561. https://doi.org/10.3201/eid2104.141723
Hu RL, Li SR, Kong FJ, Hou RJ, Guan XL, Guo F (2014) Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genet Mol Res 13(3):7022–7028. https://doi.org/10.4238/2014.March.19.2
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650. https://doi.org/10.1039/C1GC15386B
Kalikar MV, Thawani VR, Varadpande UK, Sontakke SD, Singh RP, Khiyani RK (2008) Immunomodulatory effect of Tinospora cordifolia extract in human immuno-deficiency virus positive patients. Indian J Pharm 40(3):107–110. https://doi.org/10.4103/0253-7613.42302
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine 3(1):95–101. https://doi.org/10.1016/j.nano.2006.12.001
Kudi AC, Myint SH (1999) Antiviral activity of some Nigerian medicinal plant extracts. J Ethnopharmacol 68(1–3):289–294. https://doi.org/10.1016/S0378-8741(99)00049-5
Lara HH, Ayala-Nunez NV, Ixtepan-Turrent L, Rodriguez-Padilla C (2010) Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol 8(1):1. https://doi.org/10.1186/1477-3155-8-1
Lee SH, Tang YQ, Rathkrishnan A, Wang SM, Ong KC, Manikam R, Payne BJ, Jaganath IB, Sekaran SD (2013) Effects of cocktail of four local Malaysian medicinal plants (Phyllanthus spp.) against dengue virus 2. BMC Complement Altern Med 13(192). https://doi.org/10.1186/1472-6882-13-192
Lin TP, Chen SY, Duh PD, Chang LK, Liu YN (2008) Inhibition of the epstein-barr virus lytic cycle by andrographolide. Biol Pharm Bull 31(11):2018–2023. https://doi.org/10.1248/bpb.31.2018
Liu W, Wu Y, Wang C, Li HC, Wang T, Liao CY, Cui L, Zhou QF, Yan B, Jiang GB (2010) Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology 4(3):319–330. https://doi.org/10.3109/17435390.2010.483745
Mori Y, Ono T, Miyahira Y, Nguyen VQ, Matsui T, Ishihara M (2013) Antiviral activity of silver nanoparticles/chitosan composites against H1N1 influenza A virus. Nanoscale Res Lett 8(1):93. https://doi.org/10.1186/1556-276X-8-93
Mulavney P (1996) Surface plasmon spectroscopy of nanosized metal particles. Langmuir 12(3):788–800. https://doi.org/10.1021/la9502711
Ogata T, Higuchi H, Mochida S, Matsumoto H, Kato A, Endo T, Kaji A, Kaji H (1992) HIV-1 reverse transcriptase inhibitor from Phyllanthus niruri. AIDS Res Hum Retrovir 8(11):1937–1944. https://doi.org/10.1089/aid.1992.8.1937
Pileni MP (1998) Optical properties of nanosized particles dispersed in colloidal solutions or arranged in 2D or 3D superlattices. New J Chem 22(7):693–702. https://doi.org/10.1039/A709218K
Rai MK, Deshmukh SD, Ingle AP, Gade AK (2012) Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Appl Microbiol 112(5):841–852. https://doi.org/10.1111/j.1365-2672.2012.05253.x
Reed LJ, Muench H (1938) A simple method of estimating fifty percent endpoints. Am J Epidemiol 27(3):493–497. https://doi.org/10.1093/oxfordjournals.aje.a118408
Robinson MC (1955) An epidemic of virus disease in Southern Province, Tanganyika territory, in 1952-1953. Trans R Soc Trop Med Hyg 49(1):28–32. https://doi.org/10.1016/0035-9203(55)90080-8
Rodrigues FN, Lourenço J, Cerqueira EM, Lima MM, Pybus O, Alcantara LC (2016) Epidemiology of chikungunya virus in Bahia, Brazil, 2014-2015. PLoS Curr 1(8). https://doi.org/10.1371/currents.outbreaks.c97507e3e48efb946401755d468c28b2
Rogers JV, Parkinson CV, Choi YW, Speshock JL, Hussain SM (2008) A preliminary assessment of silver nanoparticles inhibition of monkeypox virus plaque formation. Nanoscale Res Lett 3(4):129–133. https://doi.org/10.1007/s11671-008-9128-2
Salata O (2004) Applications of nanoparticles in biology and medicine. J Nanobiotechnol 2(3):3. https://doi.org/10.1186/1477-3155-2-3
Schwartz O, Albert ML (2010) Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol 8(7):491–500. https://doi.org/10.1038/nrmicro2368
Shevtsov M, Zhao L, Protzer U, Klundert MAAV (2017) Applicability of metal nanoparticles in the detection and monitoring of hepatitis B virus infection. Viruses 9(7):193. https://doi.org/10.3390/v9070193
Singh K, Panghal M, Kadyan S, Chaudhary U, Yadav JP (2014) Green silver nanoparticles of Phyllanthus amarus: as an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. J Nanobiotechnol 12(40):40. https://doi.org/10.1186/s12951-014-0040-x
Sinha SN, Paul D (2015) Phytosynthesis of silver nanoparticles using Andrographis paniculata leaf extract and evaluation of their antibacterial activities. Spectrosc Lett 48(8):600–604. https://doi.org/10.1080/00387010.2014.938756
Suresh U, Murugan K, Benelli G, Nicoletti M, Barnard DR, Panneerselvam C, Kumar PM, Subramaniam J, Dinesh D, Chandramohan B (2015) Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitol Res 114(4):1551–1562. https://doi.org/10.1007/s00436-015-4339-9
Tang LIC, Ling APK, Koh RY, Chye SM, Voon KGL (2012) Screening of anti-dengue activity in ethanolic extracts of medicinal plants. BMC Complement Altern Med 12(3). https://doi.org/10.1186/1472-6882-12-3
Trefry JC, Wooley DP (2013) Silver nanoparticles inhibit vaccinia virus infection by preventing viral entry through a macropinocytosis-dependent mechanism. J Biomed Nanotechnol 9(9):1624–1635. https://doi.org/10.1166/jbn.2013.1659
Venkateswaran PS, Millman I, Blumberg BS (1987) Effects of an extract from Phyllanthus niruri on hepatitis B and woodchuck hepatitis viruses: in vitro and in vivo studies. Proc Natl Acad Sci U S A 84(1):274–278. https://doi.org/10.1073/pnas.84.1.274
Verma H, Patil PR, Kolhapure RM, Gopalkrishna V (2008) Antiviral activity of the Indian medicinal plant extract Swertia chirata against herpes simplex viruses: a study by in-vitro and molecular approach. Indian J Med Microbiol 26(4):322–326. https://doi.org/10.4103/0255-0857.43561
Wintachai P, Kaur P, Lee RC, Ramphan S, Kuadkitkan A, Wikan N, Roytrakul S, Chu JJ, Smith DR (2015) Activity of andrographolide against chikungunya virus infection. Sci Rep 5:14179. https://doi.org/10.1038/srep14179
Yang XX, Li CM, Huang CZ (2016) Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 8(5):3040–3048. https://doi.org/10.1039/c5nr07918g
Zhang XF, Shen W, Gurunathan S (2016) Silver nanoparticle-mediated cellular responses in various cell lines: an in vitro model. Int J Mol Sci 17(10):1603. https://doi.org/10.3390/ijms17101603
Acknowledgements
We sincerely thank the Council of Scientific & Industrial Research, New Delhi, India, for providing financial support in form of fellowship to Vikrant Sharma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animal performed by any of the authors.
Rights and permissions
About this article
Cite this article
Sharma, V., Kaushik, S., Pandit, P. et al. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl Microbiol Biotechnol 103, 881–891 (2019). https://doi.org/10.1007/s00253-018-9488-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9488-1