Abstract
The highly infectious porcine transmissible gastroenteritis virus (TGEV), which belongs to the coronaviruses (CoVs), causes diarrhea and high mortality rates in piglets, resulting in severe economic losses in the pork industry worldwide. In this study, we used Lactobacillus plantarum (L. plantarum) to anchor the expression of TGEV antigen (S) to dendritic cells (DCs) via dendritic cell-targeting peptides (DCpep). The results show that S antigen could be detected on the surface of L. plantarum by different detection methods. Furthermore, flow cytometry and ELISA techniques were used to measure the cellular, mucosal, and humoral immune responses of the different orally gavaged mouse groups. The obtained results demonstrated the significant effect of the constructed L. plantarum expressing S-DCpep fusion proteins in inducing high expression levels of B7 molecules on DCs, as well as high levels of IgG, secretory IgA, and IFN-γ and IL-4 cytokines compared with the other groups. Accordingly, surface expression of DC-targeted antigens successfully induced cellular, mucosal, and humoral immunity in mice and could be used as a vaccine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Porcine transmissible gastroenteritis virus (TGEV) is classified under the genus Coronavirus, family Coronaviridae, and order Nidovirales (Jiang et al. 2016). It is one of the important determining causes of acute viral diarrhea in piglets and results in devastating economic losses in the swine industry (Xia et al. 2017b; Yu et al. 2017a). It destructs the intestinal villi epithelium, resulting in a decrease in the surface area and atrophy of the epithelial lining, intestinal disorders, and incomplete food digestion and absorption (Jiang et al. 2016; Xia et al. 2017a).
High morbidity and case fatality rates were recorded in newly born piglets, especially in cases of co-infection with the porcine epidemic diarrhea virus (PEDV) (Yu et al. 2017a). The first report of TGEV was in the USA in 1933 (Doyle and Hutchings 1946), while the first detection in China was in 1970 (Wang et al. 2010).
Four structural proteins, spike (S) proteins, membrane (M) proteins, envelope (E), and nucleoproteins (N), and five non-structural proteins are encoded by the TGEV genome (Zhang et al. 2017). The TGEV spike protein has an approximate size of 250 kDa and contains a highly conserved area (cysteine-rich motif, CRM) near the carboxy-terminal end of the transmembrane region; it combines virus particles and the interaction between them and the M protein (Gelhaus et al. 2014; Nguyen and Hogue 1997; Vennema et al. 1996). Functionally, the spike protein plays an essential role in the tropism of the virus, the binding to the host cell receptor aminopeptide N, and subsequent combination of cellular and viral membranes, as well as the pathogenicity and hemagglutination activity (Reguera et al. 2012; Sanchez et al. 1999). Additionally, the protein has a high immunogenic potency that can stimulate the host immune system to generate neutralizing antibodies (Lin et al. 2015). The antigenicity of TGEV was elucidated in different studies, where a successful spike protein-based enzyme linked immune assay (ELISA) was implemented (Lin et al. 2015) and a safe and promising vaccine was developed (Mou et al. 2016).
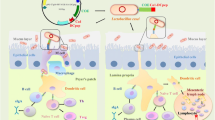
Dendritic cells (DCs) located in the gut epithelium are the most potent antigen-presenting cells (APCs). They have the unique capability of inducing T cell polarization and differentiation (Subramaniam et al. 2017), the regulation of B cell function, and differentiation into IgG-producing plasma cells (Wang et al. 2016). Furthermore, DCs limit the mucosal penetration of invasive pathogens and encourage the uptake of antigens (Owen et al. 2013) and migration into lymphoid tissues resulting in the presentation of foreign antigens to B and T cells (Mohamadzadeh et al. 2005). A specific DCpep was found to effectively protect against a lethal anthrax disease challenge in a mouse model (Mohamadzadeh et al. 2009). Probiotic feed is beneficial for the host since it prevents infection (Jiang et al. 2016). Different Lactobacillus spp. are commonly distributed in nature and are naturally found in the human and animal gastrointestinal tracts (Kaur et al. 2017; Riaz Rajoka et al. 2017). The species have been safely used as heterologous protein antigen delivery vehicles for oral vaccines owing to their characteristics of resistance against gastric secretions, ability to colonize the intestine (Landete et al. 2015; Yu et al. 2017b), relatively simple culture techniques, and suitable manipulations (Wanker et al. 1995).
The poly-γ-glutamic acid synthetase A (pgsA) protein from Bacillus subtilis, encoded by the pgsA gene, has a transmembrane region near its N-terminus (Sung et al. 2005), providing the required criteria for the implementation of a pgsA displaying expression system. The expression system is the theoretical basis for the recombination of a recent genetically engineered vaccine (Cai et al. 2016; Narita et al. 2006). For instance, Lactobacillus plantarum has been employed to display thymosin α-1 in conjunction with classical swine fever virus E2 antigen (Xu et al. 2015), SO7 of Eimeria tenella fusion DC-targeting peptide (Yang et al. 2017a), a porcine epidemic diarrhea virus S gene fused to a DC-targeting peptide (Huang et al. 2018). Surface expression of foreign antigens induced cellular, mucosal, and humoral immunity in animal and could be used as a potential vaccine. Therefore, in the current study, L. plantarum was used to express TGEV S protein and was fused to a DCpep to deliver it to mucosal DCs. Its immunogenicity was further investigated at the in vitro and in vivo levels.
Materials and methods
Synthesis of S antigen in L. plantarum
L. plantarum NC8 (CCUG 61730) bacteria and Escherichia coli-Lactobacillus harboring pSIP409 vector were supplied by Professor A. Kolandaswamy (Madurai Kamaraj University, India) (Sorvig et al. 2005). The pMD19-T-pgsA-S-DCpep or pMD19-T-pgsA-S-Ctrlpep were kept at our lab; the information of DCpep and Ctrlpep was obtained from published literature (Shi et al. 2016). The S gene fragment (GenBank accession numbers KT696544, source 20365 to 22410)/Spike Protein GenBank AMB66488, source 1 to 682) and the pMD19-T-pgsA-S-DCpep or pMD19-T-pgsA-S-Ctrlpep plasmids were digested separately by NcoI and Hind III for 4 h at 37 °C. Then, the T4 DNA ligase enzyme was used to ligate either pgsA-S-DCpep or pgsA-S-Ctrlpep into the pSIP409 vector at 4 °C for 12 h. The constructed pSIP409-pgsA-S-DCpep and pSIP409-pgsA-S-Ctrlpep vectors were confirmed and extracted using a plasmid DNA extraction kit (Omega Bio-Tek, Doraville, CA). The pSIP409-pgsA plasmid was constructed using a negative control vector. The L. plantarum was transformed with the recombinant plasmid, and positive bacteria were named Lp-pSIP-409-pgsA (pSIP409-pgsA in L. plantarum), Lp-pSIP-409-16 (pSIP409-pgsA-S-Ctrlpep in L. plantarum), and Lp-pSIP-409-17 (pSIP409-pgsA-S-DCpep in L. plantarum).
Identification of the protein anchor expression by flow cytometry and immunofluorescence
For confirmation of S protein expression on the surface of bacteria, the constructed Lp-pSIP-409-16 and Lp-pSIP-409-17 bacterial strains were cultured in De Man, Rogosa, and Sharpe (MRS) with 10 μg/ml erythromycin as a selective antibiotic. When the OD600 of the medium reached a range between 0.3 and 0.4, Sakacin P (SppIP) was added to the culture followed by inducing expression at 30 °C for 10 h. Cells from the different strains were carefully washed using phosphate buffer saline (PBS, contained 1% bovine serum albumin) and resuspended in a concentration of 5 × 105 colony forming unit (CFU) of recombined bacteria. Suspensions were stained for 12 h at 4 °C with polyclonal antibody obtained from mice vaccinated with purified S antigen. After washing twice with PBS containing 0.2% Tween-20 (PBS-T), the recombined bacteria were stained with FITC-conjugated goat anti-mouse IgG (CST, USA) for 1 h at 4 °C in the dark (Yang et al. 2017c). Finally, the cells were washed, resuspended, and tested using a flow cytometer (BD FACS LSR Fortessa™) and a laser scanning confocal microscope (LSM710; Carl Zeiss, Germany).
16S rRNA gene amplification
Bacterial genomic DNA were extracted by bacterial genomic DNA kit according to the manufacturer’s instructions (CW Biotech, China). For general bacterial identification, 16S rRNA universal primers were selected (Posman et al. 2017). The most commonly used universal primer was 27F/1492R, 27F: 5′-AGAGTTTGATCCTGGCTCAG-3′, 1492R: 5′-GGTTACCTTGTTACGACTT-3′. Polymerase chain reaction (PCR) amplification was as follows: initial pre-denaturation at 98 °C for 20 s; denaturation at 98 °C for 10 s; annealing at 50 °C for 5 s; extension at 72 °C for 15 s; and 30 cycles. After the end of the last cycle, the final extension was performed at 72 °C for 10 min to allow sufficient amplification of the reaction product. The result of 16S rRNA PCR products was checked by 0.8% agarose gel electrophoresis.
Western blot assay
For further confirmation of S protein expression, induction of expression was carried out as illustrated previously (Shi et al. 2014). Briefly, the different recombined bacteria were resuspended in 10 ml of PBS and lysed by ultrasonic crushing. After separating the protein samples with 13% SDS-PAGE, the gels were then transferred to poly (vinylidene fluoride) (PVDF) membranes. To detect the S-DCpep and S-Ctrlpep antigens, the PVDF membranes were incubated overnight at 4 °C with monoclonal antibody. The secondary antibody used was HRP-conjugated rat anti-mouse IgG (CST, USA). Finally, the samples were developed with the ECL Plus detection kit (Thermo Scientific) to visualize the bounded bands.
Ethics statements for experimental animals
Forty specific-pathogen free (SPF) 6-week-old mice were obtained from the Beijing Vital River Laboratory Animal Technology Co., Ltd. and were kept according to the rules of the Animal Care and Ethics Committees of Jilin Agriculture University. Sterile water and germ-free food were used for mice feeding at Jilin Provincial Engineering Research Center of Animal Probiotics (JLAU06201645), which provided the SPF environment facilities. Hygienic disposal of the carcasses was conducted at the end of the experiments.
Immunizations
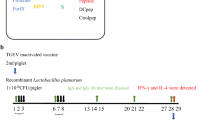
Using oral gavages, four groups of 10 randomly selected SPF mice (n = 10) were vaccinated with Lp-pSIP-409-16, Lp-pSIP-409-17, Lp-pSIP-409-pgsA, or saline. The experimental protocol was as follows: primary vaccination was administered at days 1 and 2, secondary vaccination was administered at days 15 and 16, and booster immunization doses were given at days 29, 30, and 31. All doses were 1 × 108 CFU/mouse. Furthermore, serum samples were collected from the four groups at 14, 28, and 42 days after the first immunization. Serum samples were taken from mice mail vein at 14, 28, and 42 days after the first immunization. Serum samples were collected before centrifugation at 5000×g for 10 min, and serum samples were stored at − 20 °C until detection.
Preparation of single-cell suspensions
Peyer’s patch (PPs), lamina propria (LP) cells of the small intestine, and mesenteric lymph nodes (MLNs) were obtained according to a previously published protocol (Kikuchi et al. 2014). Small intestine samples from vaccinated mice were treated with 1X PBS and spliced into pieces of 5 cm in length followed by digestion with a lymphocyte separation medium. Single LP cells were obtained after adding an LP cell digestive mix in an isotonic Percoll solution. A 70-μm sterile filter film was used to filter the cell suspension and was followed by centrifugation at 500×g for 10 min at 4 °C. After washing with cold PBS, the cells were resuspended in complete Rosewell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum (FBS; Gibco, USA) (Shi et al. 2014).
Flow cytometry
We used flow cytometry to detect the expression of costimulatory molecules on DCs and the activation of B cells according to previously published reports (Yang et al. 2016). Briefly, a single-cell suspension was prepared from the small intestine lamina propria of immunized mice and was then diluted to 1 × 106 cells/100 μl. A sample of this single-cell suspension was incubated with APC-conjugated anti-CD11c (clone HL3), fluorescein isothiocyanate (FITC)-conjugated anti-CD80 (clone 16-10A1), and phycoerythrin (PE) CD86 (clone GL1) antibodies to label dendritic cells or isotype control APC-, PE-, and FITC-conjugated antibodies (BD Pharmingen). Another sample was incubated with FITC-conjugated anti-IgA (clone C10-3) and PE-conjugated anti-B220 (clone RA3-6B2) antibodies to label B cells according to the manufacturer’s instructions. Next, the samples were incubated for 30 min at 4 °C and then washed twice with fluorescence-activated cell sorting (FACS) buffer (PBS, 1% FCS, and 0.09% sodium azide). Finally, samples were detected by flow cytometry (BD FACS LSR Fortessa™). FlowJo 7.6.1 software was used for data analysis.
ELISA
ELISA was performed by following the method of a previously published paper (Yang et al. 2017b). Specific IgG and sIgA antibodies were tested by ELISA using the purified S protein of TGEV. The end-point titers determined were two and three times higher than the background for the fecal and serum samples, respectively.
Special cytokine assay
The MLN cells were incubated with S protein (5 μg/ml) at 37 °C for 72 h, and the supernatant was collected at 72 h. To check for specific cytokines (IFN-γ and IL-4), ELISA kits (R&D Systems, USA) were used.
Serologic tests
Antibody neutralization assays (micro determination) were carried out to determine the titer of TGEV S neutralizing antibodies of the serum collected from mice immunized with recombinant L. plantarum according to a previously published protocol with few modifications (Tian et al. 2014). Briefly, 100 TCID50 TGEV was incubated at 37 °C with serially diluted sera samples. After 1 h, the admixture was plated onto Vero cells in 96-well plates. After 48 h, the neutralizing antibody titer PD50 was measured using the method detailed by Karber.
Statistical analysis
To analyze the significance of the differences between the means, statistical significance was determined by one-way analysis of variance (ANOVA) using the GraphPad Prism 5.0 graphic pad software. All data are displayed as the means ± SEM, where a p value of < 0.05 was regarded as statistically significant.
Results
TGEV S protein expression on the L. plantarum bacteria
The surface expression plasmids were constructed to encode pgsA to fuse to S-Ctrlpep or S-DCpep (Fig. 1). Expression levels of the TGEV S protein were determined by different methods. The S protein expression levels of the Lp-pSIP-409-16 and Lp-pSIP-409-17 were detected by flow cytometry (Fig. 2a). Using laser confocal microscopy, we show that the distinct green fluorescence in Fig. 2b is an example of the expression of the S antigen at the surface of Lp-pSIP-409-16 and Lp-pSIP-409-17 compared to the non-fluorescent Lp-pSIP-409-pgsA. Furthermore, a positive band was detected using the western blot technique, confirming the expression of the protein and 16S rRNA gene from bacteria as internal controls (Fig. 2c). Therefore, it could be concluded that both Lp-pSIP-409-16 and Lp-pSIP-409-17 were successfully displayed on the surface of their corresponding bacteria.
Surface displays of the S-Ctrlpep and pgsA-S-DCpep on the L. plantarum. a The image shows flow cytometric detection of foreign antigen expression after incubating with FITC-conjugated anti-mouse IgG antibodies. b The image indicates immunofluorescence observation of foreign antigen expression after incubating with FITC-conjugated anti-mouse IgG antibodies. c Western blots of S-DCpep and S-Ctrlpep synthesized proteins (Up) and 16S rRNA gene from bacteria as internal controls (Down). Lane 1: Lp-pSIP-409-pgsA. Lane 2: Lp-pSIP-409-16. Lane 3: Lp-pSIP-409-17
Constructed Lp-pSIP-409-17 elicited the activation of DCs
Flow cytometry was carried out to assess the activation degree of the costimulatory molecules induced by DCs in the four compared groups 2 weeks (w) post-booster immunization (Fig. 3a). The mean fluorescence intensity (MFI) of CD11c+CD80+ in the small intestinal LP was remarkably enhanced (p < 0.01) in the group immunized by Lp-pSIP-409-17 compared to the saline and Lp-pSIP-409-pgsA-administered groups (Fig. 3b). Additionally, a significant increase (p < 0.05) was detected between this group and the Lp-pSIP-409-16-immunized group (Fig. 3b). Additionally, the Lp-pSIP-409-17-immunized group had the ability to highly activate CD86+ production compared to the Lp-pSIP-409-16- (p < 0.05), Lp-pSIP-409-pgsA- (p < 0.01), and saline-immunized groups (p < 0.01) (Fig. 3c).
The activation of the recombinant bacterium on dendritic cell (DCs). Fourteen days after booster immunizations, B7 molecules (CD80/CD86) in the LP cells of the small intestine were tested by flow cytometry. (a) A gating strategy of CD86 expression on the DCs was employed. The mean fluorescence intensities (MFI) of CD11c+CD80+ (b) and CD11c+CD86+ (c) are shown. (*p < 0.05 and **p < 0.01)
Recombined Lp-pSIP-409-17 induced a B cell immune response
The results obtained by flow cytometry were used to determine the number of B220+ IgA+ B cells in the PP 2 w after the booster immunization (Fig. 4a). The data showed that the percentage of B220+ IgA+ B cells markedly increased in the group immunized with Lp-pSIP-409-17, compared to the groups immunized with Lp-pSIP-409-pgsA, saline (p < 0.001), and Lp-pSIP-409-16 (p < 0.05) (Fig. 4b). Furthermore, B220+ IgA+ B cells significantly decreased in the Lp-pSIP-409-pgsA-immunized group compared to the Lp-pSIP-409-16-immunized group (p < 0.01) (Fig. 4b). Generally, the obtained data elucidate that immunization with Lp-pSIP-409-17 has an important role in the activation of the humoral B cell response in immunized mice.
Recombined Lp-pSIP-409-17 enhanced specific sIgA production
ELISA was used to determine the sIgA titer in intestinal feces collected at 14, 28, and 42 days after the first vaccination (Fig. 5). At 14 days, there was no marked variance between the different groups. However, at 28 days, a remarkable difference (p < 0.05) was found only between the group immunized with Lp-pSIP-409-17 and both of the Lp-pSIP-409-pgsA- and the saline-immunized groups. Importantly, our data show that a significantly high sIgA titer was present in the Lp-pSIP-409-16-immunized group at the 42-day mark compared with the saline- (p < 0.01) and Lp-pSIP-409-pgsA-immunized groups (p < 0.05). In contrast, the sIgA production titer was significantly higher in the Lp-pSIP-409-17-immunized group compared with the saline- (p < 0.001), Lp-pSIP-409-pgsA- (p < 0.001), and Lp-pSIP-409-16-immunized groups (p < 0.05). It could be concluded that oral gavage immunization of mice with Lp-pSIP-409-17 significantly induced the secretion of sIgA.
Recombined Lp-pSIP-409-17 triggered specific IgG production
Serum samples obtained at 14, 28, and 42 days after the primary vaccination were used to detect the IgG titers using ELISA (Fig. 6). No significant difference was found between the groups immunized with Lp-pSIP-409-16 and Lp-pSIP-409-17, although there was a significant difference between the Lp-pSIP-409-17-immunized group and either the Lp-pSIP-409-pgsA- or the saline-immunized groups at 14 days (p < 0.05) or 28 days (p < 0.01), respectively. In contrast, at 42 days, a significant difference was determined between the Lp-pSIP-409-16- and the Lp-pSIP-409-17-immunized groups (p < 0.05), and a highly significant difference (p < 0.001) was recorded between the Lp-pSIP-409-17- and both of the Lp-pSIP-409-pgsA- and the saline-immunized groups. Taken together, the above data show that immunization with Lp-pSIP-409-17 has a great influence on humoral IgG production.
Production of the serum-neutralizing antibodies elicited by the Lp-pSIP-409-17
Oral vaccination of the mice with Lp-pSIP-409-17 revealed an increase in the serum antibody titer 42 days after immunization (Fig. 7). The titer was remarkably stronger than the titer induced by Lp-pSIP-409-16 (p < 0.05) and highly significantly stronger than the titers induced by Lp-pSIP-409-pgsA and saline (p < 0.01).
Effect of Lp-pSIP-409-17 on production of the cytokine
Fourteen days after the administration of the booster immunization dose, ELISA was carried out to detect the levels of the IFN-γ and IL-4 cytokines in the MLN cell secretions. The production of IFN-γ was significantly (p < 0.05) detected in the Lp-pSIP-409-17-immunized group compared with the Lp-pSIP-409-16- and Lp-pSIP-409-pgsA-immunized groups, and the production was highly significant (p < 0.01) compared with the saline-immunized group (Fig. 8a). Additionally, a significant (p < 0.001) level of IL-4 was detected in the orally vaccinated Lp-pSIP-409-17 group when compared with either the saline- or the Lp-pSIP-409-pgsA-vaccinated groups, and a marked significant difference (p < 0.05) was detected between the Lp-pSIP-409-17-immunized group and the Lp-pSIP-409-16-immunized group (Fig. 8b).
The recombinant bacterium induced S antigen-specific IFN-γ (a) and IL-4 (b) in the MLN of vaccinated mice. Two weeks after booster immunizations, single cells from the MLN of vaccinated mice were incubated with specific antigen for 72 h, and supernatants were used to detect IFN-γ (a) and IL-4 (b). (*p < 0.05, **p < 0.01, and ***p < 0.001)
Discussion
TGEV is a highly infectious coronavirus (CoV) that causes diarrhea in piglets with a high morbidity and mortality rate leading to high economic losses (Zhang et al. 2017). It causes inflammation in intestinal tissues, and usually, the death of the animal is mainly due to a sodium and potassium ion imbalance (Cruz et al. 2013).
So far, there is no effective treatment against the causative agent itself; the prescribed medication acts solely against the resulting clinical symptoms. This fact necessitates the development of an effective tool for viral control. Effective prevention and control of the TGEV and other coronaviruses can only be achieved through the use of vaccines (Gerdts and Zakhartchouk 2017).
The immune response to swine enteric coronaviruses is based on cytotoxic T cells and secretory antibodies (sIgA), which are produced by antibody-secreting cells in the lamina propria of the mucosal tissues, while systemic antibodies such as IgG and IgM are found in the serum and interstitial tissues and some isotypes can be transported across the mucosal epithelium into the lumen (Chattha et al. 2015; Horton and Vidarsson 2013).
Most present commercial TGEV vaccines are inactivated vaccines or live-attenuated vaccines that are given to the sow during the pregnancy period to provide lactogenic immunity to its offspring (Gerdts and Zakhartchouk 2017). Some parenteral vaccines did not provide effective lactogenic immunity when the vaccinated animals were experimentally challenged with the virus, resulting in a mortality rate that ranged from 44 to 80% in piglets. Furthermore, antibody titers in the milk samples obtained during the first week of lactation rapidly decreased (Voets et al. 1980).
Recent studies showed that L. plantarum was successfully used as an exogenous protein vector, adding to its probiotic nature (Yang et al. 2017a). One of the important characteristics of the anchoring protein expressed by the pgsA gene is its ability to position exogenous proteins at the bacterial surface to exhibit their function (Cai et al. 2016; Yang et al. 2017c). Moreover, effective protective immunity was provided by the DCpep constructed vaccine (Yang et al. 2017a). Additionally, the essential role of the S protein in viral tropism (Cruz et al. 2013), mainly in cell fusion and jejunal tissue infection (Almazan et al. 2000) as well as serving as a major target for neutralizing antibodies, was recorded. Mice have successfully been used as a model for vaccine studies (Jiang et al. 2017; Yang et al. 2017c; Yu et al. 2017a). Accordingly, the constructed Lp-pSIP-409-17 bacteria was developed, and its immunogenicity was tested in mice.
Dendritic cells are a type of antigen-presenting cell, and they have a significant function in delivering antigens to T cells and B cells to improve the effects of vaccines. Studies conducted by our laboratory and others show that recombined bacteria could trigger the activation of DCs at mucosal sites (Mohamadzadeh et al. 2009; Yang et al. 2016). In this study, a clear and significant increase in the production of CD11c+CD80+ and CD11c+CD86+ was found in the group immunized with Lp-pSIP-409-17 when compared to the other vaccinated groups. Similar observations have been made by other groups (Kathania et al. 2013; Wang et al. 2017). As a whole, all experimental results confirmed that oral administration of recombined bacteria can induce the activation of DCs, in turn enhancing T cell and B cell responses in order to combat pathogens in the host.
Recent studies focused on the importance of mucosal delivery of vaccine and its role in preventing viral diarrheal diseases as it can generate both mucosal and systemic immune responses. This is particularly important since TGEV infections originally arise at the intestinal mucosal surfaces (Jiang et al. 2016). The results from the current study clarify the role of the recombinant Lp-pSIP-409-17 bacteria in increasing the mucosal immune response as the number of IgA+B220+ B cells increased significantly in mice immunized with this recombinant bacteria when compared with other groups. It is notable that the mucosal immune response is the first barrier to functionally neutralizing TGEV. The obtained results are in agreement with Jiang et al. (2016).
As the neutralizing activity of the produced antibodies is an essential parameter used to evaluate vaccine efficiency and stronger antibody titers reflect higher neutralizing activities, we investigated both in this study. The results of our ELISA analysis showed that the serum IgG titers started to increase at 14 days after vaccination with a level that is significantly different than the other three vaccine groups. Additionally, the titers were markedly increased at 42 days post-primary immunization. A study recorded that the S protein DNA vaccine elicited a humoral response 21–42 days post-immunization (dpi) with the peak of the response observed at day 35 (Meng et al. 2013). Meanwhile, between 35 and 42 dpi, the antibody titers were dramatically reduced. Furthermore, the antibody titer elicited by the recombinant Lactobacillus casei oral vaccine was stronger than that triggered by the previously mentioned DNA vaccine (Yu et al. 2017a). The attenuated Salmonella typhimurium-based S gene induced an immune-level response that significantly increased at 1 month post-vaccination, peaking at 1.5 months and decreasing at 2 months after immunization (Zhang et al. 2016b). When comparing the results of using a monovalent TGEV S gene vaccine with the bivalent TGEV/PDEV S gene vaccine, the specific IgG antibody titers elicited by the single-gene vaccine were higher than those elicited by the double-gene vaccine (Zhang et al. 2016b). The difference between these results may be attributed to many factors, including the pathogen strain used, the different vector used, and the experimental host.
It has been shown that Th1 and Th2 cells have an important role in immune responses; Th1 cells have been shown to help in cytotoxic T cell differentiation, IFN-γ secretion, and macrophage cell activation (Maldonado-Lopez and Moser 2001), while Th2 cells have a major role in humoral immunity through the stimulation of B cell proliferation and antibody production (Mosmann and Coffman 1989). The ability of monocytes to differentiate into macrophages is widely affected by IL-4 secretion (Lee et al. 2009). In the present study, using specific ELISAs, MLN cell-specific cytokine analysis was used to evaluate the cellular immune response induced by the recombinant L. plantarum. Our data show that the production of IL-4 and IFN-γ was remarkably higher in the Lp-pSIP-409-17 orally immunized animal group compared with the Lp-pSIP-409-16 and other groups. These results are in agreement with previous study, which reported that recombinant DNA plasmids expressing TGEV S genes improved the number of T lymphocyte subgroups as well as the proliferation of T lymphocytes, adding to the plasmid’s ability to induce a significant production of IFN-γ in the vaccinated animal (Meng et al. 2013). Consistent with other reports, TGEV S recombinant L. casei induced IFN-γ production that was stronger than IL-4 in the vaccinated animals, and the Th1/Th2 balance was disturbed (Jiang et al. 2016). It is worth noting that TGEV has developed strategies to evade and interfere with the interferon response and that suppression of this response by many structural and non-structural viral proteins has been documented (Zhang et al. 2016a).
In conclusion, it could be concluded that the successfully constructed Lp-pSIP-409-17 shows promising results in inducing both humoral and cellular immune responses in the orally immunized mouse model. This supports the need for further assessment in a porcine model in order to optimize the immunization procedures before it can be used as an easily administered, safe, and protective mucosally delivered vaccine to control TGEV infection.
References
Almazan F, Gonzalez JM, Penzes Z, Izeta A, Calvo E, Plana-Duran J, Enjuanes L (2000) Engineering the largest RNA virus genome as an infectious bacterial artificial chromosome. Proc Natl Acad Sci U S A 97(10):5516–5521
Cai R, Jiang Y, Yang W, Yang W, Shi S, Shi C, Hu J, Gu W, Ye L, Zhou F, Gong Q, Han W, Yang G, Wang C (2016) Surface-displayed IL-10 by recombinant Lactobacillus plantarum reduces Th1 responses of RAW264.7 cells stimulated with poly(I:C) or LPS. J Microbiol Biotechnol 26(2):421–431. https://doi.org/10.4014/jmb.1509.09030
Chattha KS, Roth JA, Saif LJ (2015) Strategies for design and application of enteric viral vaccines. Annu Rev Anim Biosci 3:375–395. https://doi.org/10.1146/annurev-animal-022114-111038
Cruz JL, Becares M, Sola I, Oliveros JC, Enjuanes L, Zuniga S (2013) Alphacoronavirus protein 7 modulates host innate immune response. J Virol 87(17):9754–9767. https://doi.org/10.1128/jvi.01032-13
Doyle LP, Hutchings LM (1946) A transmissible gastroenteritis in pigs. J Am Vet Med Assoc 108:257–259
Gelhaus S, Thaa B, Eschke K, Veit M, Schwegmann-Wessels C (2014) Palmitoylation of the alphacoronavirus TGEV spike protein S is essential for incorporation into virus-like particles but dispensable for S-M interaction. Virology 464-465:397–405. https://doi.org/10.1016/j.virol.2014.07.035
Gerdts V, Zakhartchouk A (2017) Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Vet Microbiol 206:45–51. https://doi.org/10.1016/j.vetmic.2016.11.029
Horton RE, Vidarsson G (2013) Antibodies and their receptors: different potential roles in mucosal defense. Front Immunol 4:200. https://doi.org/10.3389/fimmu.2013.00200
Huang KY, Yang GL, Jin YB, Liu J, Chen HL, Wang PB, Jiang YL, Shi CW, Huang HB, Wang JZ, Wang G, Kang YH, Yang WT, Wang CF (2018) Construction and immunogenicity analysis of Lactobacillus plantarum expressing a porcine epidemic diarrhea virus S gene fused to a DC-targeting peptide. Virus Res 247:84–93. https://doi.org/10.1016/j.virusres.2017.12.011
Jiang X, Hou X, Tang L, Jiang Y, Ma G, Li Y (2016) A phase trial of the oral Lactobacillus casei vaccine polarizes Th2 cell immunity against transmissible gastroenteritis coronavirus infection. Appl Microbiol Biotechnol 100(17):7457–7469. https://doi.org/10.1007/s00253-016-7424-9
Jiang Y, Ye L, Cui Y, Yang G, Yang W, Wang J, Hu J, Gu W, Shi C, Huang H, Wang C (2017) Effects of Lactobacillus rhamnosus GG on the maturation and differentiation of dendritic cells in rotavirus-infected mice. Benef Microbes 8(4):645–656. https://doi.org/10.3920/bm2016.0157
Kathania M, Zadeh M, Lightfoot YL, Roman RM, Sahay B, Abbott JR, Mohamadzadeh M (2013) Colonic immune stimulation by targeted oral vaccine. PLoS One 8(1):e55143. https://doi.org/10.1371/journal.pone.0055143
Kaur M, Singh H, Jangra M, Kaur L, Jaswal P, Dureja C, Nandanwar H, Chaudhuri SR, Raje M, Mishra S, Pinnaka AK (2017) Lactic acid bacteria isolated from yak milk show probiotic potential. Appl Microbiol Biotechnol 101:7635–7652. https://doi.org/10.1007/s00253-017-8473-4
Kikuchi Y, Kunitoh-Asari A, Hayakawa K, Imai S, Kasuya K, Abe K, Adachi Y, Fukudome S, Takahashi Y, Hachimura S (2014) Oral administration of Lactobacillus plantarum strain AYA enhances IgA secretion and provides survival protection against influenza virus infection in mice. PLoS One 9(1):e86416. https://doi.org/10.1371/journal.pone.0086416
Landete JM, Langa S, Revilla C, Margolles A, Medina M, Arques JL (2015) Use of anaerobic green fluorescent protein versus green fluorescent protein as reporter in lactic acid bacteria. Appl Microbiol Biotechnol 99(16):6865–6877. https://doi.org/10.1007/s00253-015-6770-3
Lee YK, Mukasa R, Hatton RD, Weaver CT (2009) Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol 21(3):274–280. https://doi.org/10.1016/j.coi.2009.05.021
Lin CM, Gao X, Oka T, Vlasova AN, Esseili MA, Wang Q, Saif LJ (2015) Antigenic relationships among porcine epidemic diarrhea virus and transmissible gastroenteritis virus strains. J Virol 89(6):3332–3342. https://doi.org/10.1128/jvi.03196-14
Maldonado-Lopez R, Moser M (2001) Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin Immunol 13(5):275–282. https://doi.org/10.1006/smim.2001.0323
Meng F, Ren Y, Suo S, Sun X, Li X, Li P, Yang W, Li G, Li L, Schwegmann-Wessels C, Herrler G, Ren X (2013) Evaluation on the efficacy and immunogenicity of recombinant DNA plasmids expressing spike genes from porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. PLoS One 8(3):e57468. https://doi.org/10.1371/journal.pone.0057468
Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR (2005) Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci U S A 102(8):2880–2885. https://doi.org/10.1073/pnas.0500098102
Mohamadzadeh M, Duong T, Sandwick SJ, Hoover T, Klaenhammer TR (2009) Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc Natl Acad Sci U S A 106(11):4331–4336. https://doi.org/10.1073/pnas.0900029106
Mosmann TR, Coffman RL (1989) TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7:145–173. https://doi.org/10.1146/annurev.iy.07.040189.001045
Mou C, Zhu L, Xing X, Lin J, Yang Q (2016) Immune responses induced by recombinant Bacillus subtilis expressing the spike protein of transmissible gastroenteritis virus in pigs. Antivir Res 131:74–84. https://doi.org/10.1016/j.antiviral.2016.02.003
Narita J, Okano K, Kitao T, Ishida S, Sewaki T, Sung MH, Fukuda H, Kondo A (2006) Display of alpha-amylase on the surface of Lactobacillus casei cells by use of the PgsA anchor protein, and production of lactic acid from starch. Appl Environ Microbiol 72(1):269–275. https://doi.org/10.1128/aem.72.1.269-275.2006
Nguyen VP, Hogue BG (1997) Protein interactions during coronavirus assembly. J Virol 71(12):9278–9284
Owen JL, Sahay B, Mohamadzadeh M (2013) New generation of oral mucosal vaccines targeting dendritic cells. Curr Opin Chem Biol 17(6):918–924. https://doi.org/10.1016/j.cbpa.2013.06.013
Posman KM, DeRito CM, Madsen EL (2017) Benzene degradation by a Variovorax species within a coal tar-contaminated groundwater microbial community. Appl Environ Microbiol 83(4):e02658–e02616. https://doi.org/10.1128/aem.02658-16
Reguera J, Santiago C, Mudgal G, Ordono D, Enjuanes L, Casasnovas JM (2012) Structural bases of coronavirus attachment to host aminopeptidase N and its inhibition by neutralizing antibodies. PLoS Pathog 8(8):e1002859. https://doi.org/10.1371/journal.ppat.1002859
Riaz Rajoka MS, Shi J, Zhu J, Shao D, Huang Q, Yang H, Jin M (2017) Capacity of lactic acid bacteria in immunity enhancement and cancer prevention. Appl Microbiol Biotechnol 101(1):35–45. https://doi.org/10.1007/s00253-016-8005-7
Sanchez CM, Izeta A, Sanchez-Morgado JM, Alonso S, Sola I, Balasch M, Plana-Duran J, Enjuanes L (1999) Targeted recombination demonstrates that the spike gene of transmissible gastroenteritis coronavirus is a determinant of its enteric tropism and virulence. J Virol 73(9):7607–7618
Shi SH, Yang WT, Yang GL, Cong YL, Huang HB, Wang Q, Cai RP, Ye LP, Hu JT, Zhou JY, Wang CF, Li Y (2014) Immunoprotection against influenza virus H9N2 by the oral administration of recombinant Lactobacillus plantarum NC8 expressing hemagglutinin in BALB/c mice. Virology 464-465:166–176. https://doi.org/10.1016/j.virol.2014.07.011
Shi SH, Yang WT, Yang GL, Zhang XK, Liu YY, Zhang LJ, Ye LP, Hu JT, Xing X, Qi C, Li Y, Wang CF (2016) Lactobacillus plantarum vaccine vector expressing hemagglutinin provides protection against H9N2 challenge infection. Virus Res 211:46–57. https://doi.org/10.1016/j.virusres.2015.09.005
Sorvig E, Mathiesen G, Naterstad K, Eijsink VG, Axelsson L (2005) High-level, inducible gene expression in Lactobacillus sakei and Lactobacillus plantarum using versatile expression vectors. Microbiology 151:2439–2449. https://doi.org/10.1099/mic.0.28084-0
Subramaniam S, Cao D, Tian D, Cao QM, Overend C, Yugo DM, Matzinger SR, Rogers AJ, Heffron CL, Catanzaro N, Kenney SP, Opriessnig T, Huang YW, Labarque G, Wu SQ, Meng XJ (2017) Efficient priming of CD4 T cells by Langerin-expressing dendritic cells targeted with porcine epidemic diarrhea virus spike protein domains in pigs. Virus Res 227:212–219. https://doi.org/10.1016/j.virusres.2016.10.007
Sung MH, Park C, Kim CJ, Poo H, Soda K, Ashiuchi M (2005) Natural and edible biopolymer poly-gamma-glutamic acid: synthesis, production, and applications. Chem Rec 5(6):352–366. https://doi.org/10.1002/tcr.20061
Tian L, Zhao P, Ma B, Guo G, Sun Y, Xing M (2014) Cloning, expression and antiviral bioactivity of red-crowned crane interferon-alpha. Gene 544(1):49–55. https://doi.org/10.1016/j.gene.2014.04.036
Vennema H, Godeke GJ, Rossen JW, Voorhout WF, Horzinek MC, Opstelten DJ, Rottier PJ (1996) Nucleocapsid-independent assembly of coronavirus-like particles by co-expression of viral envelope protein genes. EMBOJ 15(8):2020–2028
Voets MT, Pensaert M, Rondhuis PR (1980) Vaccination of pregnant sows against transmissible gastroenteritis with two attenuated virus strains and different inoculation routes. Vet Q 2(4):211–219. https://doi.org/10.1080/01652176.1980.9693783
Wang C, Chen J, Shi H, Qiu HJ, Xue F, Liu S, Liu C, Zhu Y, Almazan F, Enjuanes L, Feng L (2010) Rapid differentiation of vaccine strain and Chinese field strains of transmissible gastroenteritis virus by restriction fragment length polymorphism of the N gene. Virus Genes 41(1):47–58. https://doi.org/10.1007/s11262-010-0481-8
Wang X, Wang Z, Xu H, Xiang B, Dang R, Yang Z (2016) Orally administrated whole yeast vaccine against porcine epidemic diarrhea virus induced high levels of IgA response in mice and piglets. Viral Immunol 29(9):526–531. https://doi.org/10.1089/vim.2016.0067
Wang X, Wang L, Huang X, Ma S, Yu M, Shi W, Qiao X, Tang L, Xu Y, Li Y (2017) Oral delivery of probiotics expressing dendritic cell-targeting peptide fused with porcine epidemic diarrhea virus COE antigen: a promising vaccine strategy against PEDV. Viruses 9(11). https://doi.org/10.3390/v9110312
Wanker E, Leer RJ, Pouwels PH, Schwab H (1995) Expression of Bacillus subtilis levanase gene in Lactobacillus plantarum and Lactobacillus casei. Appl Microbiol Biotechnol 43(2):297–303
Xia L, Dai L, Yu Q, Yang Q (2017a) Persistent transmissible gastroenteritis virus infection enhances enterotoxigenic Escherichia coli K88 adhesion by promoting epithelial-mesenchymal transition in intestinal epithelial cells. J Virol 91(21):e01256–e01217. https://doi.org/10.1128/jvi.01256-17
Xia L, Dai L, Zhu L, Hu W, Yang Q (2017b) Proteomic analysis of IPEC-J2 cells in response to coinfection by porcine transmissible gastroenteritis virus and enterotoxigenic Escherichia coli K88. Proteomics Clin Appl 11(11–12). https://doi.org/10.1002/prca.201600137
Xu YG, Guan XT, Liu ZM, Tian CY, Cui LC (2015) Immunogenicity in swine of orally administered recombinant Lactobacillus plantarum expressing classical swine fever virus E2 protein in conjunction with thymosin alpha-1 as an adjuvant. Appl Environ Microbiol 81(11):3745–3752. https://doi.org/10.1128/aem.00127-15
Yang WT, Shi SH, Yang GL, Jiang YL, Zhao L, Li Y, Wang CF (2016) Cross-protective efficacy of dendritic cells targeting conserved influenza virus antigen expressed by Lactobacillus plantarum. Sci Rep 6:39665. https://doi.org/10.1038/srep39665
Yang G, Yao J, Yang W, Jiang Y, Du J, Huang H, Gu W, Hu J, Ye L, Shi C, Shan B, Wang C (2017a) Construction and immunological evaluation of recombinant Lactobacillus plantarum expressing SO7 of Eimeria tenella fusion DC-targeting peptide. Vet Parasitol 236:7–13. https://doi.org/10.1016/j.vetpar.2017.01.023
Yang WT, Yang GL, Wang Q, Huang HB, Jiang YL, Shi CW, Wang JZ, Huang KY, Jin YB, Wang CF (2017b) Protective efficacy of Fc targeting conserved influenza virus M2e antigen expressed by Lactobacillus plantarum. Antivir Res 138:9–21. https://doi.org/10.1016/j.antiviral.2016.11.025
Yang WT, Yang GL, Yang X, Shonyela SM, Zhao L, Jiang YL, Huang HB, Shi CW, Wang JZ, Wang G, Zhao JH, Wang CF (2017c) Recombinant Lactobacillus plantarum expressing HA2 antigen elicits protective immunity against H9N2 avian influenza virus in chickens. Appl Microbiol Biotechnol 101(23–24):8475–8484. https://doi.org/10.1007/s00253-017-8600-2
Yu M, Qi R, Chen C, Yin J, Ma S, Shi W, Wu Y, Ge J, Jiang Y, Tang L (2017a) Immunogenicity of recombinant Lactobacillus casei-expressing F4 (K88) fimbrial adhesin FaeG in conjunction with a heat-labile enterotoxin A (LTAK63) and heat-labile enterotoxin B (LTB) of enterotoxigenic Escherichia coli as an oral adjuvant in mice. J Appl Microbiol 122(2):506–515. https://doi.org/10.1111/jam.13352
Yu M, Wang L, Ma S, Wang X, Wang Y, Xiao Y, Jiang Y, Qiao X, Tang L, Xu Y, Li Y (2017b) Immunogenicity of eGFP-marked recombinant Lactobacillus casei against transmissible gastroenteritis virus and porcine epidemic diarrhea virus. Viruses 9(10). https://doi.org/10.3390/v9100274
Zhang Q, Shi K, Yoo D (2016a) Suppression of type I interferon production by porcine epidemic diarrhea virus and degradation of CREB-binding protein by nsp1. Virology 489:252–268. https://doi.org/10.1016/j.virol.2015.12.010
Zhang Y, Zhang X, Liao X, Huang X, Cao S, Wen X, Wen Y, Wu R, Liu W (2016b) Construction of a bivalent DNA vaccine co-expressing S genes of transmissible gastroenteritis virus and porcine epidemic diarrhea virus delivered by attenuated Salmonella typhimurium. Virus Genes 52(3):354–364. https://doi.org/10.1007/s11262-016-1316-z
Zhang X, Zhu Y, Zhu X, Shi H, Chen J, Shi D, Yuan J, Cao L, Liu J, Dong H, Jing Z, Zhang J, Wang X, Feng L (2017) Identification of a natural recombinant transmissible gastroenteritis virus between Purdue and Miller clusters in China. Emerg Microbes Infect 6(8):e74. https://doi.org/10.1038/emi.2017.62
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2017YFD0501000, 2017YFD0501200), National Natural Science Foundation of China (31672528), Science and Technology Development Program of Jilin Province (20160519011JH, 20170204034NY, 20180520037JH), Special Funds for Industrial Innovation of Jilin Province (2016C063), and “Thirteen Fiveyear Plan” for Sci & Tech Research Program of Jilin Education Department of People’s Republic of China (JJKH20170318KJ).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Ethical approval
All applicable international and national guidelines for the care and use of mice were followed.
Rights and permissions
About this article
Cite this article
Yang, WT., Li, QY., Ata, E.B. et al. Immune response characterization of mice immunized with Lactobacillus plantarum expressing spike antigen of transmissible gastroenteritis virus. Appl Microbiol Biotechnol 102, 8307–8318 (2018). https://doi.org/10.1007/s00253-018-9238-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9238-4