Abstract
Poly(3-hydroxydodecanoate) [P(3HDD)], a medium-chain-length polyhydroxyalkanoate (PHA), is expected to be used as a novel type of bioplastic characterized by a soft and transparent nature. In this study, to achieve a high yield of P(3HDD), PHA synthase was modified through random mutagenesis of a region of the PHA synthase 1 gene from Pseudomonas putida KT2440 (phaC1Pp). Screening of the mutant library using a β-oxidation-deficient Escherichia coli LSBJ was performed. As a result, four mutants, designated w10, w14, w309, and w311, were selected from 10,000 mutants. The w311 mutant had two amino acid replacements (E358G and N398S), and showed the highest production of P(3HDD) with increased polymer molecular weights when compared to the native enzyme. Saturation mutagenesis at the N398 position, which was found to be highly conserved among Pseudomonas PhaCs, revealed that amino acids with hydrophobic and smaller residues either retained or increased P(3HDD) production. This study demonstrates the benefit of using the PHA synthase mutants to enhance the production of P(3HDD).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A wide variety of bacteria and archaea synthesize aliphatic polyesters called polyhydroxyalkanoates (PHAs) in unbalanced growth conditions with excess amounts of the carbon source, such as sugars and plant oils, are present (Steinbuchel and Valentin 1995; Madison and Huisman 1999; Thomson et al. 2010; Matsumoto and Taguchi, 2013; Tsuge et al. 2015; Mizuno et al. 2018). Due to their role in energy storage, PHAs get degraded during starvation to be used as cellular energy source. PHAs are also bio-based polyesters that are thermoplastic, biocompatible, and bioabsorbable; they are expected to be used as eco-friendly plastics due to their biodegradability and renewability (Sudesh et al. 2000).
Typical PHAs consist of R-3-hydroxyalkanoate (3HA) units and are classified into three groups according to side chain length of the 3HA (Steinbuchel and Valentin 1995; Rehm 2003): short-chain-length PHA (SCL-PHA), medium-chain-length PHA (MCL-PHA), and long-chain-length PHA (LCL-PHA). MCL-PHAs (3HA with 6–14 carbon numbers) are mainly produced by pseudomonads either from sugars through fatty acid biosynthesis or from fatty acids through β-oxidation (Huisman et al. 1989; Witholt and Kessler 1999). Usually, MCL-PHAs are heteropolymers consisting of 3-hydroxyoctanote (3HO, C8), 3-hydroxydecanoate (3HD, C10), and 3-hydroxydodecanoate (3HDD, C12) monomers. The copolymeric nature of these MCL-PHAs makes these materials sticky and elastomeric in nature and imparts low crystallinity (Poirier et al. 1995).
Unlike PHA heteropolymers synthesized by natural strains, MCL-PHAs comprised of an almost sole-repeating unit are novel PHA polymers that can be synthesized by metabolically engineered bacteria such as β-oxidation-deficient mutant Pseudomonas strains (Wang et al. 2009, 2011; Rai et al. 2011; Liu et al. 2011; Chung et al. 2011) and recombinant Escherichia coli (Sato et al. 2012; Tappel et al. 2012; Hiroe et al. 2016). During the synthesis of almost homopolymers in these bacteria, since the genes for the β-oxidation pathway in the host cells are absent, fatty acids supplemented are directly converted into 3HA monomers with the carbon number remaining unchanged (Sato et al. 2012; Tappel et al. 2012) (Fig. 1a). Pure fatty acids need to be supplemented to serve as a PHA precursor during these polymers production. The synthesis of almost homopolymers with carbon numbers 6–12 upon feeding the corresponding fatty acid has been previously demonstrated (Sato et al. 2012; Tappel et al. 2012). Among these MCL-PHAs, poly(3-hydroxydecanoate) [P(3HD)] and poly(3-hydroxydodecanoate) [P(3HDD)] with repeating units of C10 and C12, respectively, are materials that are easy to handle, being able to be processed into highly transparent, glossy, and flexible films (Hiroe et al. 2016). Both of these MCL-PHAs are practical materials to use; however, the P(3HDD) titer was observed to be much lower than the P(3HD) titer during fermentative production (Tappel et al. 2012; Hiroe et al. 2016). So far, strategies for enhancing the P(3HDD) production, such as selection of the best monomer-supplying enzyme, enhanced expression of biosynthetic enzymes, and optimization of medium composition have been attempted. Improvement in the yield was obtained by the optimizing medium composition (Hiroe et al. 2016), but was less than expected. A modification of PHA synthase (PhaC) represents the next logical target, since the polymerization activity of PhaC for 3HDD is lower than that for 3HD.
This study aimed to modify a PHA synthase derived from Pseudomonas putida KT2440 (PhaC1Pp) through random mutagenesis to optimize the production of P(3HDD). From this randomly mutagenized library, we screened for PhaC1Pps with increased activity and specificity for the polymerization of 3HDD. Saturation mutagenesis allowed us to optimize these enzymes to improve P(3HDD) production and provides a path forward for reliable production of this unique MCL-PHA.
Materials and methods
Construction of phaC1 Pp mutant library
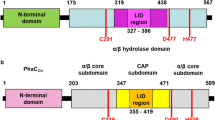
The mutant library of PHA synthase 1 gene (phaC1Pp) from P. putida KT2440 (Nelson et al., 2003) was constructed by Takara Bio Inc. (Shiga, Japan). Mutations were introduced using Diversify PCR Random Mutagenesis Kit (Takara Bio Inc.) from the region between Xba I and Bgl II sites located upstream and downstream of phaC1Pp, respectively, in pBBRC1PpJ4Pa (Fig. 1b). The pBBRC1PpJ4Pa was a pBBR1MCS2-based plasmid (Kovach et al. 1995) harboring phaC1Pp and an R-specific enoyl-CoA hydratase 4 gene (phaJ4Pa) from Pseudomonas aeruginosa DSM1707 (Tsuge et al. 2003). During PCR, the buffer condition #2 was selected to introduce 2.3-bp mutations per 1 kbp (= 3.9 bp mutations on average in phaC1Pp).
Plate-based screening of PhaC1Pp mutants
The plasmid solution of the phaC1Pp library was used to transform E. coli LSBJ (LS5218ΔfadBΔfadD) cells (Tappel et al. 2012) harboring pTTQ19ACSPp (Hiroe et al. 2016), which carries an acyl-CoA synthetase gene from P. putida KT2440; transformants were plated in Nile Red agar plates. Nile Red is a lipophilic stain that can be used as an indicator of intracellular PHA accumulation. The composition of Nile Red agar is as follows: M9 medium (Na2HPO4・12H2O 17.1 g/L, KH2PO4 3 g/L, NaCl 0.5 g/L, NH4Cl 1 g/L, MgSO4・7H2O 0.49 g/L, CaCl2 0.011 g/L) supplemented with agar (15 g/L), kanamycin (50 mg/L), carbenicillin (50 mg/L), isopropyl β-D-1-thiogalactopyranoside (IPTG) (1 mM), sodium octanoate (C8Na, 1 g/L), glucose (5 g/L), and Nile Red (0.5 mg/L). Inoculated plates were incubated at 30 °C for 1–2 days, and were cooled down at 4 °C for 2 days. Each plate was observed using a Dark Reader® transilluminator (MoBiTec, Göttingen, Germany) at 420–500 nm visible light, with an attached amber screen. Colonies exhibiting orange fluorescence were transferred to new Nile Red plates, together with the control strain expressing the parent PhaC1Pp, and were incubated again. By comparing fluorescence intensities, single colonies were selected for further screening.
Flask culture screening of PhaC1Pp mutants
As a secondary screening procedure, flask cultures were performed with sodium dodecanoate (C12Na) as the sole carbon source to identify high producing P(3HDD) strains. The colonies selected after the first round of screening were inoculated into 1.7 mL of LB medium (NaCl 10 g/L, tryptone 10 g/L, and yeast extract 10 g/L) containing kanamycin (50 mg/L) and carbenicillin (50 mg/L), and were incubated at 37 °C for 15 h. An aliquot (1 mL) of pre-culture solution was inoculated into M9 medium containing kanamycin (50 mg/L), carbenicillin (50 mg/L), glucose (5 g/L), and IPTG (1 mM), and cultivated at 30 °C. After 9 h, C12Na (0.5 g/L) and BRIJ35 (0.2 v/v %) were added to the medium to serve as a 3HDD precursor and as a detergent to dissolve fatty acids, respectively. After 72 h, cells were collected, washed with hexanes and water, and then lyophilized. Approximately 20 mg of dried cells were placed in a test tube and incubated with 2 mL of sulfuric acid/methanol (15:85) solution at 100 °C for 140 min to degrade the polymer into methyl-esterified monomers. After cooling down, 1 mL of distilled water was mixed in and the solution was left to stand overnight. The lower layer (chloroform) was filtered (0.45 μm), and 0.5 mL of the filtrate was mixed with 0.5 mL chloroform solution containing methyl n-octanoate (Kanto Chemical Co Inc., Tokyo, Japan) as an internal standard. The 3HA monomers were detected through gas chromatography (GC) equipped with a flame ionization detector (FID) using a GC-14B system (Shimadzu, Kyoto, Japan); PHA content was calculated based on peak area.
Identification of nucleic acid and amino acid mutations
DNA sequencing was performed for 140 mutants selected by plate-based screening. Overlap mutations were confirmed through bioinformatic analysis.
Analysis of the screened PhaC1Pp mutants
Four mutants were identified via the screening process that achieved high levels of PHA production and were designated w10, w14, w309, and w311. To validate the substrate specificity of these mutants, pBBRC1PpJ4Pa (w10, w14, w309, and w311) were transformed into E. coli LS5218 (Rand et al. 2017), which has a functional β-oxidation pathway, together with pTTQACSPp; transformants were cultivated using shake flasks as described previously. Copolymer compositions were determined through GC analysis.
With the w311 mutant, to elucidate the effect of each point mutation on polymer production, enzymes with single amino acid changes (E351G or N398S) were generated using site-directed mutagenesis. In the same manner, saturation mutagenesis at position N398 was also performed. Site-directed mutagenesis was carried out through the overlap extension PCR method (Ho et al. 1989) as follows: forward and reverse primers each containing a point mutation and primers containing a restriction enzyme site (Xba I or Bgl II) were designed. Using corresponding primers, amplification of the Xba I-side and Bgl II-side fragments proceeded through 3- or 2-step PCR. The resulting fragments after one round of PCR were then used as templates and PCR was carried out again using the outside Xba I and Bgl II primers. Resulting phaC1Pp fragments with point mutations were digested with Xba I and Bgl II, and were inserted into the same restriction site of pBBR1C1PpJ4Pa (Fig. 1b). Each construct was transformed into E. coli LSBJ, and flask cultivation was performed under the same conditions as described previously.
After cultivation, intracellular polymers were extracted and purified as described in the methodology of Hiroe et al. (2015). The molecular weight of the extracted PHA was determined through gel permeation chromatography (GPC) using a polystyrene calibration curve.
Results
Screening of PhaC1Pp mutants from library
As a carbon source for plate-based screening, octanoate (C8Na) was used to prevent the discoloration of the Nile Red plate by the hydrophobicity of the substrate. From 10,000 clones grown on Nile Red agar plates, 140 clones exhibiting relatively strong orange fluorescence were selected. These clones were then cultured in flasks containing C12Na as the sole carbon source. The distribution pattern of the level of PHA accumulation of the random mutant population is shown in Fig. 2. Approximately 36% of clones accumulated P(3HDD) at equal or higher levels than the parent strain (17 wt% of PHA content on average). Among 140 mutants, it was confirmed that four mutants stably produced P(3HDD) with at least 24 wt% of PHA content (Table S1).
Sequencing revealed that overlap mutants constituted only 15% of the sequenced mutants, indicating that the screening method worked while preserving diversity. In addition, the R146V, G152S, M172T, N318S, and A346V point mutations remarkably decreased the accumulation level of PHA (less than 1 wt%).
Characterization of the selected PhaC1Pp mutants
Four PhaC1Pp mutants capable of producing high PHA content were named w10, w14, w309, and w311. Sequence analysis of these four mutants revealed that each enzyme had around 2–5 mutations, which are listed in Table 1. The mutations over the whole PhaC1Pp region did not repeat and were confirmed to be unbiased, proving the efficacy of the screening method employed.
Because each mutant contained multiple mutations, it was difficult to specify the important residue that contributed to the enhancement of P(3HDD) production. Thus, amino acid sequences of the four mutants were compared with 78 amino acid sequences of PhaC1 from various Pseudomonas strains (identity = 53–100%) through homology search (Clustal W) (Fig. S1). The positions R197 (w14) and N398 (w311) were completely conserved across all 78 sequences; L32 (w10), N126 (w10), V176 (w309), I271 (w10), N333 (w10), and E358 (w311) were also highly conserved residues. On the other hand, M132 (w10), M292 (w14), A532 (w14), and Q105 (w309) were not conserved at all among the PhaC1 primary sequences examined.
The P(3HDD) production by β-oxidation-deficient E. coli LSBJ expressing four in vitro evolved PhaC1Pps are summarized in Table 2. PHA content and yield increased 1.3–1.5-fold (up to 25.6 wt%) and 1.3–1.6-fold (up to 0.35 g/L), respectively, compared to the parent enzyme. As to w311, the deviation of PHA content was relatively high (Fig. 1 and Table 2). In addition, weight average molecular weight (Mw) also increased 1.9–2.8-fold (up to 214,000 g/mol) (Table 2). The 3HDD molar fraction of each polymer was observed to be higher than 99 mol%; thus, these PhaC1Pp mutants can synthesize almost homopolymers as efficiently as the parent enzyme. These four mutants also showed an increase in both production and molecular weight of P(3HDD). The protein expression levels of three mutants (w14, w309, and w311) were almost equal to that of the parent enzyme, as confirmed through western blotting (Fig. 3). On the other hand, the expression level of the w10 mutant decreased, perhaps due to a replacement of the residue I312 with a rare codon (Table 1). These results indicated that each mutant exhibited enhanced polymerization activity.
To validate the substrate specificity of the four mutants, PHA production via the β-oxidation pathway was carried out using E. coli LS5218 with a functional β-oxidation pathway as the host strain. In this assay, the substrate specificity of PHA synthase was evaluated as the monomer composition of PHA (Takase et al. 2004). As a result, w10, w309, and w311 mutants showed increased monomer ratios of longer side chain units (3HD and 3HDD), but showed decreased monomer ratios of shorter side chain units [3-hydroxyhexanote 3HHx and 3HO (Fig. 4, Table S2)]. The w311 mutant showed a significant decrease in 3HHx ratio; nearly 40% less than the parent enzyme. In contrast, the w14 mutant showed no significant change in substrate specificity compared to the parent enzyme, indicating an enhancement of polymerization ability without changing substrate specificity.
Substrate specificity of parent PhaC1Pp and PhaC1Pp mutants (w10, w14, w309, and w311) evaluated by cultivations with the β-oxidation functional E. coli LS5218 strain and sodium dodecanoate (C12Na). Each cultivation was carried out in triplicate, and results are shown as averages with standard deviations
Examination of individual point mutations in the w311 PhaC1Pp enzyme
Among the enzymes screened from the PhaC1Pp library, the w311 mutant was the most beneficial in terms of cell growth, polymer yield, and polymer molecular weight. As for the w311 mutant, we generated a series of individual point mutations into the native PhaC1Pp primary sequence in order to identify the relevant amino acids for enhanced P(3HDD) production. As a result, the point mutations, E358G and N398S, exhibited lower PHA accumulation than the parent w311, suggesting a synergistic function between the two mutations. Furthermore, the N398S mutation seemed to contribute to increased polymer production, while the E358G mutation contributed to increased polymer molecular weight (Table 3).
The residue N398 was observed to be completely conserved across 78 PhaC sequences of Pseudomonas, demonstrating the importance of this amino acid. To obtain more beneficial mutants by replacing N398, saturation mutagenesis at this position was performed. The PHA content, dry cell weight, PHA concentration, and cell mass are listed in Fig. 5 and Fig. S2. The results indicated that the original mutation N398S was able to accumulate the highest PHA content (20.2 wt%). Aside from N398S, eight mutants did not show PHA accumulation, five mutants showed a PHA content of 1–10 wt%, and five mutants showed a PHA content of 10–20 wt%. Replacement of N398 with more hydrophilic (D, E, K, and R) and larger (W) amino acids drastically reduced PHA accumulation. Meanwhile, replacement with more hydrophobic and smaller amino acids (G, S, A, C, T, and V) either enhanced or maintained PHA accumulation.
Discussion
MCL-PHAs comprised of an almost sole repeating unit are soft and transparent materials different from conventional bioplastics such as poly(3-hydroxybutyrate) and poly(lactate). These different physical properties make the MCL-PHA polymers attractive materials for further study because of their ability to contribute to new biopolymer applications. However, the production yield of MCL-PHA homopolymers is relatively low compared to shorter chain length PHAs, especially for P(3HDD). The reason for this low P(3HDD) yield can be attributed to the low polymerization activity of PhaC for 3HDD monomers. In this study, to increase P(3HDD) yield, PhaC1Pp was modified through random mutagenesis. Since the structure of PhaC1Pp is still unknown, random mutagenesis is an efficient approach for engineering PhaC1Pp.
Efficient screening conditions were first validated to select mutants from a PhaC1Pp library. As it takes time and money to find beneficial mutants in a large library of mutants, screening should be conducted under appropriate conditions. In this study, to improve the screening efficiency, a plate assay using Nile Red dye (Greenspan et al. 1985) was used as the first screening method. Since Nile Red can stain PHA in cells dependent on PHA accumulation levels (Spiekermann et al. 1999), visual judgments can be carried out to initially select positive mutants. Nile Red agar plates supplemented with C12Na were prepared initially; however, problems involving the discoloration of the Nile Red plate due to the hydrophobicity of the substrate were encountered. Thus, C8Na was used due to its relatively lower hydrophobicity.
The Nile Red plate assay using C8Na provided an initial selection criteria and allowed us to narrow down the number of potential candidates from 10,000 to 140 clones effectively. In addition, the 140 selected clones were cultured with C12Na in the subsequent screen, and approximately 36% of the clones accumulated PHA at level equal to or greater than the parent PhaC1Pp. From these results, the viability of the screening assay was confirmed; moreover, four positive mutants, w10, w14, w309, and w311, capable of enhanced P(3HDD) production were obtained.
A number of beneficial mutations to improve the performance of the Pseudomonas sp. 61-3 class II PhaC (PhaC1Ps) have been reported. In the case of PhaC1Ps, it has been shown that E130 and S325 contribute to the enzyme activity, and Q477 and Q481 contribute to the substrate specificity (Taguchi and Tsuge 2011). In this study, 12 beneficial mutations of PhaC1Pp were discovered. Based on the results of saturation mutagenesis in this study, it is suggested that N398 is related to polymerization activity (Fig. 5). Recently, the crystal structure of the Class I PHA synthase derived from Ralstonia eutropha (PhaC1Re) was partially elucidated (Wittenborn et al. 2017; Kim et al., 2017a, b). Homology modeling (SWISS-MODEL) of Class II PhaC1Pp (180–558 aa) based on PhaCRe C-terminal domain (192–589 aa) indicated that N398 is located close to a catalytic triad, C296, D451, and H479 (Fig. S3). Additionally, according to Wittenborn et al. (2017), N398 is located in the second position of the structural motif WNXD related to the expansion and stabilization of a PHA exit channel. In this manner, experimental and structural prediction data both support the important role of N398. N333 and E358 are located in the dimerization subdomain of PhaC1Pp according to Kim et al. (2017a, b). N333 was especially close to amino acids involved in dimerization (Q334, L337, and E341); therefore, N333S may activate the dimerization and enhance the polymerization reaction. On the other hand, the E358G variant produced polymers with increased of molecular weights (Table 3), indicating the possibility that the slight change of dimer formation caused by the E358G mutation enhanced the polymerization activity. In addition, there is a possibility that the combination arrangement (E358X and N398X) would provide a better performing enzyme (PhaC1Pp). L271 is located in the active site close to catalytic triad (Fig. S3); therefore, this residue may be involved in substrate recognition and/or enzymatic activity. As for the L32, N126, V176, and R197 mutations, no structure-function relationships could be deduced from the homology model. The functional results arising from these mutations may be explained if the crystal structure of the full-length PHA synthase (including N-terminal domain) is elucidated.
In this study, we successfully generated PhaC1Pp mutants with enhanced P(3HDD) production. All amino acid mutations discovered in this study have not been reported previously. The use of mutants generated by random mutagenesis, especially w311, led to ~ 1.6-fold increased production of P(3HDD).
References
Chung AL, Jin HL, Huang LJ, Ye HM, Chen JC, Wu Q, Chen GQ (2011) Biosynthesis and characterization of poly(3-hydroxydodecanoate) by β-oxidation inhibited mutant of Pseudomonas entomophila L48. Biomacromolecules 12(10):3559–3566
Greenspan P, Mayer EP, Fowler SD (1985) Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 100(3):965–973
Hiroe A, Shiraishi M, Mizuno K, Tsuge T (2015) Behavior of different polyhydroxyalkanoate synthases in response to the ethanol level in Escherichia coli cultures. Polym J 47:767–770
Hiroe A, Ishii N, Ishii D, Kabe T, Abe H, Iwata T, Tsuge T (2016) Uniformity of monomer composition and material properties of medium-chain-length polyhydroxyalkanoates biosynthesized from pure and crude fatty acids. ACS Sustain Chem Eng 4(12):6905–6911
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77(1):51–59
Huisman GW, de Leeuw O, Eggink G, Witholt B (1989) Synthesis of poly-3-hydroxy-alkanoates is a common feature of fluorescent pseudomonads. Appl Environ Microbiol 55:1949–1954
Kim YJ, Choi SY, Kim J, Jin KY, Lee SY, Kim KJ (2017a) Structure and function of the N-terminal domain of Ralstonia eutropha polyhydroxyalkanoate synthase, and the proposed structure and mechanisms of the whole enzyme. Biotechnol J 12. https://doi.org/10.1002/biot.201600649
Kim J, Kim YJ, Choi SY, Lee SY, Kim KJ (2017b) Crystal structure of Ralstonia eutropha polyhydroxyalaknoate synthase C-terminal domain and reaction mechanisms. Biotechnol J 12. https://doi.org/10.1002/biot.201600648
Kovach ME, Elzer PH, Robertson GT, Farris MA, Roop RM II, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166(1):175–176
Liu Q, Luo G, Zhou XR, Chen GQ (2011) Biosynthesis of poly(3-hydroxydecanoate) and 3-hydroxydodecanoate dominating polyhydroxyalkanoates by β-oxidation pathway inhibited Pseudomonas putida. Metab Eng 13(1):11–17
Madison LL, Huisman GW (1999) Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63(1):21–53
Matsumoto K, Taguchi S (2013) Biosynthetic polyesters consisting of 2-hydroxyalkanoic acids: current challenges and unresolved questions. Appl Microbiol Biotechnol 97:8011–8021
Mizuno S, Endo Y, Saika A, Hiroe A, Tsuge T (2018) Biosynthesis of polyhydroxyalkanoates containing 2-hydroxy-4-methylvalerate and 2-hydroxy-3-phenylpropionate units from a related or unrelated carbon source. J Biosci Bioeng 125(3):295–300
Nelson KE, Weinel C, Paulsen IT, Dodson RJ, Hilbert H, Martins dos Santos VA, Fouts DE, Gill SR, Pop M, Holmes M, Brinkac L, Beanan M, DeBoy RT, Daugherty S, Kolonay J, Madupu R, Nelson W, White O, Peterson J, Khouri H, Hance I, Chris Lee P, Holtzapple E, Scanlan D, Tran K, Moazzez A, Utterback T, Rizzo M, Lee K, Kosack D, Moestl D, Wedler H, Lauber J, Stjepandic D, Hoheisel J, Straetz M, Heim S, Kiewitz C, Eisen JA, Timmis KN, Düsterhöft A, Tümmler B, Fraser CM (2003) Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol 4(12):799–808
Poirier Y, Nawrath C, Somerville C (1995) Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Biotechnology 13(2):142–150
Rai R, Yunos DM, Boccaccini AR, Knowles JC, Barker IA, Howdle SM, Tredwell GD, Keshavarz T, Roy I (2011) Poly-3-hydroxyoctanoate P(3HO), a medium chain length polyhydroxyalkanoate homopolymer from Pseudomonas mendocina. Biomacromolecules 12(6):2126–2136
Rand JM, Gordon GC, Mehrer CR, Pfleger BF (2017) Genome sequence and analysis of Escherichia coli production strain LS5218. Metab Eng Commun 5:78–83
Rehm BHA (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33
Sato S, Ishii N, Hamada Y, Abe H, Tsuge T (2012) Utilization of 2-alkenoic acids for biosynthesis of medium-chain-length polyhydroxyalkanoates in metabolically engineered Escherichia coli to construct a novel chemical recycling system. Polym Degrad Stab 97:329–336
Spiekermann P, Rehm BH, Kalscheuer R, Baumeister D, Steinbuchel A (1999) A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch microbiol 171(2):73–80
Steinbuchel A, Valentin HE (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett. 128:219–228
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoate: biological polyesters. Prog Polym Sci 25:1503–1555
Taguchi S, Tsuge T (2011) Natural polyester-related proteins: structure, function, evolution and engineering. In: Luts S, Bornscheuer T (eds) Protein engineering handbook. Wiley-VCH, pp 877–914
Takase K, Matsumoto K, Taguchi S, Doi Y (2004) Alteration of substrate chain-length specificity of type II synthase for polyhydroxyalkanoate biosynthesis by in vitro evolution: in vivo and in vitro enzyme assays. Biomacromolecules 5(2):480–485
Tappel RC, Wang Q, Nomura CT (2012) Precise control of repeating unit composition in biodegradable poly(3-hydroxyalkanoate) polymers synthesized by Escherichia coli. J Biosci Bioeng 113(4):480–486
Thomson N, Summers D, Sivaniah E (2010) Synthesis, properties and uses of biological storage lipid granules as naturally occurring nanoparticles. Soft Matter 6:4045–4057
Tsuge T, Taguchi K, Taguchi S, Doi Y (2003) Molecular characterization and properties of (R)-specific enoyl-CoA hydratases from Pseudomonas aeruginosa: metabolic tools for synthesis of polyhydroxyalkanoates via fatty acid β-oxidation. Int J Biol Macromol 31(4):195–205
Tsuge T, Hyakutake M, Mizuno K (2015) Class IV polyhydroxyalkanoate (PHA) synthases and PHA-producing Bacillus. Appl Microbiol Biotechnol 99:6231–6240
Wang HH, Li XT, Chen GQ (2009) Production and characterization of homopolymer polyhydroxyheptanoate P(3HHp) by a fadBA knockout mutant Pseudomonas putida KTOY06 derived from P. putida KT2442. Process Biochem 44:106–111
Wang HH, Zhou XR, Lie Q, Chen GQ (2011) Biosynthesis of polyhydroxyalkanoate homopolymers by Pseudomonas putida. Appl Microbiol Biotechnol 89(5):1497–1507
Witholt B, Kessler B (1999) Perspectives of medium chain length poly(hydroxyalkanotes), a versatile set of bacterial bioplastics. Curr Opin Biotechnol 10:279–285
Wittenborn EC, Jost M, Wei Y, Stubbe J, Drennan CL (2017) Structure of the catalytic domain of the class I polyhydroxybutyrate synthase from Cupriavidus necator. J Biol Chem 291:25264–25277. https://doi.org/10.1074/jbc.M116.756833
Acknowledgements
The authors acknowledge Biomaterials Analysis Division center Tokyo Institute of Technology for analyzing all sequencing experiments. This work was supported by JST, CREST (grant number JPMJCR12B4), Japan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animal performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 431 kb)
Rights and permissions
About this article
Cite this article
Hiroe, A., Watanabe, S., Kobayashi, M. et al. Increased synthesis of poly(3-hydroxydodecanoate) by random mutagenesis of polyhydroxyalkanoate synthase. Appl Microbiol Biotechnol 102, 7927–7934 (2018). https://doi.org/10.1007/s00253-018-9230-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9230-z