Abstract
Morels are some of the most highly prized edible and medicinal mushrooms, and the outdoor cultivation has been achieved in China in recent years. Sclerotial formation is one of the most important phases during the morel life cycle, and the number of sclerotia indicates the spawn quality during cultivation. However, the sclerotial formation and differentiation mechanisms are poorly understood. In this study, the sclerotial formation process of Morchella importuna and the effects of reactive oxygen species on scerotial formation were studied. Scerotial formation was defined as five distinctive phases, hypha early, hyphal growth, sclerotial initiation, development, and maturation. The mycelia in the sclerotium-forming area were swollen, darkened, and dense with sclerotial formation, but hydrogen peroxide accumulated in the region lacking sclerotial formation. The expression of all six genes for superoxide dismutases tested increased with sclerotial maturation. A difference in hydrogen peroxide concentration of 20 mM could promote the sclerotial initiation and induce expression of sod genes. The MAPK signaling pathway was activated, and they passed the signal from an area of high oxidative stress to a low area to initiate sclerotial formation. An understanding of the sclerotial formation mechanisms in M. importuna may help to understand the life cycle and facilitate the fruiting body cultivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sclerotia are hardened masses of hyphae that serve as survival structures for Ascomycetes and Basidiomycetes. These persistent structures help fungi survive challenging conditions such as freezing temperatures, desiccation, microbial attack, or the long-term absence of a host (Smith et al. 2015). Typically, sclerotial formation presents three stages: sclerotial initiation (SI), sclerotial development (SD), and sclerotial maturation (SM) (Papapostolou and Georgiou 2010a).
Sclerotium-forming fungi are ecologically diverse and phylogenetically distributed among 85 fungal genera in at least 20 orders of Basidiomycota and Ascomycota (Smith et al. 2015). Morels (Morchella, Ascomycota), which are commercially important edible mushrooms with a delicate taste, are sclerotium-forming fungi. Morel cultivation has been a research focus worldwide for more than 100 years, and the outdoor cultivation of Morchella belonging to the Elata Clade, including Morchella importuna, has succeeded and expanded at a large scale in China in recent years (Liu et al. 2017). The total amount of field-cultivated fresh morels was estimated to be approximately 500 t in 2015–2016 in China; however, the yield per ha fluctuated from 0 to 7620 kg (Liu et al. 2017), and this can be attributed to some unsolved biological issues.
Sclerotial formation is an important phase during the morel life cycle (Volk and Leonard 1990). Growers empirically judge the spawn quality solely based on the quantity of sclerotia in field cultivation. However, the mechanisms of sclerotial formation and differentiation are poorly understood.
Sclerotial metamorphosis is believed to be induced by oxidative stress based on the fact that the sclerotial differentiation state in Sclerotium rolfsii concurred with increasing levels of lipid peroxides (Georgiou 1997). Superoxide radical (O2-), hydrogen peroxide (H2O2), and lipid hydroperoxides (LOOH) are direct indicators of oxidative stress, and they constitute some of the most important components of reactive oxygen species (ROS). This finding was supported by a series of published data that certain hydroxyl radical scavengers, vitamin C and β-carotene inhibited the sclerotial differentiation of S. rolfsii, Sclerotinia minor, Sclerotinia sclerotiorum, and Rhizoctonia solani (Georgiou et al. 2006).
Subsequent studies have reported that H2O2 plays a role in sclerotial differentiation via induction of higher oxidative stress in sclerotiogenic filamentous phytopathogenic fungi (Papapostolou and Georgiou 2010a). During the sclerotial formation of S. minor, H2O2-reactive signals were specifically accumulated in the outer layers of the SM stage by ultrastructural analysis (Tomoyuki et al. 2017), indicating different patterns of ROS localization during sclerotial development.
Organisms possess superoxide dismutases (SODs), a group of enzymes that catalyze the dismutation of O2- to molecular oxygen (O2) and H2O2 (Sheng et al. 2014). It was reported that the antioxidant enzyme SOD levels in the sclerotiogenic fungi were significantly lower than those of their non-differentiating counterpart strains (Papapostolou and Georgiou 2010b). Superoxide radicals in association with SOD regulate the sclerotial differentiation of S. rolfsii (Papapostolou and Georgiou 2010c).
The sclerotia of morels form by repeated hyphal branching and further expansion growth of the nodules, and they are described as pseudosclerotia (Volk and Leonard 1989) because they do not have the structures typical of sclerotia as characterized in S. sclerotiorum. Whether ROS play the same role in the sclerotial formation and its mechanisms in Morchella are our concern.
There have been some studies on the effect of ROS on sclerotial formation in Morchella. Pseudosclerotial formation in Morchella steppicola Zerova was induced by oxidative burst (Király and Czövek 2007). The reactive oxygen radical scavengers, N,N-dimethyl nitrosoaniline and sodium benzoate, can reduce intracellular oxidative stress in fungi and inhibit the mycelial growth and sclerotial formation in Morchella crassipes (He et al. 2014). The inhibition was enhanced with increasing scavenger concentration, and high concentrations could completely inhibit the occurrence of sclerotia (He et al. 2014). The mechanism is worth studying further in detail.
M. importuna is the major species of morels now cultivated in China, and its cultivation accounts for approximately 80–90% of the total cultivated area. In the present study, the effects of environmental conditions including cultivation temperature, light, and medium pH on sclerotial formation were evaluated, and then the ROS level and expressions of six different genes for SOD isoenzymes during sclerotial formation were monitored in different locations. The factors affecting sclerotial formation and triggering sclerotial metamorphosis were investigated. We found that a difference in H2O2 concentration of 20 mM could promote sclerotial initiation, induce the expression of sod genes, and activate the mitogen-activated protein kinase (MAPK) signal pathway, which is a highly conserved family of serine/threonine protein kinases involved in a variety of fundamental cellular processes including proliferation, differentiation, stress response, apoptosis, and survival (Cargnello and Roux 2011).
Materials and methods
Strain and sclerotia cultivations
The strain used in this work was isolated from a Morchella fruiting body and deposited in China General Microbiological Culture Collection Center (CGMCC no. 5.2196). It was identified as M. importuna by internal transcribed spacer (ITS1-5.8S-ITS2 of nuclear ribosomal DNA) sequence analysis and the nucleotide sequence has been deposited in GenBank under accession number MH005092. M. importuna was routinely grown at 20 °C in PDA medium (2% potato, 2% glucose, and 2% agar). Mycelial plugs (ca. 8 mm in diameter) of M. importuna were inoculated at the center of Petri dishes. In the temperature assay, the Petri dishes were placed at 15, 20, 25, and 30 °C under dark conditions. Darkness, weak light (20 lx), and strong light (2000 lx, LED) at 20 °C were used in the light assay. The strain was cultivated on PDA with different pHs varying from 4 to 9 at 20 °C in the pH assay. The production of sclerotia in each treatment was evaluated with the dry weight (DW) during the experiment. The experiments were repeated at least three times. A Duncan’s multiple range test (P < 0.05) was used to evaluate the significant differences between each group. The statistical analysis and the graphs were implemented using Graphpad Prism 6.0 (Graphpad Software, Inc., San Diego, CA, USA).
Morphological observation
Microscopic preparations were made in clear lactic acid and observed with a Nikon Eclipse 80i microscope using differential interference contrast (DIC) illumination (Nikon, Minato-ku, Tokyo, Japan). ROS staining observations were conducted with a Nikon SMZ1500 dissecting microscope (Nikon, Minato-ku, Tokyo, Japan).
DAB and NBT staining
To detect ROS generation, nitro blue tetrazolium (NBT, for O2- detection) and diaminobenzidine (DAB, for H2O2 detection) staining was performed. Slices of PDA media with mycelia at different sclerotial developmental stages were cut by a scalpel from the center towards the outside with sample widths of 0.5 mm, treated with 0.01% (w/v) DAB or 0.05% (w/v) NBT stain in a 50-mM sodium phosphate buffer (pH 7.4; PBS) and incubated for 1 h at 25 °C. The buffer was then removed, and the media with mycelium were washed thrice with distilled water. The slices of the media were placed onto absorbent blot paper, observed under an anatomical lens, and photographed.
Effects of hydrogen peroxide concentrations and concentration difference on the sclerotial formation
The effects of H2O2 concentrations on sclerotial formation were determined by adding H2O2 to the PDA media from 5 to 20 mM. M. importuna was inoculated on a Petri dish, half of which was PDA and the other half of which was 20 mM H2O2 in PDA, thus producing a difference in H2O2 concentration. Plates wholly comprising a PDA medium and PDA containing 20 mM H2O2 were used as controls. The cultures were incubated at 20 °C without light. The sclerotia were scraped off with a scalpel, dried at 45 °C to a constant weight, and then weighed.
RNA isolation
Colonies of M. importuna were cultured on PDA medium at 20 °C, and approximately 0.1 g cells were collected at every stage into a 1.5-ml Eppendorf tube. Samples were mechanically broken using a Tomy Microsmash disrupter (Tomy Medico, Nerima-ku, Tokyo, Japan) by 4 rounds of 40-s bead-beating at 5500 rpm at 4 °C. Total RNA was isolated from 0.1 g mycelia using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The extracted RNA was then treated with RQ1 RNase-Free DNase (Promega, Madison, WI, USA).
RT-PCR
RNA (3 μg) was incubated with 300 ng of oligo dT-15 primer in 15 μl diethyl pyrocarbonate-treated H2O for 10 min at 70 °C and then chilled on ice. Forty-five microliters of the reaction buffer was added containing 0.6 mM dNTPs, 120 units of RNasin ribonuclease inhibitor, and 600 units of Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA). The reaction was incubated for 1 h at 37 °C and terminated by heating the tubes in boiling water for 2 min. The cDNA was stored at 4 °C. For each PCR reaction, 5 μl of the cDNA was used as a template in a 50-μl reaction mixture containing 0.25 mM dNTPs, 1× reaction buffer supplemented with 25 pmol of each PCR primer, 1.5 mM MgCl2, and 2.5 units of Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA). The PCR program used included an initial 2 min hot start at 94 °C and 30 cycles of 30 s of denaturation at 94 °C, 30 s of annealing at 55 °C, and 20 s of elongation step at 72 °C, followed by 7 min elongation at 72 °C. All PCRs were tested at varied cycle numbers to confirm that the use of 30 cycles was within the linear range of amplification.
The oligonucleotide primers used to detect the transcripts of sod genes (sod1, sod2, sod3, sod4, sod5, and sod6) and other genes by RT-PCR are listed in Table 1, and the gene sequences were searched from the JGI M. importuna site (http://genome.jgi.doe.gov/Morimp1/Morimp1.home.html). The gel images were converted to 8-bit images, and the intensity of each band was measured using ImageJ software (http://rsb.info.nih.gov/ij). The intensity ratio of a sod gene expression versus gapdh expression was calculated.
Results
M. importuna sclerotial formation is influenced by temperature, light, and pH
The M. importuna strain was cultured on a PDA medium with Petri dishes of different sizes (60, 90, and 150 mm in diameter). It was found that sclerotial formation did not initiate until mycelial growth filled the Petri dish regardless of the Petri dish size and days of growth of M. importuna (Supplemental Fig. S1).
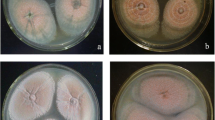
The effects of environmental conditions including temperature, light, and pH on the sclerotial formation were explored. As shown in Fig. 1a, sclerotia can form at 15, 20, and 25 °C on PDA medium, with the optimal temperature of 20 °C. Mycelial growth was unhealthy and no visible sclerotia formed at 30 °C. Cultures were grown at 20 °C for subsequent experiments.
Effect of different growth conditions on sclerotial formation in M. importuna. a Production of sclerotia at different temperatures. The strain was cultured on PDA medium at different temperatures without light for 2 weeks. b Production of sclerotia under different light conditions. The strain was cultivated in darkness, weak light (20 lx), and strong light (2000 lx) at 20 °C for 7 days. c Effect of pH on sclerotial formation. The strain was cultivated on PDA medium with different pH values at 20 °C for 10 days
Sclerotial formation was evaluated under different light conditions, including darkness, weak light (20 lx), and strong light (2000 lx) with a white light-emitting diode (LED). There was no significant difference for the sclerotium weight among the different light conditions (Fig. 1b and Supplemental Fig. S2a).
The mycelia grew well at initial pH values between 4 to 9, but the sclerotial yield at a pH value of 4 and 9 was significantly lower than that under other pH values (P < 0.05, Fig. 1c and Supplemental Fig. S2b). There was no significant difference in sclerotial weight when the initial pH was 5, 6, 7, and 8.
Sclerotial and hyphal (outside) formation process in M. importuna
The formation process of sclerotia in M. importuna was observed in Petri dishes. The growth and sclerotial formation can be divided into five distinctive phases: hypha early (HE), hyphal growth (HG), sclerotial initiation (SI), sclerotial development (SD), and sclerotial maturation (SM). A Petri dish half covered with mycelia was defined as HE (2 days) while a Petri dish full of mycelia was HG (3–4 days). The formation of morel sclerotia can be divided into three phases (Fig. 2a): SI (4–5 days), SD (5–7 days), and SM (more than 7 days).
M. importuna cells show morphological changes at different locations during sclerotial formation. a Colony morphology of M. importuna cells grown on PDA plates in different growth stages. An area of 2 cm radius around the center was divided as the inside area, and the remaining of the plate excluding the inside area was divided as the outside area. Size bar = 1 cm. b Magnified pictures of colonies in Petri dishes. Size bars = 5 mm. c Cell morphology of M. importuna in sclerotial formation. Size bars = 10 μm
White primary sclerotia were aggregated and expanded from sparse hyphae in the SI period. SD comprises dense expanded cell aggregates with a white color and SM comprises dense expanded cell aggregates with brown color. The color of mycelia was observed from white to brown, and there were two obvious regions: sclerotium-forming (inside) and hyphae (outside) regions in the SI, SD, and SM phases (Fig. 2b). In the HG period, the cells at the center and outside areas had no obvious morphological differences (Fig. 2c). During the SI period, swollen cells were observed in the center of the plate, and the outside cells still appeared as mycelia (Fig. 2c). The cells at the center of the dish at the SD period were denser than those of SI, and the outside cells appeared to contain some senescent cells exhibiting atrophy and irregular cell margins within hyphae. At the SM period, mature sclerotia were found in the center of the dish, where cell aggregation was greater, and the color was darker than that of the SD stage. The cells in the outside colony region remained as mycelia, brown with empty content, suggesting that further aging occurred in the cells in the outside region along with sclerotium maturation at the center of the dish (Fig. 2c).
Expression of genes coding for SOD isoenzymes at different locations during sclerotial formation
According to the obvious morphological differences in different locations of the dishes during sclerotial formation, the colony in the Petri dish was divided into two sections, inside and outside (Fig. 2a). The expressions of six different genes coding for SOD isoenzymes were detected in the different sections during sclerotial formation. The result showed that all six sod genes tested were expressed during the whole sclerotial formation, and that the expression of all six sod genes tested in both the inside and outside sections increased in the SM stage as compared with HE. At the SM stage, the expressions of sod genes at the inside section were much higher than those of the outside cells (Fig. 3a, b), suggesting that sclerotia have stronger antioxidant activity than mycelia and that there were different patterns of SOD expression and localization during sclerotial development.
Expressions of sod genes during sclerotial formation in M. importuna. a Expression levels of sod genes in different stages of sclerotial formation by RT-PCR. The constitutive gapdh and rRNA served as the controls. O, cells from the outside region of the Petri dish; I, cells from the inside region of the Petri dish. b The intensity ratio of expression of a sod gene versus expression of gene gapdh. Blue, inside samples; red, outside samples. Error bars, standard deviation
Hydrogen peroxide concentration in the mycelial growth region was higher than that in the sclerotium-forming region
Diaminobenzidine (DAB, for H2O2 detection) and nitro blue tetrazolium (NBT, for O2- detection) staining was used to detect ROS generation during the sclerotial formation in M. importuna. The results showed that the color of medium became deeper from the inside to the outside regions of the dishes stained with DAB at the SD and SM stages (Fig. 4); however, there was no change when dishes were stained with NBT (Fig. 4). The H2O2 concentration in the mycelial growth region (outside) was higher than that in sclerotium-forming region (inside) in the SD and SM stages. The sclerotium with NBT staining showed that the surface layer was deeper stained than in the innermost area, but this did not appear in the DAB-stained sclerotium (Supplemental Fig. S3a and b), indicating that different ROS types have different distribution patterns.
Sclerotial formation and sod expression were induced by difference in hydrogen peroxide concentration
The effect of H2O2 concentrations on sclerotial formation was determined. The results indicated that the mycelium growth rate decreased with the H2O2 concentration in the media increasing from 5 to 20 mM (Fig. 5a and Supplemental Fig. S4a). Sclerotial initiation was delayed at H2O2 concentrations greater than 10 mM in the medium (Fig. 5b) compared with the control. Low concentrations of H2O2 (5 mM) increased sclerotial formation significantly (P < 0.05), and there was no difference in sclerotium appearance time (Fig. 5b). The sclerotial yield decreased after 9 days of cultivation when the H2O2 concentration was greater than 10 mM in the medium (Supplemental Fig. S4b). This indicated that high concentration of H2O2 reduced the growth rate of hyphae and sclerotial formation and did not promote the cells to enter the SI stage.
Effects of hydrogen peroxide on sclerotial formation in M. improtuna. a Colony morphology of sclerotia on PDA containing different concentrations of H2O2. b Time to the sclerotial initiation stage under different concentrations of H2O2. c 20 mM H2O2 triggers sclerotial formation in M. importuna. Upper panel showing the colony morphology when sclerotia appeared, and the lower panel showing the magnified area circled with a red dotted line. L, left side; R, right side
Then, the effect of the H2O2 concentration difference on sclerotial initiation was studied. M. importuna was inoculated on a Petri dish, half of which was PDA and the other half 20 mM H2O2 in PDA, thus producing a difference in H2O2 concentration. PDA medium plates and PDA plates containing 20 mM H2O2 cut with a sterilized knife in the middle into two halves were used as controls. After mycelia grew from the left side with the PDA medium into the right side containing 20 mM H2O2 in the experimental group, sclerotial formation was observed (Fig. 5c). However, presclerotial structures were not observed in the two controls. These results indicate that full hyphal growth throughout the plate was unnecessary for sclerotial initiation under the presence of difference in H2O2 concentrations.
Expression of the sod genes was detected in the mycelial samples collected from cells at the left and right sides of the Petri dishes with different H2O2 concentrations and in the controls. The expression of genes coding for SODs in the right side was higher than that in that of the left side, where the fungus was inoculated in both the control and experimental groups (Fig. 6a, b). The expression of all of the tested sod genes in the group with different H2O2 concentrations in both the left and right sides was higher than in that of the control group (Fig. 6a, b), suggesting that the difference in H2O2 concentration can increase the expression of sod genes in M. importuna.
Effects of hydrogen peroxide on sod gene expression a. M. importuna cells collected from the time shown in Fig. 5c with left and right side area cells. Expression of six different sod genes analyzed by RT-PCR. The constitutively expressed gene gapdh and rRNA served as controls. L, left side; R, right side. b The intensity ratio of expression of a sod gene versus expression of gene gapdh. Blue, left samples; red, right samples. Error bars, standard deviation
MAPK pathway is likely involved in sclerotial formation in M. importuna
The MAPK signaling pathway plays an important role in H2O2 signal sensing and the formation of sclerotia (Georgiou et al. 2006). The expressions of three genes coding for proteins (hog1, spm1, and fus3) in the MAPK signaling pathway and its downstream transcription factors (msn2, prr1, and pap1) were detected in the mycelia cultured under a difference in H2O2 concentration. The results indicated that the expressions of all of the tested genes coding for MAPK proteins and its downstream transcription factors increased in the cells in the left side of a plate of the group with the H2O2 concentration difference as compared with cells of the control group (Fig. 7). As shown in Fig. 5c, sclerotial formation advanced before hyphal growth completely filled the Petri dish in the experimental group. Sclerotia formed at the left side where expressions of the gene coding for MAPK proteins were higher than in the control group, which was still in the mycelial growth stage. These results indicated that the MAPK signaling pathway was activated and its signaling probably initiated sclerotial formation. Moreover, expressions of hog1 and its downstream transcription factor msn2 were nearly double of that in the control, indicating that this H2O2-sensing pathway may play an important role in triggering cells into sclerotial initiation.
Expressions of genes coding for MAPK and downstream transcription factors after being stimulated by hydrogen peroxide. a M. importuna cells collected from the time shown in Fig. 5c with left and right area cells. The constitutively expressed gene gapdh and rRNA served as controls. L, left side; R, right side. b The intensity ratio of expression of a MAPK or transcription factor gene versus expression of gene gapdh. Blue, left samples; red, right samples. Error bars, standard deviation
Discussion
Sclerotial formation is one of the most important phases during the morel life cycle, and the number of sclerotia indicates the spawn quality during morel cultivation. However, the sclerotial formation and differentiation mechanisms are poorly understood. In the present study, the effects of environmental conditions including temperature, light, and pH on the sclerotial formation of M. importuna were evaluated, and the ROS effects and expression of sod genes during sclerotial formation were studied in different locations. The results indicated that ROS accumulation in the mycelial growth region was higher than that in the sclerotium-forming region. Additionally, sod gene expression increased with the development and maturation of sclerotia. Moreover, sclerotial formation was induced by the difference in H2O2 concentration. The MAPK signaling pathway appears to play an important role in the process of sclerotial formation.
Sclerotial formation is affected by several factors, including temperature, pH, and light in M. importuna. The sclerotia can form at 15–25 °C on PDA medium with an optimal temperature of 20 °C and an optimal pH between 5 and 8, and the lighting conditions had no significant effect. Similar results have been reported in another species of Morchella sp. (Liu et al. 2004). Sclerotium of M. importuna matured and its weight became stable when the strain was cultured on PDA medium for 7 days under the optimized conditions (temperature of 20 °C, initial pH of 5–8). However, sclerotium maturation will be delayed under the unsuitable conditions, such as low temperature or high pH. Different growth periods were used in the different experiments just to ensure the maturation of sclerotium and the stable of weight under the unsuitable conditions (Fig. 1).
In the tested M. importuna strain, distinct boundaries appeared between the formation of mycelia and sclerotia in time and location (Fig. 2), which was convenient for observing the sclerotial formation process.
The growth and sclerotial formation of M. importuan can be divided into five phases: HE, HG, SI, SD, and SM. When cultured on plates, the influence of oxidative stress on the hyphae in the outermost region was higher than on the hyphae at the center. Morphological differences between the sclerotium-forming (inside) and hyphae (outside) regions appeared during the SI, SD, and SM phases; the cells in the sclerotium-forming (inside) area were more swollen than on the outside region of the Petri dish, where no sclerotia formed. Comparing the different stages, the cells of mycelia in the sclerotium-forming (inside) area became more swollen, darker, and denser from the SI to SM stages. The cells in different regions of the plates emerged as sclerotial structures or aging mycelia as a result of the environment stimuli, which were diverse at different locations.
Several studies on various fungi have shown a correlation between the differentiation/non-differentiation process and the upregulation of specific antioxidant enzymes. A sod gene is induced during sporulation in Colletotrichum graminicola (Fang et al. 2002) and the biogenesis of microsclerotia in the slime mold Physarum polycephalum (Allen 1991; Aguirre et al. 2005). The six sod genes tested in this work were expressed during the whole sclerotial formation, and their expressions in both the inside and outside sections increased in the SM stage compared with the HE stage. At the SM stage, the expression levels of most of the sod genes in the inside section were much higher than that of the outside cells (Fig. 3a, b). The expression of the sod genes varied according to location, indicating a difference in ROS concentration in the different locations during sclerotial formation.
The ROS staining assay by DAB confirmed that the concentration of H2O2 on the outside region was higher than at the inside at the SD and SM stages (Fig. 4), indicating that ROS, mainly H2O2, accumulated in the region of no sclerotial formation. High H2O2 production rates are correlated with high oxidative stress (Georgiou and Sideri 2000). The direct indicator of oxidative stress, H2O2, has been reported to be involved in the sclerotial differentiation of S. rolfsii, S. minor, S. sclerotiorum, and R. solani throughout their developmental stages (Papapostolou and Georgiou 2010a). Increased SOD activity induced by the oxidative burst resulted in an increase of the H2O2 content in M. steppicola (Király and Czövek 2007).
The low concentration of H2O2 (5 mM) increased sclerotial formation significantly, but concentrations of greater than 10 mM resulted in the reduction of sclerotium yield and the delay of sclerotial initiation (Fig. 5b and Supplemental Fig. S4b). This indicated that high concentrations of H2O2 greater than 10 mM cannot promote the cells to enter the SI stage.
Sclerotial formation in M. importuna could not initiate until mycelia covered the full Petri dishes regardless of the size of the Petri dish (Supplemental Fig. S1). The factors triggering the sclerotial initiation appeared to accumulate in the hyphal growth stage when the Petri dish was filled with mycelium. However, in presence of a difference in H2O2 concentration, sclerotial formation was initiated when the mycelia had not yet filled the dishes (Fig. 5c). This suggests that the difference in H2O2 concentration can trigger sclerotial formation in M. importuna. Moreover, our results showed that the expressions of the sod genes were higher in the group with the difference in H2O2 concentration than in the control without (Fig. 6). This confirmed that an increased H2O2 concentration difference can increase the expression of sod genes and promote the development and maturation of sclerotia in M. importuna.
The tested M. importuna strain only formed sclerotia at the center of the Petri dishes, while the outside hyphae aged gradually and could not form sclerotia (Fig. 2), suggesting that a type of signaling between the two areas with different ROS concentrations. A conserved family of fungal MAPKs (Saccharomyces cerevisiae Hog1, Schizosaccharomyces pombe Spc1) is activated by many stress signals, including oxidative stress (Aguirre et al. 2005). MAP kinases in Candida albicans and Magnaporthe grisea have been shown to control differentiation (Sudbery 2011; Xu 2000). Other studies on Botrytis cinerea demonstrated by gene knock-out that the sclerotial formation process is controlled by the MAPK pathway (Segmüller et al. 2007). In the present study, the MAPK signaling pathways were activated, leading to cellular morphological changes, sclerotial formation in the areas of low ROS stress, and naturally aging hyphal cells in the areas of high ROS stress.
The outside cells under high oxidative stress in M. importuna cannot form sclerotia directly but rather produce signals which are passed onto cells under low oxidative stress, consequently promoting the formation of sclerotia. This may be regarded as quorum sensing throughout the whole colony specific to environmental factors, an adaptation process to environmental stimuli (Fig. 8). Other ROS except H2O2 may also play a role, which is the subject of our ongoing project. Further understanding of the sclerotial formation mechanisms in M. importuna may help to understand the life cycle and facilitate the fruiting body cultivation of this species.
Model of ROS triggering sclerotial formation in M. importuna. The cells in the high ROS stress (HRS) area gradually aged, and the cells in the low ROS stress (LRS) area formed sclerotia. A high ROS level was caused by the oxygen supply and fed through multiple upstream metabolic pathways, for example, after ROS generation from oxygen by NADPH oxidases (NOX) and scavenging by antioxidant enzymes such as SODs. H2O2 accumulated in the medium feedback to stimulate the cells growing in the HRS area. The cells stimulated by the ROS can release or pass on an unknown factor as a signal to activate the MAPK signaling pathway in the cells located in the LRS area to form sclerotia. Then, the downstream transcription factors were activated, regulating the expression of genes involved in sclerotial formation. Dotted arrows represent the approximate pathway, and solid arrows represent the pathway reported in other papers
References
Allen RG (1991) Oxygen-reactive species and antioxidant responses during development: the metabolic paradox of cellular differentiation. Proc Soc Exp Biol Med 196(2):117–129. https://doi.org/10.3181/00379727-196-43171A
Aguirre J, Ríos-Momberg M, Hewitt D, Hansberg W (2005) Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol 13(3):111–118. https://doi.org/10.1016/j.tim.2005.01.007
Cargnello M, Roux PP (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 75(1):50–83. https://doi.org/10.1128/MMBR.00031-10
Fang GC, Hanau RM, Vaillancourt LJ (2002) The sod2 gene, encoding a manganese-type superoxide dismutase, is up-regulated during conidiogenesis in the plant-pathogenic fungus Colletotrichum graminicola. Fungal Genet Biol 36(2):155–165. https://doi.org/10.1016/S1087-1845(02)00008-7
He PX, Liu W, Cai YL, Ma BJ, Chen LT, Wu XR (2014) Effects of oxidative stress on mycelial growth and sclerotial metamorphosis of Morchella crassipes. Hubei Agric Sci 53(13):3085–3089. https://doi.org/10.14088/j.cnki.issn0439-8114.2014.13.091
Liu XR, Chen FC, Tan FH, Lin Q (2004) A study of the sclerotium producing conditions of Morchella sp. J Sichuan For Sci Technol 25(3):44–47. https://doi.org/10.16779/j.cnki.1003-5508.2004.03.010
Liu QZ, Ma HS, Zhang Y, Dong CH (2017) Artificial cultivation of true morels: current state, issues and perspectives. Crit Rev Biotechnol 38(2):259–271. https://doi.org/10.1080/07388551.2017.1333082
Georgiou CD (1997) Lipid peroxidation in Sclerotium rolfsii: a new look into the mechanism of sclerotial biogenesis in fungi. Mycol Res 101:460–464. https://doi.org/10.1017/S0953756296002882
Georgiou CD, Sideri M (2000) Colorimetric method for determining hydrogen peroxide production in liquid media by filamentous fungi. Mycologia 92(5):835–840. https://doi.org/10.2307/3761578
Georgiou CD, Patsoukis N, Papapostolou L, Zervoudakis G (2006) Sclerotial metamorphosis in filamentous fungi is induced by oxidative stress. Integr Comp Biol 46(6):691–712. https://doi.org/10.1093/icb/icj034
Király I, Czövek P (2007) Oxidative burst induced pseudosclerotium formation of Morchella steppicola Zerova on different malt agar media. Can J Microbiol 53(8):975–982. https://doi.org/10.1139/W07-055
Papapostolou I, Georgiou CD (2010a) Hydrogen peroxide is involved in the sclerotial differentiation of filamentous phytopathogenic fungi. J Appl Microbiol 109(6):1929–1936. https://doi.org/10.1111/j.1365-2672.2010.04822.x
Papapostolou I, Georgiou CD (2010b) Superoxide radical is involved in the sclerotial differentiation of filamentous phytopathogenic fungi: identification of a fungal xanthine oxidase. Fungal Biol 114(5–6):387–395. https://doi.org/10.1016/j.funbio.2010.01.010
Papapostolou I, Georgiou CD (2010c) Superoxide radical induces sclerotial differentiation in filamentous phytopathogenic fungi: a superoxide dismutase mimetics study. Microbiology 156(Pt 3):960–966. https://doi.org/10.1099/mic.0.034579-0
Sudbery PE (2011) Growth of Candida albicans hyphae. Nat Rev Microbiol 9(10):737–748. https://doi.org/10.1038/nrmicro2636
Segmüller N, Ellendorf U, Tudzynski B, Tudzynski P (2007) Bcsak1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot Cell 6(2):211–221. https://doi.org/10.1128/EC.00153-06
Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller AF, Teixeira M, Valentine JS (2014) Superoxide dismutases and superoxide reductases. Chem Rev 114(7):3854–3918. https://doi.org/10.1021/cr4005296
Smith ME, Henkel TW, Rollins JA (2015) How many fungi make sclerotia? Fungal Ecol 13:211–220. https://doi.org/10.1016/j.funeco.2014.08.010
Tomoyuki O, Pyoyun P, Kenichi I (2017) Cytological analysis of the effect of reactive oxygen species on sclerotia formation in Sclerotinia minor. Fungal Biol 121(2):127–136. https://doi.org/10.1016/j.funbio.2016.11.002
Volk TJ, Leonard TJ (1989) Experimental studies on the morel. I. Heterokaryon formation between monoascosporous strains of Morchella. Mycologia 81(4):523–531. https://doi.org/10.2307/3760127
Volk TJ, Leonard TJ (1990) Cytology of the life-cycle of Morchella. Mycol Res 94(3):399–406. https://doi.org/10.1016/S0953-7562(09)80365-1
Xu JR (2000) Map kinases in fungal pathogens. Fungal Genet Biol 31(3):137–152. https://doi.org/10.1006/fgbi.2000.1237
Funding
This study was funded by the Coal-based Key Scientific and Technological Project from Shanxi Province (FT2014-03-01) and Key Research and Development Program from Guangxi Province (2016AB05317).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 490 kb)
Rights and permissions
About this article
Cite this article
Liu, Q., Zhao, Z., Dong, H. et al. Reactive oxygen species induce sclerotial formation in Morchella importuna. Appl Microbiol Biotechnol 102, 7997–8009 (2018). https://doi.org/10.1007/s00253-018-9104-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9104-4