Abstract
Avian influenza virus (AIV) can infect poultry, mammals, and other hosts and causes enormous economic losses to the global poultry industry. In this study, to develop a novel and potent oral vaccine based on Lactobacillus plantarum (L. plantarum) for controlling the spread of AIV in the poultry industry, we constructed a recombinant L. plantarum strain displaying the 3M2e-HA2 protein of the influenza virus and determined the effect of N/pgsA′-3M2e-HA2 against AIV in chicks. We first confirmed that the 3M2e-HA2 fusion protein was expressed on the surface of L. plantarum via flow cytometry and immunofluorescence experiments. Our experimental results demonstrated that chicks immunized with N/pgsA′-3M2e-HA2 could induce specific humoral, mucosal, and T cell-mediated immune responses, eliciting the host body to protect itself against AIV. Additionally, compared to oral administration, the intranasal immunization of chicks with N/pgsA′-3M2e-HA2 provided a stronger immune response, resulting in a potent protective effect that hindered the loss of body weight, decreasing pulmonary virus titers and reducing lung and throat pathological damages. Thus, our results indicate that our novel approach is an effective method of vaccine design to promote mucosal immunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Avian influenza (AI) is an acute and highly infectious disease that is widespread worldwide (Barriga et al. 2016). In the past decades, avian influenza virus (AIV) has become an important public health issue that not only causes significant economic losses to the poultry industry in China but also threatens human health (Bi et al. 2015; Pan et al. 2016). The glycoprotein covering AIV is classified into 18 hemagglutinin (HA) subtypes and 11 neuraminidase (NA) subtypes (Wu et al. 2014). At present, vaccination is still the most effective strategy to combat AI (Li et al. 2014). Nevertheless, AI prevention and control have become increasingly difficult because the commercialized inactivated vaccine can only generate antibodies to neutralize specific strains of AIV (Lee et al. 2016; Pu et al. 2015). Thus, cross-protection of various subtypes of AIV cannot be achieved. Therefore, it is of great importance that intensive research be performed to develop a universal vaccine that provides widespread protection to combat various subtypes of AIV.
Matrix protein (M) is encoded in the seventh ribonucleic acid (RNA) segment of the AIV genome, constituting the largest portion of the AIV genome, and includes M1, M2, and M42 (Matusevich et al. 2015). M2 is a 97 amino acid transmembrane matrix protein that is present on the surface of infected cells in the form of tetramers. The N-terminus consists of 23 M2 extracellular domains (M2e), which is made up of conserved amino acids (Pendzialek et al. 2017). M2 has not obviously changed from the 1918 (Spanish flu), 1957 (Asian flu), 1968 (Hong Kong flu), and 2009 (swine origin flu) pandemics and is widely spread throughout the world (Davis et al. 2015; Rappazzo et al. 2016). Currently, despite the low immunogenicity of the M2e polypeptide, it can mediate a heterogeneous subtype immune response. Thus, it plays an important role in studies of a universal vaccine against the influenza virus (Dabaghian et al. 2015; Tao et al. 2017; Yang et al. 2017c). It has been observed that the resistance against AI via M2e primarily depends on the M2e antibody and the M2e protein, which combine and are present on surfaces of cells in infected hosts (Dabaghian et al. 2014). The complement system is a major component of innate immunity that interacts to sense and respond to invading viruses. In addition, a vaccine based on M2e can induce the reaction of cytotoxic lymphocytes (CTLs) (Pendzialek et al. 2017). HA penetrates the receptor cell, and molecules, such as sialic acid of the host cells, and mediate virus adsorption and infection so that it can escape the immune surveillance of the host. The hemagglutinin polypeptide 2 (HA2) sequences encoding the C-terminus of the HA protein in various AIV subtypes are highly conserved (Yang et al. 2017a). HA2 consists of a stem structure that anchors inside the virus membrane, carrying out the function of causing the conjunction between virus membrane and cell membrane, as well as the release of the virus particle nucleocapsid (Schneemann et al. 2012). It has been shown in many studies that the antibody cross-reacting to HA2 can functionally enhance vaccines, despite the fact that its structure is not a neutralizing antibody of HA (Krammer 2016; Mallajosyula et al. 2014).
Some strains of some species of Lactic acid bacteria (LAB) are probiotics that can reside in the intestines and are widespread in nature (Mao et al. 2016). LAB can carry out probiotic functions, such as maintaining the strains of bacteria present in the intestines, adhering to deleterious substances, degrading nutrients, increasing the utilization rate of nutrients, and adjusting the immune function of organisms (Arena et al. 2017; Kaur et al. 2017; Riaz Rajoka et al. 2017). LAB are able to regulate the immune cells as well as improve the deglutition of phagocytes so that the cellular immunity level of an organism can be increased (van Baarlen et al. 2009). During the last two decades, a number of reports on LAB as foreign carriers to resist pathogenic infections have indicated that L. plantarum has an important role in LAB-related candidate vaccines (Kechaou et al. 2013; Sorvig et al. 2005). Recently, several studies showed that L. plantarum performs well as alive vector. For instance, L. plantarum has been used to express a CC chemokine ligand 3 (CCL3) in combination with human immunodeficiency virus type 1 (HIV-1) Gag-derived antigen (Kuczkowska et al. 2015), structural protein (VP2) gene of goose parvovirus (Liu et al. 2017), hemagglutinin of AIVs (Shi et al. 2016), Mycobacterium tuberculosis antigens (Kuczkowska et al. 2017), the G antigen of spring viremia of carp virus (SVCV) fused with an open reading frame (ORF) antigen (ORF81) of koi herpesvirus (KHV) (Cui et al. 2015), and the S1 antigen of porcine epidemic diarrhea virus fused with Lactobacillus-expressed nucleocapsid (N) protein (Liu et al. 2012). However, the duration of immunological protection can extend in many instances because there are no contraindications to the use of these vaccines; an additional booster can be given later. Moreover, the duration of immunological protection can be extended in the future studies.
In our previous study, we used recombinant L. plantarum expressing HA2 antigens to vaccinate chicks, which was shown to induce an immune response against AIV infection (Yang et al. 2017d). In this study, to further improve HA2 immunogenicity, we used L. plantarum to express the 3M2e and HA2 fusion protein and evaluated the immune response and protective efficacy of the recombinant 3M2e-HA2 fusion protein in chickens.
Materials and methods
Viruses, purified antigens, and vaccine
The A/Anthropoidesvirgo/Baicheng/219/2013(H9N2) (GenBank accession numbers KM245331-KM245338) and A/duck/Xuzhou/07/2003(H9N2) virus strains were provided by Jilin Agricultural University. Purified M2 and HA2 antigens from Escherichia coli (DE3) were provided by Liang Zhao. The H9N2-inactivated vaccine was commercially purchased (Weike Biotechnology).
Construction of recombinant L. plantarum
To construct the pSIP-409-pgsA′-3M2e-HA2 plasmid (Fig. 1a), the 3M2e (GenBank accession number LC120394) of A/Puerto Rico/8/1934(H1N1) and HA2 (GenBank accession number MF620131) of A/Anthropoidesvirgo/Baicheng/219/2013(H9N2) were synthesized by Shanghai Generay Biotech Co., Ltd. (Shanghai, China), and the plasmid was named pGH-3M2e-HA2. The fusion gene 3M2e-HA2 was then cloned into the pSIP-409-pgsA′ vector by digestion with the restriction enzymes Xba I and Hind III to construct the pSIP-409-pgsA′-3M2e-HA2 plasmid. The positive plasmid was transformed into L. plantarum strain NC8 (CCUG 61730) and identified and sequenced by Shanghai Generay Biotech Co., Ltd. (Shanghai, China), after which the positive strain was named N/pgsA′-3M2e-HA2.
Plasmid generation and protein expression. a The pSIP409-pgsA′-3M2e-HA2 plasmid was constructed using methods described in a previously published protocol. b Western blot analyses of 3M2e-HA2 expression. Lane 1: N/pgsA′; Lane 2 and 3: N/pgsA′-3M2e-HA2. c Flow cytometry assay (left picture represented Anti-HA Ab, right picture represented anti-M2 Ab). d Immunofluorescence detection was performed to detect the 3M2e-HA2 fusion protein expressed on the surface of L. plantarum
Western blotting
The fusion protein 3M2e-HA2 from recombinant L. plantarum was synthesized and identified using our previously described methods (Yang et al. 2016). The 3M2e-HA2 antigens were separated by SDS-PAGE (12% acrylamide) and transferred to nylon membranes. The 3M2e-HA2 protein was identified using an anti-M2 protein monoclonal antibody (Abcam 14C2, USA) and a polyclonal mouse anti-HA antibody. The samples were then incubated with affinity-purified horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody (Sigma, USA). Subsequently, the samples were developed using a chemiluminescence (ECL) Plus detection kit (Thermo Scientific, USA).
Flow cytometry assay and immunofluorescence detection
The 3M2e-HA2 fusion protein displayed on the surface of N/pgsA′-3M2e-HA2 was detected by flow cytometry analysis and an immunofluorescence assay. Briefly, 106 cells of recombinant bacteria were stained with monoclonal mouse anti-M2 or anti-HA antibodies at 4 °C for 1 h. Next, cells were stained with FITC-conjugated goat anti-mouse IgG at 4 °C for 1 h. After washing with PBST, recombinant bacteria cells were assessed using flow cytometry (BD LSRFortessa™, USA) and a fluorescence microscope (Leica Microsystems, German).
Experimental animals
Specific pathogen-free (SPF) white leghorn layer chickens (3 weeks of age) were obtained from the Beijing Merial Vital Laboratory Animal Technology Co., Ltd., China. All animal experiments were approved and supervised by the Animal Care and Ethics Committees of Jilin Agriculture University.
Chicken vaccination and challenge
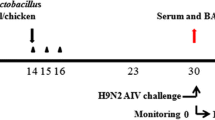
N/pgsA′-3M2e-HA2 was cultured as previously described (Shi et al. 2014). The birds were randomly divided into 5 groups, each containing 20 birds, as follows: PBS-challenge group, oral N/pgsA′ group, oral N/pgsA′-3M2e-HA2 group, intranasal N/pgsA′-3M2e-HA2, and positive group (AIV-inactivated vaccine). Chickens from three groups were orally gavaged with PBS (300 μl), N/pgsA′ (2 × 109 CFU/300 μl), and N/pgsA′-3M2e-HA2 (2 × 109 CFU/300 μl), and one group was intranasally vaccinated with N/pgsA′-3M2e-HA2 (2 × 109 CFU/300 μl) at 1, 2, and 3 days. The booster vaccinations of chickens were performed at 15, 16, and 17 days after the primer dosing. One group was intramuscularly injected with a commercial vaccine (200 μl/chicken). All groups of birds were challenged intranasally with 106.5 EID50 of A/Anthropoidesvirgo/Baicheng/219/2013(H9N2) and A/duck/Xuzhou/07/2003(H9N2) as previously described (Yang et al. 2017b) 14 days after immunization. The weights and survival rates of chickens were monitored for 10 days after challenge. For assessing virus titers in the lungs, the samples were obtained from the lungs of chickens after the AIV challenge, and the virus was titrated in SPF embryonated chicken eggs.
Flow cytometry
Single-cell suspensions from spleens were prepared, and flow cytometry was performed using a published protocol (Shi et al. 2016). In brief, spleens were excised with scissors and filtered through a 70-μm nylon membrane (BD Biosciences). Next, the erythrocytes were removed using Ficoll prior to density centrifugation (1000×g for 20 min at 4 °C with low acceleration and no brake). After washing with FACS buffer (D-PBS, 2%FBS, and 0.1% sodium azide), 1 × 106 of splenic single-cell suspensions were incubated with monoclonal anti-chicken CD4 (clone CT4), anti-chicken CD8 (clone CT8), and anti-chicken CD3 (clone CT3) antibodies. Finally, the samples were detected using a flow cytometry (BD LSRFortessa™, USA), and the data was analyzed using FlowJo 7.6.1 software.
T cell stimulatory index
Splenic single-cell suspensions were prepared as described previously in the “Methods” section. To perform the proliferation assay, 2 × 105 splenic cells were seeded in 96-well plates with 4 μg/mL M2 and HA proteins (final concentration) at 37 °C, 5% CO2 for 72 h. Four hours before the end of the incubation, 20 μL of (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS) was plated to each well, and the OD values were determined at 570 nm and performed to calculate the stimulation index (SI).
Hemagglutination inhibition titer
To test recombinant N/pgsA-3M2e-HA2-specific neutralizing antibodies, a previously published hemagglutination inhibition (HI) detection assay was used (Shi et al. 2016).
Enzyme-linked immunosorbent assay
The presence of antigens that specifically bound to IgG and IgA antibodies in serum and bronchoalveolar lavage fluid (BALF) was assessed by Enzyme-linked immunosorbent assay (ELISA) as described previously, with some minor alterations (Shi et al. 2014). The wells were coated with M2 and HA2 (5 μg/well, respectively) and incubated at 4 °C overnight. After three washes, 150 μL of PBS containing 1% BSA was plated to each sample well and the plate was treated overnight at 4 °C. Afterwards, the plates were washed as described above. Using 100 μL/well of serum samples diluted from 1/2 to 1/1024 with PBST, the plate was then statically incubated at 37 °C for 1.5 h after washing, which was performed three times for 3 min each time. Subsequently, the plates were incubated for an additional 1.5 h at 37 °C with goat anti-chicken IgG antibodies conjugated with HRP (1:5000) (Invitrogen) that was added to each well. To develop the reaction, 100 μL of 3,3-,5,5-tetramethylbenzidine (TMB) was then plated each well. The absorbance was recorded at 492 nm. For samples in which the absorbance was twofold higher than the background, the endpoint titers were regarded as producing a positive result.
Histopathology
The lungs and throats of vaccinated chickens were obtained 5 days after AIV infection. All samples were fixed with 4% paraformaldehyde in the subsequent steps. Sections with a thickness of 5 μm were stained with hematoxylin and eosin for examining pathological changes.
Statistical analysis
All statistical analyses in this study were carried out using GraphPad Prism version 5. The data is represented as the means ± standard error of the mean (SEM). Two-tailed t tests and ANOVA were performed for the statistical analyses, and P < 0.05 was considered significant.
Results
Synthesis of 3M2e-HA2 on L. plantarum
A recombinant pSIP409-pgsA′-3M2e-HA2 plasmid expressing 3M2e-HA2 from the influenza virus was generated (Fig. 1a). The fusion proteins were synthesized on L. plantarum and assessed by western blotting using anti-HA or anti-M2 antibodies. As shown in Fig. 1b, a positive band was observed in N/pgsA′-3M2e-HA2 cells using special antibodies, but a band was not observed in the N/pgsA′ control cells. To determine if the 3M2e-HA2 fusion was expressed on the surface of recombinant L. plantarum, the 3M2e-HA2 fusion antigen displayed on the surface of recombinant L. plantarum was detected by flow cytometry analysis and an immunofluorescence assay. The results showed that N/pgsA′-3M2e-HA2 cells produced a positive fluorescence signal compared to the negative control cells (N/pgsA′) (Fig. 1c, d). These results demonstrated that the 3M2e-HA2 fusion protein was expressed on the surface of recombinant L. plantarum.
N/pgsA′-3M2e-HA2 induced HI titers in the sera of vaccinated chicks
To detect the specific HI titers in birds after vaccination with N/pgsA′-3M2e-HA2, the antibody titers were detected using an HI assay 2 weeks after the final vaccination. As shown in Fig. 2, a higher HI titer from experimental birds that had been orally immunized with N/pgsA′-3M2e-HA2 was observed compared to the PBS and N/pgsA′ groups (P < 0.001). The results showed a notable difference between the inactivated H9N2 and the N/pgsA′-3M2e-HA2 orally vaccinated groups (P < 0.01) (Fig. 2). Interestingly, a remarkably higher HI titer was observed in the N/pgsA′-3M2e-HA2 intranasally immunized chickens compared to the orally immunized group (P < 0.05) (Fig. 2).
The HI titers elicited by N/pgsA′-3M2e-HA2 2 weeks after booster vaccination neutralize the H9N2 subtype of avian influenza (A/Anthropoidesvirgo/Baicheng/219/2013). Serum obtained from chicks in all groups was investigated by an HI assay. The data are shown as the means ± S.E.M (n = 3) and were tested using a one-way ANOVA (*P < 0.05; **P < 0.01; ***P < 0.001)
N/pgsA′-3M2e-HA2 induced specific antibody level of IgG in serum after immunization
To determine whether vaccination of recombination N/pgsA′-3M2e-HA2 affects the specific level of IgG antibodies in the serum of chicks after immunization, all groups were tested after the booster vaccination at week 2. The results showed that the N/gsA′-3M2e-HA2 orally vaccinated group had higher IgG levels than the PBS and N/pgsA′ groups (P < 0.001). However, chicks that were intranasally vaccinated with N/pgsA′-3M2e-HA2 had no significant difference of IgG than the orally vaccinated group (P > 0.05) (Fig. 3).
The specific production of IgG antibodies in serum elicited by recombinant vaccines after immunization. Chicks were booster immunized with N/pgsA′-3M2e-HA2, and serum samples were obtained after 14 days. The results are displayed as the means ± S.E.M (n = 3) and were tested using a one-way ANOVA (***P < 0.001)
N/pgsA′-3M2e-HA2 induced specific sIgA production
To further assess the effect of vaccination of N/pgsA′-3M2e-HA2 on the specific sIgA secretion in chicks, we used ELISA to detect the sIgA titer of BALF in each group of chicks 2 weeks after the booster vaccination. Our data demonstrated that the vaccination of N/pgsA′-3M2e-HA2 resulted in a significantly higher sIgA titer than the other control groups (P < 0.001) (Fig. 4). As expected, the BALF of chicks that were intranasally vaccinated with N/pgsA′-3M2e-HA2 was notably higher than that of the oral immunization group (P < 0.05) (Fig. 4).
The specific production of sIgA antibodies in bronchoalveolar lavage fluids (BALF) stimulated by N/pgsA′-3M2e-HA2. BALF was taken from all chicks that were booster vaccinated with N/pgsA′-3M2e-HA2 after 2 weeks. The results are shown as the means ± S.E.M (n = 3) and were tested using a one-way ANOVA (*P < 0.05; ***P < 0.001)
N/pgsA′-3M2e-HA2 improved the T cell responses in chickens after immunization
To explore the function of N/pgsA′-3M2e-HA2 on lymphocyte proliferation by oral or intranasal administration, lymphocyte proliferation was assessed in chicks after booster vaccinations at week 2. As the data shows, the oral N/pgsA′-3M2e-HA2 group had a higher level of lymphocyte proliferation than the PBS and N/pgsA′ (P < 0.001) groups, but intranasal immunization with N/pgsA′-3M2e-HA2 resulted in a higher level than oral group (P < 0.05) (Fig. 5).
To further investigate N/pgsA′-3M2e-HA2-triggered T cell responses in the spleens of vaccinated chicks, a flow cytometry analysis was utilized to detect the frequencies of T cell subsets (Fig. 6a). A higher frequency of CD3+CD4+ T cells were observed in the oral vaccination N/pgsA′-3M2e-HA2 group compared to the N/pgsA′ (P < 0.05) and PBS (P < 0.001) groups. Intranasal vaccination with N/pgsA′-3M2e-HA2 recombinant bacteria was not different from oral administration, and the positive group (inactivated vaccine) showed the highest percentages of CD3+CD4+ T cells in all groups (Fig. 6b). In addition, the data indicated birds that were orally immunized with N/pgsA′-3M2e-HA2 had enhanced percentages of CD3+CD8+ T cells compared with the PBS groups (P < 0.001) and N/pgsA′ (P < 0.05), but there were higher percentages of CD3+CD8+ T cells from chickens intranasally vaccinated with N/pgsA′-3M2e-HA2 than was observed in orally administered birds (P < 0.05) (Fig. 6c).
The T cell-mediated immune responses elicited by recombinant N/pgsA′-3M2e-HA2. a Gating strategy for T cells subset. b CD3+CD4+ T cells and c CD3+CD8+ T cells from chickens were tested 14 days after booster immunization. The data is shown as the means ± S.E.M (n = 3) and was analyzed using a one-way ANOVA (*P < 0.05; **P < 0.01; ***P < 0.001)
N/pgsA′-3M2e-HA2 induced protection for the chicks
To determine whether N/pgsA′-3M2e-HA2-elicited immune responses could provide protection to an AIV challenge, groups of experimental birds were booster vaccinated with N/pgsA′-3M2e-HA2 and challenged 14 days later with A/Anthropoidesvirgo/Baicheng/219/2013(H9N2) and changes in the body weight of chicks were observed and recorded. The results showed that the weight loss of chicks in the PBS and N/pgsA′ groups-exhibited a certain level of decline after being challenged 1–7 days later (Fig. 7a). In addition, a lower weight loss in the N/pgsA′-3M2e-HA2 oral administration group was observed compared to the PBS (P < 0.001) and N/pgsA′ group (P < 0.001) but was higher than the positive group (inactivated vaccine) (P < 0.05) at 6 and 7 days post-infection (Fig. 7b). As expected, the weight loss of chicks in the N/pgsA′-3M2e-HA2 intranasal administration group was notably lower than the group that was oral administered N/pgsA′-3M2e-HA2 at 6 and 7 days post-infection (P < 0.05) (Fig. 7b). Furthermore, lower lung virus titers were observed in birds that were orally immunized with the N/pgsA′-3M2e-HA2 and H9N2 inactivated vaccine compared with the N/pgsA′ and PBS groups (P < 0.001) (Fig. 7e). Importantly, birds in the intranasally administered N/pgsA′-3M2e-HA2 group showed lower lung virus titers than oral administration (Fig. 7e). Consistent with these findings, the degree of lung and throat damage after infection with AIV was alleviated in the experimental birds orally vaccinated with N/pgsA′-3M2e-HA2 compared with that detected in the birds vaccinated with N/pgsA′ or PBS (Figs. 8 and 9). Furthermore, our data showed that the degree of lung and throat damage was reduced in the birds intranasally administered N/pgsA′-3M2e-HA2 compared with oral administration.
Protective effect provided by N/pgsA′-3M2e-HA2. Fourteen days after finally immunization, birds were challenged with A/Anthropoidesvirgo/Baicheng/219/2013 (106.0 EID50) or A/duck/Xuzhou/07/2003(H9N2) (106.0 EID50). a–d The weight loss of animals was monitored after infection. e, f Five days after infection, the virus titers in lung tissue of chickens were determined in MDCK cells. The data were expressed as the means ± S.E.M (n = 3) and analyzed using a one-way ANOVA (*P < 0.05; **P < 0.01; ***P < 0.001)
The pathological damage in lungs or animals was reduced by recombinant N/pgsA′-3M2e-HA2 after challenge with A/Anthropoides virgo/Baicheng/219/2013 (106.0 EID50). Three days after infection with AIVs, the samples were taken and assessed by histopathological observations. Sample sections were made using hematoxylin-eosin (H&E) staining; scale bar,10 μm (magnification at × 100)
The pathological damage in throats of animals was reduced by recombinant N/pgsA′-3M2e-HA2 after challenge with A/Anthropoides virgo/Baicheng/219/2013 (106.0 EID50). Three days after infection with AIVs, the samples were taken and assessed by histopathological observations. Sample sections were made using hematoxylin-eosin (H&E) staining; scale bar 10 μm (magnification at × 100)
Next, all groups of chicken were challenged with A/duck/Xuzhou/07/2003(H9N2) (92% amino acid similarity of HA with A/Anthropoidesvirgo/Baicheng/219/2013) after final immunization for 2 weeks. The results revealed that the weight loss of chicks in the PBS-treated control group and the N/pgsA′ group exhibited a remarkable decline after challenge, while the orally and intranasally administered N/pgsA′-3M2e-HA2 groups and the positive group (H9N2 inactivated vaccine) had no change after infection (Fig. 7c). In addition, a lower weight loss was observed in the group that was orally administered N/pgsA′-3M2e-HA2 compared to the PBS (P < 0.001) and N/pgsA′ groups (P < 0.001) but was higher than the inactivated vaccine group (P < 0.05) at 7 and 8 days post-infection (Fig. 7d). As expected, the weight loss of chicks in the N/pgsA′-3M2e-HA2 intranasal administration group was significantly lower than in the N/pgsA′-3M2e-HA2 oral administration group at 7 and 8 days post-infection (P < 0.05) (Fig. 7d). Furthermore, our data showed that significantly lower virus titers were present in lung homogenates of chicks that were orally administered N/pgsA′-3M2e-HA2, compared with the pulmonary virus titers in chicks of the PBS-treated control group and the N/pgsA′ group (P < 0.001) (Fig. 7f). Despite the lower virus titers detected in the inactivated vaccine group compared with group that was orally administered N/pgsA′-3M2e-HA2 (P < 0.001), the pulmonary virus titers of chicks that were intranasally vaccinated with N/pgsA′-3M2e-HA2 was significantly lower than that of the orally immunized group (P < 0.05) (Fig. 7f).
Discussion
The use of L. plantarum strains as live delivery vectors has gained increased attention over the last several years as an option for vaccination. Such an approach is particularly attractive to prevent the massive spread of disease in animals, such as avian influenza. The administration of antigens expressed by L. plantarum to the gut mucosa via the oral route has been reported to be effectual in activating both local and systemic immune responses (Liu et al. 2017). It is hypothesized that mucosally administered vaccines via oral or nasal routes require adjuvants to draw out specific protective responses. With respect to the need for this aspect of vaccines to be improved, L. plantarum strains are an attractive antigen-expressing vector, as they have been revealed to have inherent adjuvant properties (Cai et al. 2016; Liu et al. 2017; Shi et al. 2014; Yao et al. 2016). Specifically, for the L. plantarum strain NC8, our recent studies demonstrated its potential to induce an immune response by the induction of cytokines and the maturation of dendritic cells in chickens and mice (Yang et al. 2016; Yang et al. 2017b).
In this study, we assessed the effectiveness of the expression of 3M2e-HA2 from the influenza virus. In our previous work, we showed that engineering an L. plantarum NC8 strain to generate protein of the H9N2 avian influenza virus in chickens is a promising approach (Shi et al. 2016). In this study, we further developed this approach. To reduce the potential protein toxicity, the expression of 3M2e-HA2 was carried out using a prokaryotic expression system, because its regulation allows for stable expression of proteins of various functions and origin (Sorvig et al. 2005). Western blot assays revealed the presence of a 37 kDa immunospecific band that corresponded to 3M2e-HA2. This size is most likely related to the protein produced in other studies. Flow cytometry and immunofluorescence analyses were conducted and confirmed the expression of this protein on the surface of L. plantarum. The obtained data confirmed the construction of the 3M2e-HA2 protein of the avian influenza virus H9N2 in the L. plantarum strain. The immunogenicity of the recombinant antigen was assessed using chickens as an animal model. Using ELISA assays, we observed the specific serum IgG and sIgA titers of BALF between individual chickens that were nasally or orally immunized with N/pgsA′-3M2e-HA2. We observed notably high IgG and sIgA antibody titers in intranasally inoculated animals compared to orally inoculated animals. These observations resemble the results of those who reported that after inoculation of recombinant influenza virus, IgG and sIgA antibody levels were induced by recombinant LAB (Li et al. 2015).
In addition, we observed an increase in the infection rate after a challenge infection with an antigen by nasal oral administration of N/pgsA′-3M2e-HA2. The most pronounced protection was observed for chickens that were intranasally immunized compared to orally immunized chickens, as the degree of lung and throat damage was reduced in the former chickens compared to the latter ones (Figs. 7, 8, and 9). Intranasal immunizations revealed a difference in the response levels compared to oral administration, which was likely due to the immune response after oral administration of an antigen being weaker than that of intranasal immunization. Variations in responses between intranasally immunized chickens and orally immunized chicken reflects the differences in the immunological responses resulting from the immunization routes, and our results were similar to several published studies (Li et al. 2015; Yang et al. 2017b).
The difference in immune responses of L. plantarum expressed 3M2e-HA2 by intranasal and oral routes of administration as could account for the differences in the responses that were observed in other studies during immunization of chickens (Kuczkowska et al. 2017). Oral immunization was envisaged as being particularly beneficial for extensive immunizations of farm animals, such as chickens, where the recombinant L. plantarum strain could be administered in drinking water or food (simplicity of administration). This approach does not require sterile syringes, needles, or trained personnel compared to intranasal administration. However, in this study, we showed low responses to immunity production resulting from oral immunization compared to the intranasal rout, which we assume results from the orally administered antigens being inactivated by digestive enzymes and gastric acid.
The higher number of chickens with observable sIgA titers after being intranasally immunized compared to those that were orally immunized with N/pgsA′-3M2e-HA2 suggests that, in this form, the antigen is more immunogenic. It is apparent that higher overall specific antibody titers could be acquired by expressing recombinant LAB, where the 3M2e-HA2 antigen is attached to L. plantarum. A series of studies confirmed that chickens exposed to L. plantarum-produced antigens are produced at higher levels, are more stable, and elicit stronger immune responses after mucosal vaccination than their cytoplasmic forms (Kuczkowska et al. 2015; Li et al. 2015). Our results suggest that recombinant N/pgsA′-3M2e-HA2 may reduce cell lysis when intranasally administered, which is reflected by the higher anti-HA-specific antibody titers compared to that obtained by oral administration. It was assumed that the 3M2e-HA2 antigen was enveloped by epithelial cells in the intestine and that N/pgsA′-3M2e-HA2 was taken up by mesenteric lymph nodes or another immune tissue. With this in mind, intranasal inoculation resulted in greater quantities of IgG in serum and was produced in greater quantities than that observed by oral administration of N/pgsA′-3M2e-HA2 or N/pgsA′. In addition, it is assumed that intranasal immunization with N/pgsA′-3M2e-HA2 induces the production of some cytokines in epithelial cells in the lung alveoli, increasing the speed of the immune response and antibody production in response to absorbed N/pgsA′-3M2e-HA2 (Youn et al. 2012).
Consistent with previous studies, the M2e and HA2 antigens, singly or in combination, were able to induce T cell immune responses in animals and confer cross-protection against divergent influenza subtypes (Chowdhury et al. 2017; Guo et al. 2017; Lee et al. 2013). In this study, we provided evidence that N/pgsA′-3M2e-HA2 contributes to the production of T cells using different immune pathways, and the T cell-mediated immune response could play an important role in protecting the host against AIVs (Yang et al. 2016). Thus, the vaccine-induced T cell response is an important strategy for the future design of vaccines.
In conclusion, our study clearly shows that it is of significant importance to test the effects of potential vaccine formulations using more than one route of immunization. Moreover, we achieved higher antibody responses, T cell responses, and effective protection via intranasal rather than oral administration. Additional amendments of the immunization method are expected to determine the most effective route of immunization by the recombinant bacteria. The bacterium is as safe as an adjuvant for intranasal vaccination, and N/pgsA′-3M2e-HA2 may be used in the host to develop new intranasal vaccine.
References
Arena MP, Capozzi V, Spano G, Fiocco D (2017) The potential of lactic acid bacteria to colonize biotic and abiotic surfaces and the investigation of their interactions and mechanisms. Appl Microbiol Biotechnol 101(7):2641–2657. https://doi.org/10.1007/s00253-017-8182-z
van Baarlen P, Troost FJ, van Hemert S, van der Meer C, de Vos WM, de Groot PJ, Hooiveld GJ, Brummer RJ, Kleerebezem M (2009) Differential NF-кB pathways induction by Lactobacillus plantarum 554 in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci U S A 106(7):2371–2376. https://doi.org/10.1073/pnas.0809919106
Barriga GP, Boric-Bargetto D, San Martin MC, Neira V, van Bakel H, Thompsom M, Tapia R, Toro-Ascuy D, Moreno L, Vasquez Y, Sallaberry M, Torres-Perez F, Gonzalez-Acuna D, Medina RA (2016) Avian influenza virus H5 strain with north American and Eurasian lineage genes in an Antarctic penguin. Emerg Infect Dis 22(12):2221–2223. https://doi.org/10.3201/eid2212.161076
Bi Y, Mei K, Shi W, Liu D, Yu X, Gao Z, Zhao L, Gao GF, Chen J, Chen Q (2015) Two novel reassortants of avian influenza A (H5N6) virus in China. J Gen Virol 96(Pt 5):975–981. https://doi.org/10.1099/vir.0.000056
Cai R, Jiang Y, Yang W, Yang W, Shi S, Shi C, Hu J, Gu W, Ye L, Zhou F, Gong Q, Han W, Yang G, Wang C (2016) Surface-displayed IL-10 by recombinant Lactobacillus plantarum reduces Th1 responses of RAW264.7 cells stimulated with poly (I:C) or LPS. J Microbiol Biotechnol 26(2):421–431. https://doi.org/10.4014/jmb.1509.09030
Chowdhury MY, Kim TH, Uddin MB, Kim JH, Hewawaduge CY, Ferdowshi Z, Sung MH, Kim CJ, Lee JS (2017) Mucosal vaccination of conserved sM2, HA2 and cholera toxin subunit A1 (CTA1) fusion protein with poly gamma-glutamate/chitosan nanoparticles (PC NPs) induces protection against divergent influenza subtypes. Vet Microbiol 201:240–251. https://doi.org/10.1016/j.vetmic.2017.01.020
Cui LC, Guan XT, Liu ZM, Tian CY, Xu YG (2015) Recombinant Lactobacillus expressing G protein of spring viremia of carp virus (SVCV) combined with ORF81 protein of koi herpesvirus (KHV): a promising way to induce protective immunity against SVCV and KHV infection in cyprinid fish via oral vaccination. Vaccine 33(27):3092–3099. https://doi.org/10.1016/j.vaccine.2015.05.002
Dabaghian M, Latify AM, Tebianian M, Nili H, Ranjbar AR, Mirjalili A, Mohammadi M, Banihashemi R, Ebrahimi SM (2014) Vaccination with recombinant 4×M2e.HSP70c fusion protein as a universal vaccine candidate enhances both humoral and cell-mediated immune responses and decreases viral shedding against experimental challenge of H9N2 influenza in chickens. Vet Microbiol 174(1–2):116–126. https://doi.org/10.1016/j.vetmic.2014.09.009
Dabaghian M, Latifi AM, Tebianian M, Dabaghian F, Ebrahimi SM (2015) A truncated C-terminal fragment of Mycobacterium tuberculosis HSP70 enhances cell-mediated immune response and longevity of the total IgG to influenza A virus M2e protein in mice. Antivir Res 120:23–31. https://doi.org/10.1016/j.antiviral.2015.05.002
Davis AS, Taubenberger JK, Bray M (2015) The use of nonhuman primates in research on seasonal, pandemic and avian influenza, 1893-2014. Antivir Res 117:75–98. https://doi.org/10.1016/j.antiviral.2015.02.011
Guo Y, He L, Song N, Li P, Sun S, Zhao G, Tai W, Jiang S, Du L, Zhou Y (2017) Highly conserved M2e and hemagglutinin epitope-based recombinant proteins induce protection against influenza virus infection. Microbes Infect 19:641–647. https://doi.org/10.1016/j.micinf.2017.08.010
Kaur M, Singh H, Jangra M, Kaur L, Jaswal P, Dureja C, Nandanwar H, Chaudhuri SR, Raje M, Mishra S, Pinnaka AK (2017) Lactic acid bacteria isolated from yak milk show probiotic potential. Appl Microbiol Biotechnol 101:7635–7652. https://doi.org/10.1007/s00253-017-8473-4
Kechaou N, Chain F, Gratadoux JJ, Blugeon S, Bertho N, Chevalier C, Le Goffic R, Courau S, Molimard P, Chatel JM, Langella P, Bermudez-Humaran LG (2013) Identification of one novel candidate probiotic Lactobacillus plantarum strain active against influenza virus infection in mice by a large-scale screening. Appl Environ Microbiol 79(5):1491–1499. https://doi.org/10.1128/aem.03075-12
Krammer F (2016) Novel universal influenza virus vaccine approaches. Curr Opin Virol 17:95–103. https://doi.org/10.1016/j.coviro.2016.02.002
Kuczkowska K, Mathiesen G, Eijsink VG, Oynebraten I (2015) Lactobacillus plantarum displaying CCL3 chemokine in fusion with HIV-1 gag derived antigen causes increased recruitment of T cells. Microb Cell Factories 14:169. https://doi.org/10.1186/s12934-015-0360-z
Kuczkowska K, Kleiveland CR, Minic R, Moen LF, Overland L, Tjaland R, Carlsen H, Lea T, Mathiesen G, Eijsink VG (2017) Immunogenic properties of Lactobacillus plantarum producing surface-displayed Mycobacterium tuberculosis antigens. Appl Environ Microbiol 83(2):e02782–e02716. https://doi.org/10.1128/aem.02782-16
Lee JS, Chowdhury MY, Moon HJ, Choi YK, Talactac MR, Kim JH, Park ME, Son HY, Shin KS, Kim CJ (2013) The highly conserved HA2 protein of the influenza A virus induces a cross protective immune response. J Virol Methods 194(1–2):280–288. https://doi.org/10.1016/j.jviromet.2013.08.022
Lee DH, Fusaro A, Song CS, Suarez DL, Swayne DE (2016) Poultry vaccination directed evolution of H9N2 low pathogenicity avian influenza viruses in Korea. Virology 488:225–231. https://doi.org/10.1016/j.virol.2015.11.023
Li C, Bu Z, Chen H (2014) Avian influenza vaccines against H5N1 ‘bird flu’. Trends Biotechnol 32(3):147–156. https://doi.org/10.1016/j.tibtech.2014.01.001
Li R, Chowdhury MY, Kim JH, Kim TH, Pathinayake P, Koo WS, Park ME, Yoon JE, Roh JB, Hong SP, Sung MH, Lee JS, Kim CJ (2015) Mucosally administered Lactobacillus surface-displayed influenza antigens (sM2 and HA2) with cholera toxin subunit A1 (CTA1) induce broadly protective immune responses against divergent influenza subtypes. Vet Microbiol 179(3–4):250–263. https://doi.org/10.1016/j.vetmic.2015.07.020
Liu DQ, Ge JW, Qiao XY, Jiang YP, Liu SM, Li YJ (2012) High-level mucosal and systemic immune responses induced by oral administration with Lactobacillus-expressed porcine epidemic diarrhea virus (PEDV) S1 region combined with Lactobacillus-expressed N protein. Appl Microbiol Biotechnol 93(6):2437–2446. https://doi.org/10.1007/s00253-011-3734-0
Liu YY, Yang WT, Shi SH, Li YJ, Zhao L, Shi CW, Zhou FY, Jiang YL, Hu JT, Gu W, Yang GL, Wang CF (2017) Immunogenicity of recombinant Lactobacillus plantarum NC8 expressing goose parvovirus VP2 gene in BALB/c mice. J Vet Sci 18(2):159–167. https://doi.org/10.4142/jvs.2017.18.2.159
Mallajosyula VV, Citron M, Ferrara F, Lu X, Callahan C, Heidecker GJ, Sarma SP, Flynn JA, Temperton NJ, Liang X, Varadarajan R (2014) Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc Natl Acad Sci U S A 111(25):E2514–E2523. https://doi.org/10.1073/pnas.1402766111
Mao R, Wu D, Wang Y (2016) Surface display on lactic acid bacteria without genetic modification: strategies and applications. Appl Microbiol Biotechnol 100(22):9407–9421. https://doi.org/10.1007/s00253-016-7842-8
Matusevich OV, Egorov VV, Gluzdikov IA, Titov MI, Zarubaev VV, Shtro AA, Slita AV, Dukov MI, Shurygina AP, Smirnova TD, Kudryavtsev IV, Vasin AV, Kiselev OI (2015) Synthesis and antiviral activity of PB1 component of the influenza A RNA polymerase peptide fragments. Antivir Res 113:4–10. https://doi.org/10.1016/j.antiviral.2014.10.015
Pan M, Gao R, Lv Q, Huang S, Zhou Z, Yang L, Li X, Zhao X, Zou X, Tong W, Mao S, Zou S, Bo H, Zhu X, Liu L, Yuan H, Zhang M, Wang D, Li Z, Zhao W, Ma M, Li Y, Li T, Yang H, Xu J, Zhou L, Zhou X, Tang W, Song Y, Chen T, Bai T, Zhou J, Wang D, Wu G, Li D, Feng Z, Gao GF, Wang Y, He S, Shu Y (2016) Human infection with a novel, highly pathogenic avian influenza a (H5N6) virus: virological and clinical findings. J Inf Secur 72(1):52–59. https://doi.org/10.1016/j.jinf.2015.06.009
Pendzialek J, Roose K, Smet A, Schepens B, Kufer P, Raum T, Baeuerle PA, Muenz M, Saelens X, Fiers W (2017) Bispecific T cell engaging antibody constructs targeting a universally conserved part of the viral M2 ectodomain cure and prevent influenza A virus infection. Antivir Res 141:155–164. https://doi.org/10.1016/j.antiviral.2017.02.016
Pu J, Wang S, Yin Y, Zhang G, Carter RA, Wang J, Xu G, Sun H, Wang M, Wen C, Wei Y, Wang D, Zhu B, Lemmon G, Jiao Y, Duan S, Wang Q, Du Q, Sun M, Bao J, Sun Y, Zhao J, Zhang H, Wu G, Liu J, Webster RG (2015) Evolution of the H9N2 influenza genotype that facilitated the genesis of the novel H7N9 virus. Proc Natl Acad Sci U S A 112(2):548–553. https://doi.org/10.1073/pnas.1422456112
Rappazzo CG, Watkins HC, Guarino CM, Chau A, Lopez JL, DeLisa MP, Leifer CA, Whittaker GR, Putnam D (2016) Recombinant M2e outer membrane vesicle vaccines protect against lethal influenza A challenge in BALB/c mice. Vaccine 34(10):1252–1258. https://doi.org/10.1016/j.vaccine.2016.01.028
Riaz Rajoka MS, Shi J, Zhu J, Shao D, Huang Q, Yang H, Jin M (2017) Capacity of lactic acid bacteria in immunity enhancement and cancer prevention. Appl Microbiol Biotechnol 101(1):35–45. https://doi.org/10.1007/s00253-016-8005-7
Schneemann A, Speir JA, Tan GS, Khayat R, Ekiert DC, Matsuoka Y, Wilson IA (2012) A virus-like particle that elicits cross-reactive antibodies to the conserved stem of influenza virus hemagglutinin. J Virol 86(21):11686–11697. https://doi.org/10.1128/jvi.01694-12
Shi SH, Yang WT, Yang GL, Cong YL, Huang HB, Wang Q, Cai RP, Ye LP, Hu JT, Zhou JY, Wang CF, Li Y (2014) Immunoprotection against influenza virus H9N2 by the oral administration of recombinant Lactobacillus plantarum NC8 expressing hemagglutinin in BALB/c mice. Virology 464-465:166–176. https://doi.org/10.1016/j.virol.2014.07.011
Shi SH, Yang WT, Yang GL, Zhang XK, Liu YY, Zhang LJ, Ye LP, Hu JT, Xing X, Qi C, Li Y, Wang CF (2016) Lactobacillus plantarum vaccine vector expressing hemagglutinin provides protection against H9N2 challenge infection. Virus Res 211:46–57. https://doi.org/10.1016/j.virusres.2015.09.005
Sorvig E, Mathiesen G, Naterstad K, Eijsink VG, Axelsson L (2005) High-level, inducible gene expression in Lactobacillus sakei and Lactobacillus plantarum using versatile expression vectors. Microbiology 151(Pt 7):2439–2449. https://doi.org/10.1099/mic.0.28084-0
Tao W, Hurst BL, Shakya AK, Uddin MJ, Ingrole RS, Hernandez-Sanabria M, Arya RP, Bimler L, Paust S, Tarbet EB, Gill HS (2017) Consensus M2e peptide conjugated to gold nanoparticles confers protection against H1N1, H3N2 and H5N1 influenza A viruses. Antivir Res 141:62–72. https://doi.org/10.1016/j.antiviral.2017.01.021
Wu Y, Wu Y, Tefsen B, Shi Y, Gao GF (2014) Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol 22(4):183–191. https://doi.org/10.1016/j.tim.2014.01.010
Yang WT, Shi SH, Yang GL, Jiang YL, Zhao L, Li Y, Wang CF (2016) Cross-protective efficacy of dendritic cells targeting conserved influenza virus antigen expressed by Lactobacillus plantarum. Sci Rep 6:39665. https://doi.org/10.1038/srep39665
Yang JR, Cheng CY, Chen CY, Lin CH, Kuo CY, Huang HY, Wu FT, Yang YC, Wu CY, Liu MT, Hsiao PW (2017a) A virus-like particle vaccination strategy expands its tolerance to H3N2 antigenic drift by enhancing neutralizing antibodies against hemagglutinin stalk. Antivir Res 140:62–75. https://doi.org/10.1016/j.antiviral.2017.01.010
Yang WT, Yang GL, Shi SH, Liu YY, Huang HB, Jiang YL, Wang JZ, Shi CW, Jing YB, Wang CF (2017b) Protection of chickens against H9N2 avian influenza virus challenge with recombinant Lactobacillus plantarum expressing conserved antigens. Appl Microbiol Biotechnol 101(11):4593–4603. https://doi.org/10.1007/s00253-017-8230-8
Yang WT, Yang GL, Wang Q, Huang HB, Jiang YL, Shi CW, Wang JZ, Huang KY, Jin YB, Wang CF (2017c) Protective efficacy of Fc targeting conserved influenza virus M2e antigen expressed by Lactobacillus plantarum. Antivir Res 138:9–21. https://doi.org/10.1016/j.antiviral.2016.11.025
Yang WT, Yang GL, Yang X, Shonyela SM, Zhao L, Jiang YL, Huang HB, Shi CW, Wang JZ, Wang G, Zhao JH, Wang CF (2017d) Recombinant Lactobacillus plantarum expressing HA2 antigen elicits protective immunity against H9N2 avian influenza virus in chickens. Appl Microbiol Biotechnol 101(23–24):8475–8484. https://doi.org/10.1007/s00253-017-8600-2
Yao JY, Yuan XM, Xu Y, Yin WL, Lin LY, Pan XY, Yang GL, Wang CF, Shen JY (2016) Live recombinant Lactococcus lactis vaccine expressing immobilization antigen (i-Ag) for protection against Ichthyophthirius multifiliis in goldfish. Fish Shellfish Immu 58:302–308. https://doi.org/10.1016/j.fsi.2016.09.037
Youn HN, Lee DH, Lee YN, Park JK, Yuk SS, Yang SY, Lee HJ, Woo SH, Kim HM, Lee JB, Park SY, Choi IS, Song CS (2012) Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antivir Res 93(1):138–143. https://doi.org/10.1016/j.antiviral.2011.11.004
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2017YFD0501000, 2017YFD0500400), National Natural Science Foundation of China (31672528, 31602092), Science and Technology Development Programof Jilin Province (20160519011JH, 20170204034NY, 20180520037JH), Special Funds for Industrial Innovation of Jilin Province (2016C063), “Thirteen Five-year Plan” for Sci&Tech Research Program of Jilin Education Department of P.R. China (JJKH20170318KJ), and the Doctoral Project sponsored by the Scientific Research Foundation of Jilin Agricultural University of China (201601).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
All applicable international and national guidelines for the care and use of chickens were followed.
Rights and permissions
About this article
Cite this article
Yang, WT., Yang, GL., Zhao, L. et al. Lactobacillus plantarum displaying conserved M2e and HA2 fusion antigens induces protection against influenza virus challenge. Appl Microbiol Biotechnol 102, 5077–5088 (2018). https://doi.org/10.1007/s00253-018-8924-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8924-6