Abstract
Microorganisms play a significant role in uranium(VI) biogeochemistry and influence U(VI) transformation through biomineralization. In the present work, the process of uranium mineralization was investigated by Saccharomyces cerevisiae. The toxicity experiments showed that the viability of cell was not significantly affected by 100 mg L−1 U(VI) under 4 days of exposure time. The batch experiments showed that the phosphate concentration and pH value increased over time during U(VI) adsorption. Meanwhile, thermodynamic calculations demonstrated that the adsorption system was supersaturated with respect to UO2HPO4. The X-ray powder diffraction spectroscopy (XRD), field emission scanning electron microscopy (FE-SEM) equipped with energy dispersive spectroscopy (EDX), Fourier transform infrared spectroscopy (FTIR), and X-ray photoelectron spectroscopy (XPS) analyses indicated that the U(VI) was first attached onto the cell surface and reacted with hydroxyl, carboxyl, and phosphate groups through electrostatic interactions and complexation. As the immobilization of U(VI) transformed it from the ionic to the amorphous state, lamellar uranium precipitate was formed on the cell surface. With the prolongation of time, the amorphous uranium compound disappeared, and there were some crystalline substances observed extracellularly, which were well-characterized as tetragonal-chernikovite. Furthermore, the size of chernikovite was regulated at nano-level by cells, and the perfect crystal was formed finally. These findings provided an understanding of the non-reductive transformation process of U(VI) from the amorphous to crystalline state within microbe systems, which would be beneficial for the U(VI) treatment and reuse of nuclides and heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium (U) is a heavy metal contaminant widely distributed all over the world (Boyanov et al. 2017). It mainly exists in two environmentally important oxidation states (+ 4 and + 6), among which U(IV) is considered to be more stable and sparingly soluble, while U(VI) [i.e., UO22+ and/or UO2(CO3) n (2 − 2n)] is considered to be soluble within aquatic systems, and easier to discharge into the near-surface environment from different sources, such as by weathering of U-mine tailing, milling, as well as the failing geological disposal of radioactive waste (Zhou and Gu 2005; Rui et al. 2013; Yan et al. 2016). However, the in-depth understanding of uranium for biodiversity and human health has raised considerable attentions regarding its potentially harmful effects (Martinez et al. 2007). In vitro and in vivo studies have confirmed that the hazardous effect of uranium on animals is derived from both chemical and radiological toxicity (Pierrefite-Carle et al. 2017; Asic et al. 2017). Moreover, exposure to the byproducts of uranium radioactive decay (i.e., radon and radium) might increase the incidence of cancer (Bjørklund et al. 2017). Therefore, the remediation of uranium waste is of particular importance. Compared with the traditional treatment procedures, such as membrane filtration (Schulte-Herbrüggen et al. 2016), co-precipitation (Pidchenko et al. 2017), ion exchange (Moon et al. 2017; Barrett et al. 2017), extraction, etc. (Singhal et al. 2017; Li et al. 2017), biological remediation is an ecofriendly and promising alternative method due to its efficiency, simplicity, and low operating costs (Goksungur et al. 2005). Currently, the most developed microbial remediation techniques are biosorption (Liu et al. 2010; Lu et al. 2013; Wang et al. 2017a, b), bioreduction (Istok et al. 2004; Gu et al. 2005; Wu et al. 2006, 2007; Watson et al. 2013), and biomineralization (Ohnuki et al. 2005; Pan et al. 2015; Sun et al. 2016; Zheng et al. 2017). Biosorption is limited to the bioavailability of metal-binding sites, and bioreduction is also constrained by the strict anaerobic conditions as well as a complex recirculation system, while biomineralization is less restricted. Hereby, biomineralization continues to be one of the more attractive remedial technologies for metal removal from contaminated sediments and groundwater (Merroun et al. 2011).
Over the past several decades, a variety of microbes have been investigated for use in uranium immobilization. It has been reported that fermentative fungi as well as sulfate- and iron-reducing bacteria have the reduction ability to convert hexavalent uranium [U(VI)] to tetravalent uranium [U(IV)], and form uranium(IV)-oxide minerals, uranium(IV)-phosphate minerals, and/or multidentate complexes (Lovley and Phillips 1992; Ganesh et al. 1997; Lee et al. 2014; Gilson et al. 2015; Wang et al. 2017a), such as uraninite (UO2) (Lovley and Phillips 1992; Lee et al. 2014), ningyoite [CaU(PO4)2·2H2O] (Bernier-Latmani et al. 2010; Rui et al. 2013; Newsome et al. 2015), and diuranium oxide phosphate [U2O(PO4)2] (Bernier-Latmani et al. 2010). In addition, other researchers have also found that the majority of biomineralization are not involved in the reduction of U(VI). Previous studies have been demonstrated that the uranium might be precipitated with phosphate upon the surface of microbe ligands to form uranyl-phosphate minerals, such as autunite [Ca(UO2)2(PO4)2·6H2O] (Merroun et al. 2011), uramphite [(NH4)(UO2)PO4·3H2O] (Liu et al. 2010; Pan et al. 2015; Liang et al. 2016), chernikovite (UO2HPO4·4H2O) (Macaskie et al. 1992; Liang et al. 2016; Zheng et al. 2017), and meta-ankoleite (KUO2PO4·3H2O) (Liang et al. 2015b, 2016). In order to overcome the phosphate limitations associated with microorganisms, aqueous phase phosphate has even been directly added to promote the immobilization of uranium in previous studies (Shelobolina et al. 2009; Sousa et al. 2013; Liang et al. 2015b). In general, UO22+ has been considered soluble as a major pollutant in aqueous phase, while the feasibility of formation and their stability conditions of uranyl precipitates have not been well studied. Therefore, it is crucial to systematically evaluate the interactions between U(VI) and indigenous microorganisms.
Saccharomyces cerevisiae is a representative fungus that is ubiquitously distributed in the aerobic lithosphere. Due to poor survivability of the yeast in subsurface and soil, it is not the most ideal candidate for the in situ remediation of uranium. However, inherently safe, easy to cultivate, widely accepted, and a high U(VI) accumulation capacity made it more appreciated in investigating the interactions of U-microbe under laboratory conditions (Wang and Chen 2006). In this study, the transformation process of soluble U(VI) from an amorphous state to a crystalline substance by S. cerevisiae and the newly formed secondary U(VI) mineral were investigated by a multidisciplinary approach combining batch adsorption experiments, microscopy, thermodynamic calculation, as well as a microbiological staining technique. These results provide new insight on the mineralization process of microbes with U(VI).
Materials and methods
Yeast strain and regents
S. cerevisiae used in this work was purchased from the Angel Yeast Co., Ltd., Yichang, China. After 2 h of activation and 16 h of first-generation culture at 30 °C, the second-generation cells were cultured in the pre-sterilized yeast extract peptone dextrose (YPD) liquid medium (1% yeast extract, 2% peptone, and 2% dextrose) for 30 h and centrifuged (5000×g, 5 min). Then, the cell suspension was washed three times with sterilized deionized water and preserved at 4 °C. Before the adsorption experiments, the concentration of the cell suspension was determined using the method of cell dry weight (Lu et al. 2013) and gradually diluted to the desired concentration (10.0, 5.0, and 2.0 g L−1).
All chemicals were purchased from Aihua Chemical Co., Ltd., Lanzhou, China, and of analytical grade unless otherwise stated. Uranyl stock solution was prepared by dissolving uranyl nitrate hexahydrate (UO2(NO3)2·6H2O) in 1.0 mol L−1 nitrate acid (HNO3). The stock solution was then gradually diluted to the desired concentration (1000, 500, and 200 mg L−1) with 0.01 mol L−1 HNO3 solution and stored at pH 2.0.
Batch adsorption experiments
The prepared cell suspension and deionized water were transferred into a 50-mL polyethylene tube, and then, the uranyl stock solution was placed into the test tube. The final yeast concentration of 1.6 g L−1 (dry weight) and the U(VI) concentration of 100 mg L−1 were set to meet the need of the microscopic and spectroscopic techniques. The initial pH of the system was adjusted to 5.0 using a negligible volume of 0.01 or 1.0 mol L−1 sodium hydroxide (NaOH) solution. Subsequently, the mixtures were shaken in a rotary shaker stirring at 200 rpm, 30 °C. At the predetermined contact time intervals (60, 120, 360, and 480 min), cells were separated by centrifugation (10,000×g, 15 min), the supernatant was assayed for residual uranium, and the cells were subjected to characterization. The cell-free control experiments were conducted using the same procedures to assure that the amount of uranium adsorption on the tube wall was negligible (Lu et al. 2013). The final uranium concentration was measured by the pulsed-laser induced fluorescence method due to its sensitivity and selectivity to UO22+ (Ganesh et al. 2012). The inorganic phosphate (Pi) concentration was assessed by the ammonium molybdate spectrophotometric method (Shyla et al. 2011).
Measurement of cell viability
Methylene blue (MB, C16H18ClN3S), which is used as a biological indicator for monitoring the viability of eukaryotic cells, has a basic dye skeleton of a thiazine group that can be reduced to colorless by high-metabolism living cells. Conversely, the dead or senescent cells have a slight reduction ability that is unable to decolorize the oxidized MB and the cells are stained blue. Therefore, the numbers of live and dead cell can be counted using viable count method thus calculated the viability of S. cerevisiae. The principle that blue-MB was reduced to the colorless leuco-MB by living cells was illustrated by Eq. (1) (Pande et al. 2006):

Since Fabrizio and Longo (2003) have reviewed the mean chronological lifespan of S. cerevisiae and confirmed that cells could survive under water or YPD culture conditions for 15–20 days, it was reasonable to select 8 days for monitoring the U(VI)-dependent mortality. After the batch experiments, 0.5 mL of yeast suspension was added into staining solution (0.3-mL phosphate buffer saline, 0.2 mL of 0.1% MB dye solution, and 1 mL deionized water), and then dropped onto a standard hemocytometer (XB.K.25., Qiujing Instrumentation Co., Ltd., Shanghai, China) by a pipette (Eppendorf, Hamburg, Germany). After covering the samples with a microscope cover slip and removing the excess water, the cells on the hemocytometer grid were observed and counted after 3 min under the transflective polarizing microscope (XPT-150, Bingyu Instrument Co., Ltd., Shanghai, China) at 100 times magnification. Parallel assays without U(VI) were conducted to ensure that decrease in cell viability induced by temperature and metabolism was negligible. All experiments were run in triplicate and counts were averaged.
XRD analysis
The mineralogy of patterns was confirmed via X-ray powder diffraction spectroscopy (XRD, Rigaku D/Max-2400, Tokyo, Japan) using Cu-Kα radiation (λ = 0.154056 nm) over the range of 5° to 60° (2θ) with a scan speed of 2° min−1 and a step size of 0.02°. The crystallographic structure was identified using the PDF database of the International Centre for Diffraction Data (ICDD), and the size of newly formed crystallite was calculated by the Debye-Scherrer equation (Wadgaonkar et al. 2018):
where τ (nm) is the mean crystal thickness, K is the dimensionless shape factor (typical value of 0.89), λ (nm) is the X-ray wavelength, β is the full width at half maximum (FWHM) of the maximum intensity peak (radians), and θ is the corresponding Bragg angle (radians). In the process of crystallization, the level of crystallinity of secondary U mineral is provided by the MDI’s Jade 6.0 software (Mehta et al. 2014).
Surface analysis
The cell surface functional groups were identified by Fourier transform infrared spectroscopy (FTIR, Thermo Nicolet NEXUS670, Madison, USA) recorded in the frequency range of 4000–400 cm−1. For FTIR analysis, the air-dried and powdered cells were added into the KBr powder and then pressed into the disk for the test.
The surface chemical composition and the valence of uranium precipitation were determined by X-ray photoelectron spectroscopy (XPS, Kratos AXIS UltraDLD, Kyoto, Japan) analysis using Al-Kα radiation (hν = 1486.6 eV). To overcome the surface charging effects, all XPS spectra were referenced by the standard C1s neutral carbon peak (Binding energy = 284.6 eV). For XPS analysis, the collected cells were air-dried naturally and pulverized.
Mesoscopic investigation
The morphology of S. cerevisiae was recorded using field emission scanning electron microscopy (FE-SEM, HITACHI SU8020, Tokyo, Japan) equipped with energy dispersive spectroscopy (EDX). Samples for FE-SEM analysis were prepared by using the methodology described previously (Zheng et al. 2017). The collected cells were washed three times with 0.1-mol L−1 sodium chloride (NaCl) solutions for purification and maintain the cells’ osmotic pressure. Samples were then fixed with 2.5% glutaraldehyde (C5H8O2) for 1 h at 4 °C and washed with 0.1-mol L−1 NaCl solutions. After being dehydrated using graded ethanol series (30, 50, 70, 90, and 100%), the suspensions were transferred onto the cover slip and air-dried for 12 h. Before analysis, samples were sputter-coated with nanometer-sized platinum particles.
Thermodynamic calculation of the U(VI) mineral saturation
Since the XRD results determined that major uranium product by S. cerevisiae is chernikovite [H2(UO2PO4)2·8H2O] (seen in XRD results), in order to track the process of the precipitation reaction under experimental conditions, the saturation index (SI) of the U(VI) mineral for adsorption system and the distribution of dominant soluble uranium species were calculated using Visual MINTEQ 3.0 software package (https://vminteq.lwr.kth.se/). The SI value is defined as the ratio of ion activity product (Q) to the solubility product (Ksp) and is given by Eq. (3):
An SI value less than zero indicated that the reaction was in the state of disequilibrium. After excluding other secondary minerals such as gummite, H-autunite, and schoepite, the dissolution reaction of chernikovite was expressed by Eq. (4):
The log Ksp value of chernikovite was obtained from recent reviews and updated experimental data (Grenthe et al. 1992; Haverbeke et al. 1996; Johnson 2000; Gorman-Lewis et al. 2008). Multiple log Ksp values of chernikovite are available in the literatures under different temperatures, pH values, and ionic strength of the solutions (Haverbeke et al. 1996). For chernikovite, the log Ksp value varied from − 22.73 to − 25.50. In our calculation, we followed the value of − 24.225 which was provided by MINTEQ 3.0 at 30 °C. In the computation, carbonate is assumed to be in equilibrium under atmospheric conditions.
Results
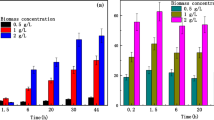
Fungal viability upon U(VI) exposure and toxicity of uranium
Figure 1a illustrates the total number of S. cerevisiae cells as a function of preservation time at initial pH 5.0, the yeast counts maintained at a stable level under nutrient-free conditions [with a density of (7.50 ± 0.49) × 109 cell g−1 at 4 °C and (7.57 ± 0.57) × 109 cell g−1 at 30 °C] after 8-day preservation, indicated that the cells could survive in a low-metabolism state, and the autolysis of S. cerevisiae was negligible. To investigate the tolerance of S. cerevisiae to uranium, the yeast cell viability was measured indirectly by the MB dyeing rate at different initial uranium concentrations (100, 200, and 400 mg L−1) and initial pH 5.0. As shown in Fig. 1b, less than 5% mortality was measured in U-free control samples at 4 and 30 °C, indicating that the cell viability was independent of the ambient temperature. In contrast, the S. cerevisiae cells under different uranium concentrations exhibited distinct survival rates that were diverse from the control samples at 30 °C and initial pH 5.00 ± 0.10. At 100 mg L−1 of uranium, cell viability was not impacted within the first 3 days. As the exposure time prolonged, the survival rate drastically decreased, and cells could keep 63.78 ± 3.42% viability in the presence of 100 mg L –1 U(VI) after 4 days. At 200 mg L−1, the cell viability was barely affected within the first 24 h, while 40.28 ± 6.27% of total cells was not viable after 3-day exposure. For comparison, when the uranium concentration reached 400 mg L−1, cells were devitalized quickly only within 6 h (80.66 ± 1.14% of the population died).
Time-course accumulation of U(VI) by S. cerevisiae
Figure 2 plots the variation of the uranium concentration, pH, and Pi concentration on the uranium supernatants as a function of contact time at the initial pH 5.00 ± 0.10. The cell-free control revealed that U(VI) remains stable (~ 100 mg L−1) within 3960 min of exposure and confirmed that U(VI) removal resulted exclusively from biotic interaction. It was observed that uranium accumulation by the yeast increased rapidly within 360 min. After 360 min, the U accumulation rate slowed down (Fig. 2a). The adsorption equilibrium was established after 1440 min with an accumulation of 56.21 ± 2.35 mg U g−1 (dry weight), which represented 89.94 ± 0.39% of U(VI) removal. Noticeably, the pH value of the experimental samples gradually increased from 5.00 to 6.95 within the first 2160 min and eventually stabilized at ~ 7.00, while the pH values of the cell-free control were almost unchanged.
The concentration of Pi released into solution from S. cerevisiae rose with increasing exposure time to U(VI) (Fig. 2b). At 30 °C, a constant and low quantity of Pi (7.34 ± 1.83 mg L−1 in average) was measured in the U-free control sample. Within the first 480 min, the Pi concentration in the U-free samples was slightly higher than for the experimental samples. However, the trend was reversed latter after 960 min of exposure, where the maximum Pi was measured at 3960 min (26. 25 ± 1.31 mg L−1).
XRD analysis
To further determine the uranyl phosphate speciation and the chemical structure, test strains were subjected to X-ray powder diffraction analysis. As shown from the XRD patterns in Fig. 3a, there were no obvious diffraction peaks for raw biomass, indicating that the yeasts were crystal-free samples. When exposed to 100 mg L−1 U for 2 days, there were no obvious diffraction peaks either, suggesting that the U(VI) was simply absorbed onto the cell surface and was still in the amorphous state. However, after 2 days of exposure, a series of weak diffraction peaks appeared, suggesting that the uranyl crystallite began to form. With increasing exposure time, the 2θ values of diffraction peaks tended to be consistent. The observed diffraction peaks of uranium complexes were well-indexed as chernikovite [H2(UO2PO4)2·8H2O] (ICDD-PDF #08-0296). Comparably, at 200 mg L−1 of U(VI), there were obvious intense crystalline peaks after U(VI) exposure for 4 days (Fig. 3b).
Thermodynamic of chernikovite
Figure 4a illustrates the values of SI calculated for chernikovite. At the beginning of adsorption, the SI value was negative, indicating that the solution was undersaturated with respect to the chernikovite. Meanwhile, the SI value became positive in range of 120 to 3960 min because the Pi was gradually released and the U(VI) concentration on the cell surface was increased rapidly to be up to 30 times as much as that in the solution.
a The saturation index of chernikovite and pH values over time during yeast cell (1.6 g L−1) exposure to 100 mg L−1 U(VI) at 30 °C (calculations were carried out under the materials and charge balance of U and Pi in the solutions). b Distribution diagram for the aqueous species in the adsorption system under atmospheric conditions [pCO2 = 3.16 × 10−4 atm, U(VI) = 100 mg L−1, Pi = 25 mg L−1]. All calculations were performed with Visual MINTEQ 3.0
However, within 120–2880 min, the XRD result is inconsistent with the thermodynamic calculation, in which no chernikovite is detectable. Hereby, the species distribution of uranyl ions and pH changes should be taken into account. The U(VI) hydrolysis speciation in an aqueous 100-mg L−1 solution as a function of pH (2–10), ambient atmosphere (pCO2 = 3.16 × 10−4 atm), and the presence of 25 mg L−1 Pi is illustrated in Fig. 4b. The calculation was originated from Visual MINTEQ 3.0 and as many as seven different isomers and/or coexisting polymeric hydroxo species appeared. Remarkably, in the batch experiments, the relative content of soluble UO2HPO4(aq) was dramatically decreased with rising pH values (5.00–7.00), while under circumneutral pH conditions, the sparingly soluble UO2HPO4(s) predominated over the soluble UO2HPO4(aq) (Haverbeke et al. 1996; Gorman-Lewis et al. 2008; Mehta et al. 2014; Cumberland et al. 2016).
Surface analysis
FTIR analysis is a valid method to monitor structural and architectural cell wall alterations at the molecular level. The S. cerevisiae cell wall is made of 30–60% polysaccharides (β-glucans and mannan sugar polymers), 15–30% proteins, 5–20% lipids, and a small amount of chitin (as % dry weight) (Galichet et al. 2001). The polysaccharides consist of three layers: mannan, protein, and β-glucan. Most of the protein is linked to the mannan-oligo-saccharides (MOS) and is referred to as the mannoprotein complex (Fleet and Manners 1976). These chemical components can provide a large amount of functional groups such as carboxyl, hydroxyl, phosphoryl, and amino groups. Peaks in the FTIR spectra corresponded to the above groups and bonds consistent with their respective wavenumbers (cm−1) as reported in the literatures and are summarized in Table 1 (Liu et al. 2010; Lu et al. 2013; Du et al. 2017).
The IR spectrum of S. cerevisiae (Fig. 5) includes the stretching vibration of –OH/–NH groups at 3310.5 cm−1 and a C=O stretching vibration from carboxyl groups (-COO−) at 1398.3 cm−1. Meanwhile, there are three dominant protein characteristic peaks: amide band I (1654.9 cm−1, α-helical structure), amide II (1543.9 cm−1), and amide III (1241.1 cm−1). Additionally, there is a strong symmetrical stretching vibration of the phosphodiester group [νs(–PO2−)] (1075.5 cm−1) and an asymmetrical stretching vibration of PO43− (533.2 cm−1), which reveals that the phospholipid bilayers also exist in yeast cells (Du et al. 2017). Compared with the raw biomass, after uranium treatment, the great blueshift of the –OH, CH2, and PO43− peak indicated that uranyl ions were achieved on the yeast surface. Particularly, the intense peak at 1075.5 cm−1 that belonged to the –PO2− was redshifted to a low level (1045.1–998.5 cm−1) after 15-day uranium exposure, and the peak at 1398.2 cm−1 was disappeared completely, which indicated that the cell surface has changed greatly. The appearance of a new peak in the fingerprint region at approximately 915 cm−1 was assigned to the asymmetrical stretching vibration of uranyl ions and complexation between uranyl ions and functional groups such as peptides and phospholipids on cells (Lu et al. 2013).
XPS was employed to verify the valence states of uranium precipitation and recognize the surface chemical states. Here, we further identified whether there was reduction of U(VI) during the mineralization process by S. cerevisiae (Fig. 6). After U loaded, a new peak assigned to the antisymmetric vibration of the [O=UVI=O]2+ was observed, which indicated that uranium was absorbed onto the cell surface according to the chemical bonding (Li et al. 2016). Moreover, the U4f spectra (Fig. 6b) of samples showed that two distinct peaks at 379.5 eV (U4f7/2) and 390.4 eV (U4f5/2) appeared after 0.25 and 3 days of exposure, respectively. No satellite peaks at the position of U4f7/2 and U4f5/2 were observed, which indicated that the uranium in the yeast was still present in the U(VI) state and that S. cerevisiae could not reduce U(VI) (Kushwaha et al. 2012). Additionally, the P2p spectra (Fig. 6c) showed that the binding energy of P(V) downshift (from 131.15 to 131.06 eV) gradually after uranium adsorbed onto S. cerevisiae, and the normalized intensity of phosphorus increased dramatically (area from 326.48 to 454.13), which is ascribed to uranyl-phosphorus interaction and the release of intracellular phosphate (Zheng et al. 2017). These results suggested that the interaction between cells and uranium was primarily attributed to the complexation of phosphate on the cell surface, and the uranium biomineralization by S. cerevisiae was a non-reductive process throughout.
Mesoscopic analysis
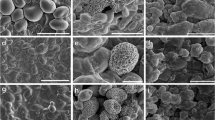
The microstructure of U(VI) precipitation and S. cerevisiae was characterized by FE-SEM technique. As shown from the FE-SEM images in Fig. 7, the surface of live S. cerevisiae cells was clean and intact (Fig. 7a). However, the yeast surfaces were no longer smooth after 6-h uranium exposure, and a layer of lamellar precipitation was uniformly attached onto the cell surface (Fig. 7b, c). After 24-h exposure, the edges of the uranium compound became blurred (Fig. 7d). Moreover, the precipitates gradually disappeared after 3-day exposure, which were only localized on the part of cell surface (Fig. 7e). With the extension of time, the amorphous uranium precipitation disappeared completely and crystalline particles were visualized on the surface of cells (Fig. 7f, arrow). Furthermore, the EDX spectrum was employed to confirm the component of uranium compound (Fig. 7g), the normalized spectral results showed that after U(VI) bonded with cells, two distinct peaks of uranium appeared. Meanwhile, the relative content of phosphorus obviously increased with increasing exposure time to U(VI). The high silicon content was due to the thin yeast cell thickness on the carrier of cover slip (Liu et al. 2010).
Field emission scanning electron microscopy and energy dispersive spectroscopy of a S. cerevisiae and U-loaded S. cerevisiae; the exposure times of patterns b, d, e, f were 0.25, 1, 3, 7 days, respectively. c The enlarged regions denoted by rectangles in b. White bar = 2 μm [initial pH: 5.00 ± 0.10, biomass: 1.6 g L−1, U(VI):100 mg L−1, 30 °C]
Discussion
In this work, we described the interaction between U(VI) and S. cerevisiae using a multidisciplinary approach involving a combination of the batch adsorption experiments, microscopy characterization (XRD, FTIR, XPS, and FESEM-EDX), thermodynamic calculation, and microbiological staining. According to the study of morphology and crystallology of the U(VI) product and the changes of the liquid phase environment, we found the phenomenon of Pi release, U(VI) precipitation, and pH ascent, as well as mediation of crystallization by cells. Based on the above analysis, the possible process of U mineralization by S. cerevisiae, surface adsorption, amorphous precipitation, and crystallization was proposed.
Resistance of S. cerevisiae to U(VI)
Biomineralization is a self-assembly process induced by living organisms, which is carried out with the exquisite control of crystal morphology and compositional specificity under physiological conditions (Liang et al. 2015a). Among lots of factors affecting bio-induced mineralization, cell viability is the most important one. In this work, our data strongly demonstrated that the S. cerevisiae can survive in the radioactively contaminated aqueous environment despite 4-day exposure to 100 mg L−1 U(VI). The resistance of S. cerevisiae to U(VI) was also investigated under culture conditions in other studies (Ohnuki et al. 2005; Liu et al. 2010). Results showed that cells could even remain 60% viability after 96 h when exposed to 4 × 10−4 mol L−1 (~ 95.2 mg L−1) U(VI) solution. Therefore, within the range of period and uranium concentration, the accumulation of uranium by S. cerevisiae is completely feasible.
Pi release, pH ascent, and U(VI) precipitation
Intracellularlly synthesized polyphosphate granules, lipopolysaccharide, and phospholipid bilayers contribute to the phosphate storage of cells, which can be used in associating metal and alleviating the metal toxicity to cells (Achbergerová and Nahálka 2011). In this study, we found that the Pi concentration in the cell-free sample remained stable after ~ 3-day exposure. However, in the biotic system, Pi was increased significantly within the first 1440 min, and near linearly increased after 2880 min under the stress of 100 mg L−1 U, indicating that Pi release was an active process (Theodorakopoulos et al. 2015). Furthermore, the released Pi could affect the extracellular accumulation of U due to its strong ligand effect on sequestering heavy metals. This response mechanism of Pi released was illustrated as “self-protection” or “detoxification” previously (Theodorakopoulos et al. 2015; Wang et al. 2017a). In the spectra characterizations, the great peak shift of phosphodiester group (77.0 cm−1 of wavenumbers) and phosphate (9.4 cm−1 of wavenumbers) in the IR spectrum, the downshift of P(V) binding energy in XPS spectra, and the high content of phosphorus in EDX spectra also evidenced the uranyl-phosphorus interaction according to chemical bonding (Kushwaha et al. 2012), complexation, and electrostatic interactions (Lu et al. 2013), as well as the release of phosphate from inside of the cells (Zheng et al. 2017).
pH is another important factor that impact the U(VI) mineralization, because it not only controls protonation and deprotonation when interacting with cells, but also influences the adsorption ability and the structure of uranium precipitation (Zhou 1999; Huang et al. 2017). The results of XRD and thermodynamic calculation showed that as the pH value of the solution increased from slight acid to neutral, the chernikovite was gradually detectable, which was ascribed to the ascending pH value that decreased the solubility of UO2HPO4 (with the lowest solubility from pH 6.3–7.0), and resulted in the supersaturation of chernikovite (Haverbeke et al. 1996; Gorman-Lewis et al. 2008; Mehta et al. 2014; Cumberland et al. 2016).
For U(VI), at the beginning of adsorption, the U(VI) concentration in the solution decreased significantly, suggesting that U(VI) was associated with cells rapidly. Meanwhile, the FE-SEM images illustrated the lamellar precipitation distributed on the surface of cells, and the XRD results also suggested that the U(VI) was simply absorbed onto the cells, which was still in the amorphous state. In the IR spectra, the great peak shift of –OH, –CH2, –PO2−, –COO−, –PO43− indicated that the functional groups bonded with U(VI) were mainly hydroxyl, methylene, carboxyl, and phosphate. In contrast, after a long-term exposure, the appearance of crystal substance (Fig. 3) and the disappearance of irregular precipitation (Fig. 7) confirmed the great phase transition of U(VI) from the amorphous to crystalline state. Considering the constant U(VI) concentration in the solution after ~ 3-day exposure (Fig. 2), we suspected that the only approach for U(VI) was to enter cells with metabolism-dependent active transport or metabolism-independent membrane permeability (Pan et al. 2015; Theodorakopoulos et al. 2015).
Cell-mediated crystallization
The XRD results showed that a series of weak diffraction peaks appeared after 2 days of exposure, which was well-characterized as chernikovite (Fig. 3). Thus, it is of significance to evaluate the stability and mobility of materials through further study of the mineral phase. The peak at the 2θ~9.9° shows the primary phosphate reflection of the (001) plane, indicating that the chernikovite structure is perpendicular to the c axis. For ambient conditions, chernikovite exists in a tetragonal structure with a P4/ncc space group. In this structure, four oxygen atoms were coordinated with the uranyl group equatorially to form a distorted octahedron, and each of the octahedrons is connected to the phosphate groups through the sharing of oxygen corners, thus forming a two-dimensional (2D) layered structure. The layered structure is separated by water molecules and randomly disordered hydronium ions (H3O+) (Clavier et al. 2016). Other distinct diffraction peaks at 2θ values of approximately 16.1°, 18.0°, 20.5°, 23.5°, 25.6°, and 27.4° correspond to the (101), (110), (111), (102), (200), and (201) planes, respectively. Table 2 shows that the mean thickness values of chernikovite for the (001), (101), and (110) planes gradually increased within 5 days of exposure according to the Debye-Scherrer equation; however, the thickness of the crystals was then decreased and finally distributed in the range of 16.71 ± 1.46 to 39.73 ± 7.26 nm, suggesting that the micron-sized S. cerevisiae could mediate the transformation of amorphous uranium species into the nano-sized crystalline phase. Moreover, Table 3 gives the crystallinity of tetragonal chernikovite with 20 days of 100 and 200 mg L−1 uranium contact. As the exposure time increases, the crystallinity of chernikovite increased simultaneously, and after 15 days of 100 mg L−1 U(VI) exposure, the chernikovite crystallinity decreased slightly, which is ascribed to the surface change of cells (Fig. 5) and the dissolution of the newly formed crystal. However, the crystallinity of 200 mg L−1 patterns neared 100% after 15 days of exposure and no longer changed. This suggested that the crystal grew gradually after forming in the solid-liquid interface and nano-chernikovite was perfectly crystallized with the extension of time. Previous studies have obtained similar results and demonstrated that the Bacillus thuringiensis could mediate the U to form uramphite particles, while the leakage of cytoplasm could not play a major role in the formation of the U-mineral (Pan et al. 2015). These results showed that living cells could mediate the transformation of U(VI) from ionic U(VI) to amorphous U(VI) and finally precipitate it to nano-chernikovite, with the Pi released by the cells facilitating the uranium nucleation and precipitation in a crystalline state.
Possible process of uranium mineralization by S. cerevisiae
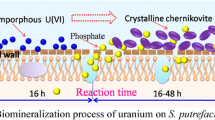
Based on the above analyses, we proposed the possible process of uranium mineralization by S.cerevisiae (Fig. 8). The biomineralization process of U(VI) by S. cerevisiae can be divided into the following three stages: surface adsorption, amorphous precipitation, and crystallization. At the beginning of adsorption, uranyl ions were rapidly attached onto the surface of the cells (Fig. 2a) and then bonded with the carbohydrates and phospholipids according to electrostatic interactions and complexation (Figs. 5 and 6). Meanwhile, the Pi was gradually released from inside the cells (Fig. 2b). With the increasing of the phosphorus concentration and pH value, the solubility of uranyl phosphate decreased (Fig. 4), and the uranium was transformed from the ionic U(VI) state to amorphous uranium precipitate. In the crystallization stage, the amorphous U(VI) disappeared from the cell surface, and the nano-chernikovite was nucleated extracellularly (Fig. 5) with a non-reductive process (Fig. 6). The possible pathway for U(VI) that enters the inside of cells might include active transport and membrane permeability, and the morphology (Fig. 7), size (Table 2), as well as crystallinity (Table 3) of chernikovite were regulated by cells. These results provided us a visualization of yeast bound crystalline uranium deposits. Meanwhile, extracellular mineralization of U(VI) as nano-chernikovite secondary mineral also inspired us to use biomineralization to remediate uranium-contaminated environments technically. However, because the biomineralization is a complex and dynamic process, the mechanism of cell-modulated uranium crystallization and the in situ biomineralization of U by microbes need to be studied further.
Schematic of the proposed U(VI) biomineralization process by S. cerevisiae: (i) surface adsorption, UO22+ was absorbed onto the cell surface; (ii) amorphous precipitation, U(VI) was reacted with the released Pi as well as carbohydrate, and formed the amorphous U(VI) compound; (iii) crystallization, the amorphous U(VI) precipitation was disappeared and entered cells, and the crystalline chernikovite was formed extracellularly. The tetragonal chernikovite, linear UO22+, and its octahedral complex structure were obtained from Clavier et al. (2016), Huang et al. (2017), and Rui et al. (2013), respectively
References
Achbergerová L, Nahálka J (2011) Polyphosphate—an ancient energy source and active metabolic regulator. Microb Cell Factories 10:63. https://doi.org/10.1186/1475-2859-10-63
Asic A, Kurtovic-kozaric A, Besic L, Mehinovic L, Hasic A, Kozaric M, Hukic M, Marjanovicaf D (2017) Chemical toxicity and radioactivity of depleted uranium: the evidence from in vivo and in vitro studies. Environ Res 156:665–673. https://doi.org/10.1016/j.envres.2017.04.032
Barrett CA, Chouyyok W, Speakman RJ, Olsen KB, Addleman RS (2017) Rapid extraction and assay of uranium from environmental surface samples. Talanta 173:69–78. https://doi.org/10.1016/j.talanta.2017.05.052
Bernier-Latmani R, Veeramani H, Vecchia ED, Junier P, Lezama-Pacheco JS, Suvorova EI, Sharp JO, Wigginton NS, Bargar JR (2010) Non-uraninite products of microbial U(VI) reduction. Environ Sci Technol 44:9456–9462. https://doi.org/10.1021/es101675a
Bjørklund G, Christophersen OA, Chirumbolo S, Selinus O, Aaseth J (2017) Recent aspects of uranium toxicology in medical geology. Environ Res 156:526–533. https://doi.org/10.1016/j.envres.2017.04.010
Boyanov MI, Latta DE, Scherer MM, O'Loughlin EJ, Kemner KM (2017) Surface area effects on the reduction of UVI in the presence of synthetic montmorillonite. Chem Geol 464:110–117. https://doi.org/10.1016/j.chemgeo.2016.12.016
Clavier N, Crétaz F, Szenknect S, Mesbah A, Poinssot C, Descostes M, Dacheux N (2016) Vibrational spectroscopy of synthetic analogues of ankoleite, chernikovite and intermediate solid solution. Spectrochim Acta A 156:143–150. https://doi.org/10.1016/j.saa.2015.11.035
Cumberland SA, Douglas G, Grice K, Moreau JW (2016) Uranium mobility in organic matter-rich sediments: a review of geological and geochemical processes. Earth-Sci Rev 159:160–185. https://doi.org/10.1016/j.earscirev.2016.05.010
Du ZJ, Zhang Y, Li ZJ, Chen H, Wang Y, Wang GT, Zou P, Chen HP, Zhang YS (2017) Facile one-pot fabrication of nano-Fe3O4/carboxyl-functionalized baker's yeast composites and their application in methylene blue dye adsorption. Appl Surf Sci 392:312–320. https://doi.org/10.1016/j.apsusc.2016.09.050
Fabrizio P, Longo VD (2003) The chronological life span of Saccharomyces cerevisiae. Aging Cell 2:73–81. https://doi.org/10.1046/j.1474-9728.2003.00033.x
Fleet GH, Manners DJ (1976) Isolation and composition of an alkali-soluble glucan from the cell walls of Saccharomyces cerevisiae. J Gen Microbiol 94:180–192. https://doi.org/10.1099/00221287-94-1-180
Galichet A, Sockalingum GD, Belarbi A, Manfait M (2001) FTIR spectroscopic analysis of Saccharomyces cerevisiae cell walls: study of an anomalous strain exhibiting a pink-colored cell phenotype. FEMS Microbiol Lett 197:179–186. https://doi.org/10.1016/S0378-1097(01)00101-X
Ganesh R, Robinson KG, Reed GD, Sayler GS (1997) Reduction of hexavalent uranium from organic complexes by sulfate- and iron-reducing bacteria. Appl Environ Microbiol 63:4385–4391
Ganesh S, Khan F, Ahmed MK, Velavendan P, Pandey NK, Mudali UK, Pandey SK (2012) Determination of ultra traces amount of uranium in raffinates of Purex process by laser fluorimetry. J Radioanal Nucl Chem 292:331–334. https://doi.org/10.1007/s10967-011-1431-1
Gilson ER, Huang S, Jaffé PR (2015) Biological reduction of uranium coupled with oxidation of ammonium by Acidimicrobiaceae bacterium A6 under iron reducing conditions. Biodegradation 26:475–482. https://doi.org/10.1007/s10532-015-9749-y
Goksungur Y, Uren S, Guvenc U (2005) Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour Technol 96:103–109. https://doi.org/10.1016/j.biortech.2003.04.002
Gorman-Lewis D, Burns PC, Fein JB (2008) Review of uranyl mineral solubility measurements. J Chem Thermodyn 40:335–352. https://doi.org/10.1016/j.jct.2007.12.004
Grenthe I, Fuger J, Konings RJM, Lemire RJ, Muller AB, Nguyen-Trung C, Wanner H (1992) Chemical thermodynamics of uranium. Elsevier, Amsterdam, pp 605–606
Gu BH, Wu WM, Ginder-Vogel MA, Yan H, Fields MW, Zhou JZ, Fendorf S, Criddle CS, Jardine PM (2005) Bioreduction of uranium in a contaminated soil column. Environ Sci Technol 39:4841–4847. https://doi.org/10.1021/es050011y
Haverbeke LV, Vochte R, Springe KV (1996) Solubility and spectrochemical characteristics of synthetic chernikovite and meta-ankoleite. Mineral Mag 60:759–766. https://doi.org/10.1180/minmag.1996.060.402.05
Huang WB, Cheng WC, Nie XQ, Dong FQ, Ding CC Liu MX, Li Z, Hayat T, Alharbi NS (2017) Microscopic and spectroscopic insights into uranium phosphate mineral precipitated by Bacillus mucilaginosus. ACS Earth Space Chem 1:483–492. https://doi.org/10.1021/acsearthspacechem.7b00060
Istok JD, Senko JM, Krumholz LR, Watson D, Bogle MA, Peacock A, Chang YJ, White DC (2004) In situ bioreduction of technetium and uranium in a nitrate-contaminated aquifer. Environ Sci Technol 38:468–475. https://doi.org/10.1021/es034639p
Johnson J (2000) Geochemist’s workbench, database thermocom Lawrence Livermore National Laboratory, Livermore, California V8R6230
Kushwaha S, Sreedhar B, Padmaja P (2012) XPS, EXAFS, and FTIR as tools to probe the unexpected adsorption-coupled reduction of U(VI) to U(V) and U(IV) on Borassus flabellifer-based adsorbents. Langmuir 28:16038–16048. https://doi.org/10.1021/la3013443
Lee SY, Cha WS, Kim J-G, Baik MH, Jung EC, Jeong JT, Kim K, Chung SY, Lee YJ (2014) Uranium(IV) remobilization under sulfate reducing conditions. Chem Geol 370:40–48. https://doi.org/10.1016/j.chemgeo.2014.01.020
Li FZ, Li DM, Li XL, Liao JL, Li SJ, Yang JJ, Yang YY, Tang J, Liu N (2016) Microorganism-derived carbon microspheres for uranium removal from aqueous solution. Chem Eng J 284:630–639. https://doi.org/10.1016/j.cej.2015.09.015
Li JQ, Gong LL, Feng XF, Zhang L, Wu HQ, Yan CS, Xiong YY, Gao HY, Luo F (2017) Direct extraction of U(VI) from alkaline solution and seawater via anion exchange by metal-organic framework. Chem Eng J 316:154–159. https://doi.org/10.1016/j.cej.2017.01.046
Liang K, Ricco R, Doherty CM, Styles MJ, Bell S, Kirby N, Mudie S, Haylock D, Hill AJ, Doonan CJ, Falcaro P (2015a) Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat Commun 6:1–8. https://doi.org/10.1038/ncomms8240
Liang XJ, Hillier S, Pendlowski H, Gray N, Ceci A, Gadd GM (2015b) Uranium phosphate biomineralization by fungi. Environ Microbiol 17:2064–2075. https://doi.org/10.1111/1462-2920.12771
Liang XJ, Csetenyi L, Gadd GM (2016) Uranium bioprecipitation mediated by yeasts utilizing organic phosphorus substrates. Appl Microbiol Biotechnol 100:5141–5151. https://doi.org/10.1007/s00253-016-7327-9
Liu MX, Dong FQ, Yan XY, Zeng WM, Hou LY, Pang XF (2010) Biosorption of uranium by Saccharomyces cerevisiae, and surface interactions under culture conditions. Bioresour Technol 101:8573–8580. https://doi.org/10.1016/j.biortech.2010.06.063
Lovley DR, Phillips EJ (1992) Reduction of uranium by Desulfovibrio desulfuricans. Appl Environ Microbiol 58:850–856
Lu X, Zhou XJ, Wang TS (2013) Mechanism of uranium(VI) uptake by Saccharomyces cerevisiae, under environmentally relevant conditions: batch, HRTEM, and FTIR studies. J Hazard Mater 262:297–303. https://doi.org/10.1016/j.jhazmat.2013.08.051
Macaskie LE, Empson RM, Cheetham AK, Grey CP, Skarnulis AJ (1992) Uranium bioaccumulation by a Citrobacter sp as a result of enzymically mediated growth of polycrystalline HUO2PO4. Science 257:782–784. https://doi.org/10.1126/science.1496397
Martinez RJ, Beazley MJ, Taillefert M, Arakaki AK, Skolnick J, Sobecky PA (2007) Aerobic uranium (VI) bioprecipitation by metal-resistant bacteria isolated from radionuclide and metal-contaminated subsurface soils. Environ Microbiol 9:3122–3133. https://doi.org/10.1111/j.1462-2920.2007.01422.x
Mehta VS, Maillot F, Wang ZM, Catalano JG, Giammar DE (2014) Effect of co-solutes on the products and solubility of uranium(VI) precipitated with phosphate. Chem Geol 364:66–75. https://doi.org/10.1016/j.chemgeo.2013.12.002
Merroun ML, Nedelkova M, Ojeda JJ, Reitz T, Fernández ML, Arias JM, Romero-González M, Selenska-Pobella S (2011) Bio-precipitation of uranium by two bacterial isolates recovered from extreme environments as estimated by potentiometric titration, TEM and X-ray absorption spectroscopic analyses. J Hazard Mater 197:1–10. https://doi.org/10.1016/j.jhazmat.2011.09.049
Moon EM, Ogden MD, Griffith CS, Wilson A, Mata JP (2017) Impact of chloride on uranium(VI) speciation in acidic sulfate ion exchange systems: towards seawater-tolerant mineral processing circuits. J Ind Eng Chem 51:255–263. https://doi.org/10.1016/j.jiec.2017.03.009
Newsome L, Morris K, Trivedi D, Bewsher A, Lloyd JR (2015) Biostimulation by glycerol phosphate to precipitate recalcitrant uranium(IV) phosphate. Environ Sci Technol 49:11070–11078. https://doi.org/10.1021/acs.est.5b02042
Ohnuki T, Ozaki T, Yoshida T, Sakamoto F, Kozai N, Wakai E, Francis AJ, Iefuji H (2005) Mechanisms of uranium mineralization by the yeast Saccharomyces cerevisiae. Geochim Cosmochim Acta 69:5307–5316. https://doi.org/10.1016/j.gca.2005.06.023
Pan XH, Chen Z, Chen FB, Cheng YJ, Lin Z, Guan X (2015) The mechanism of uranium transformation from U(VI) into nano-uramphite by two indigenous Bacillus thuringiensis strains. J Hazard Mater 297:313–319. https://doi.org/10.1016/j.jhazmat.2015.05.019
Pande S, Kumar Ghosh S, Nath S, Praharaj S, Jana S, Panigrahi S, Basu S, Pal T (2006) Reduction of methylene blue by thiocyanate: kinetic and thermodynamic aspects. J Colloid Interf Sci 299:421–427. https://doi.org/10.1016/j.jcis.2006.01.052
Pidchenko I, Kvashnina KO, Yokosawa T, Finck N, Bahl S, Schild D, Polly R, Bohnert E, Rossberg A, Göttlicher J, Dardenne K, Rothe J, Schäfer T, Geckeis H, Vitova T (2017) Uranium redox transformations after U(VI) coprecipitation with magnetite nanoparticles. Environ Sci Technol 51:2217–2225. https://doi.org/10.1021/acs.est.6b04035
Pierrefite-Carle V, Santucci-Darmanin S, Breuil V, Gritsaenko T, Vidaud C, Creff G, Solari PL, Pagnotta S, Al-Sahlanee R, Auwer CD, Carle GF (2017) Effect of natural uranium on the UMR-106 osteoblastic cell line: impairment of the autophagic process as an underlying mechanism of uranium toxicity. Arch Toxicol 91:1903–1914. https://doi.org/10.1007/s00204-016-1833-5
Rui X, Kwon MJ, O’Loughlin EJ, Dunham-Cheatham S, Fein JB, Bunker B, Kemner KM, Boyanov MI (2013) Bioreduction of hydrogen uranyl phosphate: mechanisms and U(IV) products. Environ Sci Technol 47:5668–5678. https://doi.org/10.1021/es305258p
Schulte-Herbrüggen HMA, Semião AJC, Chaurand P, Graham MC (2016) Effect of pH and pressure on uranium removal from drinking water using NF/RO membranes. Environ Sci Technol 50:5817–5824. https://doi.org/10.1021/acs.est.5b05930
Shelobolina ES, Konishi H, Xu HF, Roden EE (2009) U(VI) sequestration in hydroxyapatite produced by microbial glycerol 3-phosphate metabolism. Appl Environ Microbiol 75:5773–5778. https://doi.org/10.1128/AEM.00628-09
Shyla B, Mahadevaiah, Nagendrappa G (2011) A simple spectrophotometric method for the determination of phosphate in soil, detergents, water, bone and food samples through the formation of phosphomolybdate complex followed by its reduction with thiourea. Spectrochim Acta A 78:497–502. https://doi.org/10.1016/j.saa.2010.11.017
Singhal P, Jha SK, Pandey SP, Neogy S (2017) Rapid extraction of uranium from sea water using Fe3O4 and humic acid coated Fe3O4 nanoparticles. J Hazard Mater 335:152–161. https://doi.org/10.1016/j.jhazmat.2017.04.043
Sousa T, Chung AP, Pereira A, Piedade AP, Morais PV (2013) Aerobic uranium immobilization by Rhodanobacter A2-61 through formation of intracellular uranium-phosphate complexes. Metallomics 5:390–397. https://doi.org/10.1039/c3mt00052d
Sun YB, Zhang R, Ding CC, Wang XX, Cheng WC, Chen CL, Wang XK (2016) Adsorption of U(VI) on sericite in the presence of Bacillus subtilis: a combined batch, EXAFS and modeling techniques. Geochim Cosmochim Acta 180:51–65. https://doi.org/10.1016/j.gca.2016.02.012
Theodorakopoulos N, Chapon V, Coppin F, Floriani M, Vercouter T, Sergeant C, Camilleri V, Berthomieu C, Février L (2015) Use of combined microscopic and spectroscopic techniques to reveal interactions between uranium and Microbacterium sp A9, a strain isolated from the Chernobyl exclusion zone. J Hazard Mater 285:285–293. https://doi.org/10.1016/j.jhazmat.2014.12.018
Wadgaonkar SL, Mal J, Nancharaiah YV, Maheshwari NO, Esposito G, Lens PNL (2018) Formation of Se(0), Te(0), and Se(0)-Te(0) nanostructures during simultaneous bioreduction of selenite and tellurite in a UASB reactor. Appl Microbiol Biotechnol 102:2899–2911. https://doi.org/10.1007/s00253-018-8781-3
Wang JL, Chen C (2006) Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol Adv 24:427–451. https://doi.org/10.1016/j.biotechadv.2006.03.001
Wang TS, Zheng XY, Wang XY, Lu X, Shen YH (2017a) Different biosorption mechanisms of uranium(VI) by live and heat-killed Saccharomyces cerevisiae under environmentally relevant conditions. J Environ Radioact 167:92–99. https://doi.org/10.1016/j.jenvrad.2016.11.018
Wang XY, Wang TS, Zheng XY, Shen YH, Lu X (2017b) Isotherms, thermodynamic and mechanism studies of removal of low concentration uranium (VI) by Aspergillus niger. Water Sci Technol 75:2727–2736. https://doi.org/10.2166/wst.2017.055
Watson DB, Wu WM, Mehlhorn T, Tang GP, Earles J, Lowe K, Gihring TM, Zhang GX, Phillips J, Boyanov MI, Spalding BP, Schadt C, Kemner KM, Criddle CS, Jardine PM, Brooks SC (2013) In situ bioremediation of uranium with emulsified vegetable oil as the electron donor. Environ Sci Technol 47:6440–6448. https://doi.org/10.1021/es3033555
Wu WM, Carley J, Gentry T, Ginder-Vogel MA, Fienen M, Mehlhorn T, Yan H, Caroll S, Pace MN, Nyman J, Luo J, Gentile ME, Fields MW, Hickey RF, Gu BH, Watson D, Cirpka OA, Zhou JZ, Fendorf S, Kitanidis PK, Jardine PM, Criddle CS (2006) Pilot-scale in situ bioremedation of uranium in a highly contaminated aquifer. 2. Reduction of U(VI) and geochemical control of U(VI) bioavailability. Environ Sci Technol 40:3986–3995. https://doi.org/10.1021/es051960u
Wu WM, Carley J, Luo J, Ginder-Vogel MA, Cardenas E, Leigh MB, Hwang CC, Kelly SD, Ruan CM, Wu LY, Joy VN, Gentry T, Lowe K, Mehlhorn T, Carroll S, Luo WS, Fields MW, Gu BH, Watson D, Kemner KM, Marsh T, Tiedje J, Zhou JZ, Fendorf S, Kitanidis PK, Jardine PM, Criddle CS (2007) In situ bioreduction of uranium (VI) to submicromolar levels and reoxidation by dissolved oxygen. Environ Sci Technol 41:5716–5723. https://doi.org/10.1021/es062657b
Yan X, Luo XG, Zhao M (2016) Metagenomic analysis of microbial community in uranium-contaminated soil. Appl Microbiol Biotechnol 100:299–310. https://doi.org/10.1007/s00253-015-7003-5
Zheng XY, Wang XY, Shen YH, Lu X, Wang TS (2017) Biosorption and biomineralization of uranium(VI) by Saccharomyces cerevisiae—crystal formation of chernikovite. Chemosphere 175:161–169. https://doi.org/10.1016/j.chemosphere.2017.02.035
Zhou JL (1999) Zn biosorption by Rhizopus arrhizus and other fungi. Appl Microbiol Biotechnol 51:686–693. https://doi.org/10.1007/s002530051453
Zhou P, Gu BH (2005) Extraction of oxidized and reduced forms of uranium from contaminated soils: effects of carbonate concentration and pH. Environ Sci Technol 39:4435–4440. https://doi.org/10.1021/es0483443
Acknowledgements
This study was supported by the Fundamental Research Funds for the Central Universities (Grant Number: lzujbky-2017-it37). The authors also acknowledge Fanrui Kong, Yu Luo, and Chen Zeng, for the great help in designing the cellular graphics, thermodynamic calculations, and English proofreading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Shen, Y., Zheng, X., Wang, X. et al. The biomineralization process of uranium(VI) by Saccharomyces cerevisiae — transformation from amorphous U(VI) to crystalline chernikovite. Appl Microbiol Biotechnol 102, 4217–4229 (2018). https://doi.org/10.1007/s00253-018-8918-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8918-4