Abstract
Acetaminophen (APAP) overdose is currently the leading cause of acute liver disease, but therapeutic treatment strategies are commonly limited. Although dihydroquercetin (DHQ) is an attractive botanical antioxidant, its protective potential for liver disease remains elusive. The present study investigated the protective effects of DHQ against APAP-induced hepatic cytotoxicity. Primary mouse hepatocytes were treated with different concentrations of DHQ followed by APAP administration. Our data showed that DHQ relieved APAP-induced growth inhibition and lactate dehydrogenase (LDH) release in a dose-dependent manner, as well as inhibited APAP-induced necrosis and extracellular signal regulated kinase-c-Jun-N-terminal kinase (ERK-JNK) stress. In addition, reactive oxygen species (ROS) accumulation and mitochondria dysfunction were also reversed by DHQ treatment. Further study revealed that DHQ induced phosphorylation of Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) cascade and thus modulated expression of anti-apoptotic Bcl-2 family proteins. Moreover, DHQ induced autophagy which mediated its protective effects in hepatocytes. The protection was abrogated through pharmacological blockage of autophagy by chloroquine (CQ). These studies demonstrated, for the first time, that DHQ possessed hepatocellular protective effects in the context of APAP-induced cytotoxicity and subsequently revealed that the mechanisms comprised activation of JAK2/STAT3 signaling pathway and autophagy. These altogether highlighted the significant therapeutic potential of this agent during acute liver failure and other types of liver diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dihydroquercetin (DHQ) is a typical flavanone constituent of Pseudotsuga taxifolia, Taxus chinensis, Cedrus deodara, and so on (Kuang et al. 2017). It is described to possess series of biological capacities including antioxidant, anti-inflammation, anti-tumor, and cardiovascular protective properties (Sun et al. 2014). Although barely discernable, DHQ is simultaneously found in the approved drug Silymarin, which as a hepatoprotective product used for centuries in the world (Kroll et al. 2007; Theriault et al. 2000). As the efficacy of Silymarin is always attributed to the major flavonolignan constituents, DHQ is ignored as an impurity and its property to protect against liver injury is rarely investigated (Weidmann 2012). In fact, DHQ is described as “particularly noteworthy” in a study screening the potency of pure compounds derived from Silymarin extract against hepatitis C virus infection (Polyak et al. 2010). Previous studies have also revealed the protective actions of DHQ in tetrachloromethane-induced hepatitis and concanavalin A-induced autoimmune hepatitis (Teselkin et al. 2000; Zhao et al. 2015). However, the precise mechanisms remain elusive and whether DHQ can be explicated as an ideal hepatoprotective agent needs further exploration.
Acetaminophen (APAP) overdose is the leading cause of acute liver disease. Although the pathogenesis of APAP-induced toxicity has been well studied, there are still lack of efficient methods to cure this acute disease clinically (Bunchorntavakul and Reddy 2013). Mechanistically, the majority of APAP undergoes phase II metabolism to nontoxic compounds and then excreted, but some proportions are bioactivated by cytochrome P-450 (CYP450) enzyme system to reactive N-acetyl-p-benzoquinone imine (NAPQI). NAPQI in turn depletes glutathione (GSH) and causes oxidative stress, mitochondrial dysfunction, DNA fragmentation, and finally cell necrosis (Antoine et al. 2008; McGill et al. 2012; Jaeschke et al. 2012). Currently, the best therapeutic option for APAP overdose is N-acetyl-l-cysteine (NAC), a cysteine prodrug which supports the detoxification of NAPQI via restoring GSH pool (Saito et al. 2010). However, NAC possesses several side effects and it must be administered very early considering its short-time window (Athuraliya and Jones 2009). Further research for alternative and efficacious antidotes against APAP toxicity is warranted.
In the present study, we investigated the protective function of DHQ on APAP-induced hepatic cytotoxicity using primary mouse hepatocytes. Our results demonstrated that DHQ effectively ameliorated APAP-induced hepatocellular necrosis via free radical elimination and mitochondrial protection. Further investigation revealed an involvement of Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) cascade and autophagy in DHQ-mediated hepatocellular protective actions. Thus, DHQ was proved to be a promising therapeutic candidate for liver diseases.
Materials and methods
Reagents and antibodies
Dihydroquercetin and acetaminophen were purchased from MedChem Express (Shanghai, China); NAC was obtained from Beyotime Institute of Biotechnology (Jiangsu, China); and chloroquine (CQ) and rapamycin were obtained from Sigma-Aldrich (St. Louis, MO, USA). The antibodies including anti-β-Actin, anti-phospho-JNK (Thr183/Tyr204), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), anti-phospho-JAK2, anti-phospho-STAT3 (Tyr705), anti-Bax, anti-Bcl-2, anti-LC3B, and anti-p62 were all purchased from Cell Signaling Technology (Danvers, MA, USA) (1:1000 dilutions). The anti-CYP2E1 antibody was purchased from Sangon Biotech (Shanghai, China) (1:300 dilutions). Horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from MR Biotech (Shanghai, China).
Primary mouse hepatocyte isolation and cell culture
Primary mouse hepatocytes were isolated from C57BL/6 mice by retrograde perfusion (a two-step isolation procedure) with 0.05% Collagenase IV (Yeasen Institute of Biotechnology, Shanghai, China); then, the isolated cells were purified with 40% Percoll (GE Healthcare Life Sciences, Shanghai, China). Cells were suspended in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin and then seeded in collagen-coated plates subsequently. Hepatoma-derived HepG2 cell line was obtained from Cell Bank of Chinese Academy of Science, Shanghai branch (Shanghai, China), and cultured in complete DMEM. After plating overnight, the medium was replaced with serum-free DMEM medium and proposed compounds were added for further detection.
Cell viability and LDH release assay
Cells were seeded in 96-well plates overnight; different concentrations of DHQ (containing 0.1% DMSO) or NAC were added 1 h before APAP (10 mM) treatment. Cell viability was measured by 3-(4, 5-dimethylthizaol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. LDH release was detected via CytoTox 96® Non-Radio Cytotoxicity Assay (Promega, WI, USA) according to the manufacture’s instruction.
Determination of necrosis
After indicated treatment, cells were doubled stained with Hoechst 33342 and propidium iodide (PI) dye (Beyotime, Jiangsu, China) for 15 min and then observed by a fluorescent microscopy (Zeiss Inc., MN, USA). The proportion of necrosis cells was quantified via ImageJ software (National Institutes of Health, MD, USA).
Analysis of intracellular GSH content
GSH concentration was measured with a GSH assay kit (Nanjing JianCheng Bioengineering Institute, Jiangsu, China). After treatment, cells were lysed and subjected to a cycling reaction; the GSH levels were determined by spectrophotometry (Sigma, MO, USA). The concentration of total protein was measured by bicinchoninic acid (BCA) protein assay kit.
ROS detection
2′,7′-Dichlorofluorescein diacetate (DCFH-DA) assay kit (Beyotime, Jiangsu, China) was used for detection of intracellular ROS levels. Primary mouse hepatocytes were seeded in a 96-well black plate and were incubated overnight for attachment. After treatment with intensive drugs, the medium was replaced with serum-free DMEM containing fluorescent probes for 30 min at 37 °C. The DCF fluorescent intensity was detected at Ex./Em. = 488/525 nm using a fluorescence spectrophotometer (Tecan). Cells were stained with MitoSox™ Red Dye (Beyotime, Jiangsu, China) and Hoechst 33342 for the observation by confocal microscopy (Carl Zeiss LSM710, Carl Zeiss, Germany) following the manufacturer’s instructions. Relative fluorescent intensity was quantified by ImageJ software.
MMP measurement
MMP was measured via JC-1 assay kit (Beyotime, Jiangsu, China). After treatment, cells were stained with JC-1 at 37 °C for 30 min, according to the manufacture’s protocol, and then photographed using fluorescence microscopy (Zeiss Inc., MN, USA). JC-1 aggregates fluorescent red, representing normal membrane potential, whereas monomers green, indicating the depolarized mitochondria. The fluorescent intensities were calculated using ImageJ software.
Autophagy detection via confocal microscopy
Cells were seeded in glass bottom culture dishes. After indicated treatments, cells were disposed with Cyto-ID® Autophagy Detection Kit (Enzo Life Science, Inc., Farmingdale, NY, USA) following the manufacture’s instruction and then observed via confocal microscopy.
Western Blot analysis
Equivalent amounts of protein extracted from primary hepatocytes were loaded and separated by sodium dodecyl sulfate (SDS) polyacrylamide gels. The protein was subsequently transferred to polyvinylidene difluoride (PVDF) membranes and blocked with 5% BSA in Tris-buffered saline (containing 0.1% Tween 20) (TBST). The blots were then probed with primary antibodies for 4 h followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies for another 2 h in room temperature. The membranes were visualized by enhanced chemiluminescent detection kit (Pierce, Rockford, IL, USA). Intensity of protein bands was quantified by ImageJ software.
Statistical analysis
Data was analyzed using one-way analysis of variance (ANOVA) or two-tailed Student’s test and presented as mean ± standard deviations (S.D.). Values of P < 0.05 were considered statistically significant.
Results
DHQ inhibited APAP-induced cytotoxicity in hepatocytes
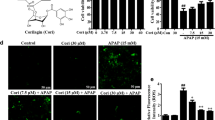
Cultured primary mouse hepatocytes were pretreated with different concentrations of DHQ for 1 h and then incubated with APAP (10 mM) for another 24 h. The result depicted a potent suppression of hepatocytes triggered by APAP, whereas DHQ prevented the cytotoxicity in a concentration-dependent manner (Fig. 1a). NAC was used here as a positive control. Meanwhile, APAP treatment increased the percentage of LDH release in hepatocytes, which was significantly ameliorated by co-treatment with DHQ (Fig. 1b). Cytotoxicity dose-response experiment showed that DHQ was well tolerated by primary mouse hepatocytes up to 200 μM (Fig. S1A). Similar protection of DHQ was also detected in HepG2 cell lines (Fig. S1B).
Dihydroquercetin inhibited APAP-induced cytotoxicity in hepatocytes. a Primary mouse hepatocytes were pretreated with different concentrations of DHQ or NAC (2.5 mM) for 1 h and then incubated with APAP (10 mM) for another 24 h. Cell viability was measured via MTT assay. b Primary mouse hepatocytes were incubated with DHQ, NAC, and/or APAP (10 mM); the LDH release was quantified using LDH assay. Values were expressed as mean ± S.D. (n = 3). **P < 0.01, *P < 0.05 compared to APAP; ## P < 0.01 compared to APAP
These results suggested that DHQ could prevent APAP-induced cellular injury both in primary mouse hepatocytes and in hepatoma carcinoma cells.
DHQ relieved APAP-induced necrosis and suppressed ERK/JNK stress responses
Exposure of hepatocytes to APAP led to a remarkable increase in propidium iodide (PI)-positive cell numbers, indicating cell death and DNA damage, while DHQ dramatically relieved the extent of PI staining (Fig. 2a, b). In addition, APAP treatment also caused a significant phosphorylated activation of c-Jun-N-terminal kinase (JNK) but this effect was drastically attenuated by DHQ in a dose-dependent manner (Fig. 2c). The densitometric analysis of p-JNK1/2 protein bands confirmed the above alternation (Fig. 2d). Similarly, extracellular signal regulated kinase (ERK) was activated as a consequence of APAP toxicity, which was reversed by co-treatment with DHQ (Fig. 2e, f).
Dihydroquercetin relieved APAP-induced necrosis and suppressed ERK/JNK stress responses. a Primary mouse hepatocytes were pretreated with DHQ (100 μM) for 1 h and then incubated with APAP (5 mM) for another 12 h. After treatment, cells were subjected to Hoechst 33342 and PI staining to detect cell necrosis. b The statistics of PI-positive cells. Primary mouse hepatocytes were pretreated with DHQ (0–100 μM) for 1 h, followed by treatment with APAP (5 mM) for 24 h. c Western Blot analysis for phosphor-JNK1/2, β-Actin as a lading control. d Densitometric values of p-JNK1/2 were qualified by ImageJ software. e Western Blot analysis for phosphor-Erk1/2, β-Actin as a lading control. f Densitometric values of p-Erk1/2. Values were expressed as mean ± S.D. (n = 3). **P < 0.01, *P < 0.05 compared to APAP, ## P < 0.01 compared to normal
These data further confirmed the hepato-protective action of DHQ in the context of APAP toxicity.
DHQ prevented APAP-triggered ROS accumulation and mitochondria dysfunction
GSH depletion by NAPQI was the early step of redox balance disruption and could result in further mitochondrial dysfunction (Lee et al. 2015). APAP (5 mM) treatment for 6 h caused a consumption of GSH in hepatocytes, not DHQ (100 μM) but NAC (2.5 mM) restored GSH levels (Fig. S2A). Similarly, cytochrome P450 (CYP)2E1, the major CYP form that mediated the metabolism of APAP, remained unchanged after the addition of DHQ (Fig. S2B, C). These results indicated that the protection of DHQ was not simply due to the modulation of APAP metabolism. We next examined the function of DHQ on APAP-triggered oxidative stress. The intracellular ROS levels were measured via quantification of fluorescent DCFH by a spectrometer. Hepatocytes showed a marked ROS accumulation after 24 h treatment of 5 mM APAP, while DHQ ameliorated cellular ROS levels in a dose-depended manner (Fig. 3a). To confirm the anti-oxidative function of DHQ, the superoxide production was measured with MitoSOX Red dye and analyzed by confocal microscopy. Similarly, MitoSOX Red-derived fluorescence in APAP-treated cells distinctively increased, which was significantly attenuated by DHQ through eliminating ROS (Fig. 3b, c). Mitochondria dysfunction was a major contributor of ROS, and early oxidative stress could further amplify mitochondria damage (Lin et al. 2014). The result exhibited that JC-1 dye formed red fluorescent aggregates in normal cells, whereas APAP treatment caused an increase of JC-1 green monomer and a decrease of JC-1aggregates, indicating the loss of mitochondria membrane potential (MMP). As expected, DHQ improved the proportion of red fluorescence via reversing the depolarization of MMP (Fig. 3d, e).
Dihydroquercetin prevented APAP-triggered ROS accumulation and mitochondria dysfunction. a Primary mouse hepatocytes were incubated with DHQ (0~200 μM), NAC (2.5 mM), and/or APAP (10 mM) for 24 h; the intracellular ROS levels were measured by DCFH-DA assay. b Hepatocytes were incubated with DHQ (100 μM) for 1 h, followed by APAP (5 mM) treatment for 12 h; the cells were then stained by MitoSOX and Hoechst 33342 to visualize intracellular ROS. c The statistics of MitoSOX-positive staining compared to Hoechst 33342. d Hepatocytes were treated with DHQ (100 μM) and/or APAP (5 mM) for 12 h and then stained by JC-1 to detect the cellular MMP. e The statistics of JC-1 aggregates compared to JC-1 monomers. Values were expressed as mean ± S.D. (n = 3). **P < 0.01, *P < 0.05; ## P < 0.01 compared to APAP
Altogether, these data showed that DHQ could prevent against APAP-triggered ROS accumulation and mitochondrial dysfunction.
DHQ activated JAK2/STAT3 pathway in APAP-treated hepatocytes
Multiple evidence showed that JAK2/STAT3 cascade was closely related to the regulation of oxidative stress responses and mitochondrial function (Cai et al. 2016; Yu et al. 2011). The action of DHQ on JAK2/STAT3 signaling against APAP toxicity was further studied by Western Blot analysis. The expression of p-JAK2 and p-STAT3 was slightly increased after APAP treatment, while DHQ pretreatment further increased JAK2 and STAT3 phosphorylation levels (Fig. 4a–d). As STAT3 was an anti-apoptotic factor regulating the expression of Bcl-2 family protein (Tian et al. 2011), the pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-2 were next detected. Our results showed that the expression of Bax was increased by APAP treatment, whereas the expression of Bcl-2 was decreased. But these changes were dramatically attenuated in the presence of DHQ (Fig. 4e–h).
Dihydroquercetin activated JAK2/STAT3 pathway in APAP-treated hepatocytes. Primary mouse hepatocytes were incubated with DHQ (0~100 μM) and/or APAP (5 mM) for 24 h; Western Blot analysis for a phosphor-JAK2, c phosphor-STAT3, e Bax, and g Bcl-2; and β-Actin as a lading control. Densitometric values of b p-JAK2, d p-STAT3, f Bax, and h Bcl-2. Values were expressed as mean ± S.D. (n = 3). **P < 0.01, *P < 0.05 compared to APAP, ## P < 0.01 compared to normal
Collectively, these data suggested that the protective effects of DHQ were partly the result of activating JAK2/STAT3 cascade.
DHQ attenuated APAP toxicity via activation of autophagy
Previous studies revealed that autophagy served as an adaptive response to counteract APAP-induced liver injury, mainly via autophagic removal of damaged mitochondria as well as toxic protein aggregates (Ni et al. 2012, 2016). We next investigated whether autophagy was participated in the protective effect of DHQ against APAP toxicity. As evidenced by Fig. 5a, a punctuated pattern of Cyto-ID labeled autophagic vacuoles was detected via confocal microscopy after DHQ treatment. Further autophagy induction was ensured by Western Blot analysis of light chain 3 (LC3) expression (Fig. 5b). In consistent with previous studies, APAP treatment induced the elevation of LC3-II levels and Cyto-ID-positive vacuoles. Our results showed that DHQ further increased LC3-II levels and induced the formation of autophagosomes (Fig. 5c–e). To determine the role of autophagy in DHQ-mediated hepatoprotection, CQ was applied to pharmacologically suppress autophagy. As expected, the protective function of DHQ was significantly compromised when autophagy was inhibited (Fig. 6).
Dihydroquercetin attenuated APAP toxicity via activation of autophagy. a Primary mouse hepatocytes were incubated with DHQ (100 μM) or rapamycin (50 nM) for 24 h; then, cells were stained with Cyto-ID® Green autophagy dye and Hoechst 33342. b Primary mouse hepatocytes were incubated with various concentrations of DHQ (0~200 μM) for 24 h, Western Blot analysis for LC3, and β-Actin as a lading control. c Hepatocytes were treated with DHQ (100 μM) and/or APAP (5 mM) for 24 h and then detected for autophagy by confocal microscopy. d Primary mouse hepatocytes were pretreated with DHQ (0~100 μM) for 1 h and then treated with APAP (5 mM) for another 24 h, Western Blot analysis for LC3, and β-Actin as a lading control. e Densitometric values of LC3. Values were expressed as mean ± S.D. (n = 3). **P < 0.01, *P < 0.05 compared to APAP
Suppression of autophagy blocked the effect of dihydroquercetin against APAP toxicity. Primary mouse hepatocytes were pretreated with DHQ (100 μM) and/or autophagy inhibitor CQ (10 μM) for 1 h and then treated with APAP (5 mM) for another 24 h. a Cell viability was measured via MTT assay. b LDH release by LDH assay. c Western Blot analysis for Bax and p62 and β-Actin as a lading control. Values were expressed as mean ± S.D. (n = 3). *P < 0.05 compared to APAP; ## P < 0.01, ## P < 0.01 compared to normal
Consequently, autophagy induced by DHQ was likely participated in the protection against APAP-induced hepatotoxicity.
Discussion
Dihydroquercetin (DHQ), also known as taxifolin, is a widely distributed flavonoid and is attractive for its potent antioxidant property (Xie et al. 2017). Although this compound exhibits multiple healthy benefits such as anti-inflammatory, anti-allergic, antibacterial, anticancer activity, and so on (Kuang et al. 2017), its protective potential for liver injury remains neglected. To our best knowledge, this study is unique in determining the hepatocellular protective function of DHQ in the context of APAP-induced cytotoxicity. This botanical extract DHQ displayed potent activity against APAP-mediated lethal cellular injury as evidenced by cell viability and LDH release assay. In consistent with these results, further PI staining showed that DHQ significantly decreased the degree of APAP-triggered hepatocyte necrosis. Besides, we also found that DHQ could provide protection against palmitic acid and poly-amidoamine (PAMAM) dendrimer-induced hepatocyte injury (Fig. S3). Given its safety and easy availability, DHQ may be a potential therapeutic candidate for liver diseases.
APAP overdose-induced hepatotoxicity is the most common acute liver injury in the world, but this severe fatal disease lacks efficient antidotes. Mitogen-activated protein kinases (MAPK) such as JNK and ERK are reported to participate in the development of hepatic damage and amplify APAP toxicity (Latchoumycandane et al. 2007; Xie et al. 2016; McGarry et al. 2015). Activation of JNK is an important mediator of APAP toxicity, by which ER stress could be conveyed to mitochondria triggering further necrosis (Zhang et al. 2016; Saberi et al. 2014). In this study, exposure to APAP induced activation of ERK-JNK cascade in cultured hepatocytes, which was attenuated by DHQ co-treatment. These results confirmed the hepatocellular protective function of this plant-derived antioxidant DHQ in vitro.
Previous studies have demonstrated that the metabolic product NAPQI contributes to the major pathogenesis of APAP overdose-induced liver injury. GSH depletion by NAPQI renders uncontrolled generation of reactive oxygen species and mitochondria dysfunction (Pang et al. 2016). Oxidative stress is proven to be the key factor of APAP-induced hepatotoxicity. Consistently, mitochondrial membrane potential collapse further amplifies oxidative stress signaling pathways and leads to the necrotic cell death (Wang et al. 2016). In the current study, pretreatment with DHQ did not inhibit the consumption of GSH or inhibit the CYP2E1 expression, indicating that its protection was not mediated via modulation of APAP metabolism. Instead, DHQ abrogated APAP-induced ROS accumulation in hepatocytes, suggesting that the hepatoprotective properties of DHQ were partly ascribed to its antioxidant ability. Moreover, DHQ restored the depolarization of mitochondria membrane potential, which further blocked mitochondrial oxidative stress cascade.
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway is an evolutionarily conserved pathway and is well studied in tissue homoeostasis modulation (Chen et al. 2017). It participates in different cellular processes, such as growth, survival, development, and differentiation (Mahmoud and Abd EI-Twab 2017). Previous studies have demonstrated that JAK2/STAT3 pathway plays a vital role both in myocardial and hepatic ischemia/reperfusion injury (Yu et al. 2011). Activated STAT3 not only exerts antioxidant property, but also stabilizes mitochondrial membrane (Wu et al. 2016). Furthermore, STAT3 is also recognized as an anti-apoptotic factor as it regulates several apoptosis-related genes, such as Bcl-2 and Bcl-xL (Tian et al. 2011). During cellular stress, JAK2/STAT3 could be phosphorylated thus induce protective and survival signals and restrict the extent of damage (Boengler et al. 2008). The present study showed that APAP treatment caused a minor increase of p-JAK2/p-STAT3 expression and probably triggered by sensing cellular oxidative stress, while pretreatment of DHQ significantly increased the phosphorylation of JAK2/STAT3 cascade. This finding could be supported by a previous study detecting the potential molecular target of DHQ via silico screening and the docking score of DHQ against JAK2 is 0.82 (Oi et al. 2012). Further study demonstrated that expression of anti-apoptotic protein Bcl-2 was elevated whereas the pro-apoptotic protein Bax decreased. These results suggested that the protective effects of DHQ might be related to the activation of the JAK2/STAT3 pathway.
Autophagy is a highly controlled metabolic process which is induced by different stress conditions and is responsible for bulk proteolytic degradation (Zhang et al. 2017). Accumulating evidence supports that autophagy plays an important role in liver physiology and pathology. Selective autophagic degradation of damaged cellular component contributes to quality control of proteins and organisms in hepatocytes, whereas malfunction of autophagy leads to pathological problems (Ueno and Komatsu 2017; Li et al. 2015). Recent studies show that autophagy is activated in response of APAP treatment and serves as an adaptive mechanism to protect against APAP-induced liver injury (Ni et al. 2012). This protection is mediated mainly via removal of damaged mitochondria and toxic APAP-adducted proteins (Williams et al. 2015; Baulies et al. 2015). Autophagy can also relieve oxidative stress and ER stress (Zhang et al. 2016). We next investigated whether autophagy was involved in the protection of DHQ against APAP toxicity. Our results demonstrated that APAP treatment induced elevation of LC3-II protein levels as well as Cyto-ID-positive autophagy vacuoles, while DHQ pretreatment further enhanced the formation of autophagosomes. Suppression of autophagy by CQ remarkably abrogated DHQ-mediated hepatocyte protection, as shown by cell viability and LDH release experiment. These results implied the participation of autophagy in the protective effects of DHQ on APAP-induced cytotoxicity. But the exact mechanisms need further study.
In summary, this is the first report demonstrating that DHQ could be a novel hepatocellular protective candidate against APAP-induced lethal hepatotoxicity. The protective action of DHQ is concerned with its capacity of scavenging oxidative stress, restoring mitochondrial function, activating JAK2/STAT3 cascade, and enhancing autophagy (Fig. 7). Further exploration of DHQ as a therapeutic agent for liver diseases is well worthy. However, the formulation or structural modification of this botanical compound must be solved, because of its poor solubility and bioactivity (Zu et al. 2012). This could also explain why there is a dearth of in vivo assessment of DHQ among existing studies (Weidmann 2012).
Potential mechanisms underlying the protective effects of dihydroquercetin in APAP-induced hepatotoxicity. Metabolic NAPQI depletes liver GSH, leading to the accumulation of oxidative stress and mitochondrial dysfunction. DHQ, as a scavenger of ROS, relieves the ERK/JNK activation caused by oxidative stress. JAK2/STAT3 signaling pathways are involved in the protection of DHQ. Moreover, autophagy is activated by DHQ and subsequently ameliorates APAP-induced hepatotoxicity
References
Antoine DJ, Williams DP, Park BK (2008) Understanding the role of reactive metabolites in drug-induced hepatotoxicity: state of the science. Expert Opin Drug Metab Toxicol 4(11):1415–1427. https://doi.org/10.1517/17425255.4.11.1415
Athuraliya TN, Jones AL (2009) Prolonged N-acetylcysteine therapy in late acetaminophen poisoning associated with acute liver failure—a need to be more cautious? Crit Care 13(3):144. https://doi.org/10.1186/cc7800
Baulies A, Ribas V, Núñez S, Torres S, Alarcón-Vila C, Martínez L, Suda J, Ybanez MD, Kaplowitz N, García-Ruiz C, Fernández-Checa (2015) JC lysosomal cholesterol accumulation sensitizes to acetaminophen hepatotoxicity by impairing mitophagy. Sci Rep 5(1):18017. https://doi.org/10.1038/srep18017
Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R (2008) The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther 120(2):172–185. https://doi.org/10.1016/j.pharmthera.2008.08.002
Bunchorntavakul C, Reddy KR (2013) Acetaminophen-related hepatotoxicity. Clin Liver Dis 17(4):587–607. https://doi.org/10.1016/j.cld.2013.07.005
Cai W, Yang X, Han S, Guo H, Zheng Z, Wang H, Guan H, Jia Y, Gao J, Yang T, Zhu X, Hu D (2016) Notch1 Pathway Protects against Burn-Induced Myocardial Injury by Repressing Reactive Oxygen Species Production through JAK2/STAT3 Signaling. Oxid Med Cell Longev 2016:5638943. https://doi.org/10.1155/2016/5638943
Chen W, Zhang X, Fang J, Zai W, Luan J, Li Y, Wang S, Chen Q, Wang Y, Liang Y, Ju D (2017) Tethering Interleukin-22 to apolipoprotein A-I ameliorates mice from acetaminophen-induced liver injury. Theranostics 7(17):4135–4148. https://doi.org/10.7150/thno.20955
Jaeschke H, McGill MR, Ramachandran A (2012) Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev 44(1):88–106. https://doi.org/10.3109/03602532.2011.602688
Kroll DJ, Shaw HS, Oberlies NH (2007) Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther 6(2):110–119. https://doi.org/10.1177/1534735407301825
Kuang, H, Tang Z, Zhang C, Wang Z, Li W, Yang C, Wang Q, Yang B, Kong A (2017) Taxifolin activates the Nrf2 anti-oxidative stress pathway in mouse skin epidermal JB6 P+ cells through epigenetic modifications. Int J Mol Sci 18(7). doi:https://doi.org/10.3390/ijms18071546
Latchoumycandane C, Goh CW, Ong MM, Boeisterli UA (2007) Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology 45(2):412–421. https://doi.org/10.1002/hep.21475
Lee KK, Imaizumi N, Chamberiand SR, Alder NN, Boelsterli UA (2015) Targeting mitochondria with methylene blue protects mice against acetaminophen-induced liver injury. Hepatology 61(1):326–336. https://doi.org/10.1002/hep.27385
Li Y, Zeng X, Wang S, Sun Y, Wang Z, Fan J, Song P, Ju D (2015) Inhibition of autophagy protects against PAMAM dendrimers-induced hepatotoxicity. Nanotoxicology 9(3):344–355. https://doi.org/10.3109/17435390.2014.930533
Lin Z, Wu F, Lin S, Pan X, Jin L, Lu T, Shi L, Wang Y, Xu A, Li X (2014) Adiponectin protects against acetaminophen-induced mitochondrial dysfunction and acute liver injury by promoting autophagy in mice. J Hepatol 61(4):825–831. https://doi.org/10.1016/j.jhep.2014.05.033
Mahmoud AM, Abd EI-Twab SM (2017) Caffeic acid phenethyl ester protects the brain against hexavalent chromium toxicity by enhancing endogenous antioxidants and modulating the JAK/STAT signaling pathway. Biomed Pharmacother 91:303–311. https://doi.org/10.1016/j.biopha.2017.04.073
McGarry DJ, Chakravarty P, Wolf CR, Henderson CJ (2015) Altered protein S-glutathionylation identifies a potential mechanism of resistance to acetaminophen-induced hepatotoxicity. J Pharmacol Exp Ther 355(2):137–144. https://doi.org/10.1124/jpet.115.227389
McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H (2012) The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest 122(4):1574–1583. https://doi.org/10.1172/JCI59755
Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX (2012) Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology 55(1):222–232. https://doi.org/10.1002/hep.24690
Ni HM, McGill MR, Chao X, Du K, Williams JA, Xie Y, Jaeschke H, Ding WX (2016) Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J Hepatol 65(2):354–362. https://doi.org/10.1016/j.jhep.2016.04.025
Oi N, Cheng H, Ok Kim M, Lubet RA, Bode AM, Dong Z (2012) Taxifolin suppresses UV-induced EGFR and PI3-K. Cancer Prev Res (Phila) 5(9):1103–1114. https://doi.org/10.1158/1940-6207
Pang C, Zheng Z, Shi L, Sheng Y, Wen H, Wang Z, Ji L (2016) Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic Biol Med 91:236–246. https://doi.org/10.1016/j.freeradbiomed.2015.12.024
Polyak SJ, Morishima C, Lohmann V, Pal S, Lee DY, Liu Y, Graf TN, Oberlies NH (2010) Identification of hepatoprotective flavonolignans from silymarin. Proc Natl Acad Sci U S A 107(13):5995–5999. https://doi.org/10.1073/pnas.0914009107
Saberi B, Ybanez MD, Johnson HS, Gaarde WA, Han D, Kaplowitz N (2014) Protein kinase C (PKC) participates in acetaminophen hepatotoxicity through c-jun-N-terminal kinase (JNK)-dependent and -independent signaling pathways. Hepatology 59(4):1543–1554. https://doi.org/10.1002/hep.26625
Saito C, Zwingmann C, Jaeschke H (2010) Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 51(1):246–254. https://doi.org/10.1002/hep.23267
Sun X, Chen RC, Yang ZH, Sun GB, Wang M, Ma XJ, Yang LJ, Sun XB (2014) Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem Toxicol 63:221–232. https://doi.org/10.1016/j.fct.2013.11.013
Teselkin YO, Babenkova IV, Kolhir VK, Baginskaya AI, Tjukavkina NA, Kolesnik YA, Selivanova IA, Eichholz AA (2000) Dihydroquercetin as a means of antioxidative defence in rats with tetrachloromethane hepatitis. Phytother Res 14(3):160–162. https://doi.org/10.1002/(SICI)1099-1573(200005)14:3<160::AID-PTR555>3.0.CO;2-Y
Theriault A, Wang Q, Van Iderstine SC, Chen B, Franke AA, Adeli K (2000) Modulation of hepatic lipoprotein synthesis and secretion by taxifolin, a plant flavonoid. J Lipid Res 41(12):1969–1979
Tian Y, Zhang W, Xia D, Modi P, Liang D, Wei M (2011) Postconditioning inhibits myocardial apoptosis during prolonged reperfusion via a JAK2-STAT3-Bcl-2 pathway. J Biomed Sci 18(1):53. https://doi.org/10.1186/1423-0127-18-53
Ueno T, Komatsu M (2017) Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol 14(3):170–184. https://doi.org/10.1038/nrgastro.2016.185
Wang L, Zhang S, Cheng H, Lv H, Cheng G, Ci X (2016) Nrf2-mediated liver protection by esculentoside A against acetaminophen toxicity through the AMPK/Akt/GSK3β pathway. Free Radic Biol Med 101:401–412. https://doi.org/10.1016/j.freeradbiomed.2016.11.009
Weidmann AE (2012) Dihydroquercetin: more than just an impurity? Eur J Pharmacol 684(1–3):19–26. https://doi.org/10.1016/j.ejphar.2012.03.035
Williams JA, Ni HM, Haynes A, Manley S, Li Y, Jaeschke H, Ding WX (2015) Chronic deletion and acute knockdown of Parkin have differential responses to acetaminophen-induced mitophagy and liver injury in mice. J Biol Chem 290(17):10934–10946. https://doi.org/10.1074/jbc.M114.602284
Wu J, Guo W, Lin SZ, Wang ZJ, Kan JT, Chen SY, Zhu YZ (2016) Gp130-mediated STAT3 activation by S-propargyl-cysteine, an endogenous hydrogen sulfide initiator, prevents doxorubicin-induced cardiotoxicity. Cell Death Dis 7(8):e2339. https://doi.org/10.1038/cddis.2016.209
Xie W, Wang M, Chen C, Zhang X, Melzig MF (2016) Hepatoprotective effect of isoquercitrin against acetaminophen-induced liver injury. Life Sci 152:180–189. https://doi.org/10.1016/j.lfs.2016.04.002
Xie X, Feng J, Kang Z, Zhang S, Zhang L, Zhang Y, Li X, Tang Y (2017) Taxifolin protects RPE cells against oxidative stress-induced apoptosis. Mol Vis 23:520–528
Yu HC, Qin HY, He F, Wang L, Fu W, Liu D, Guo FC, Liang L, Dou KF, Han H (2011) Canonical notch pathway protects hepatocytes from ischemia/reperfusion injury in mice by repressing reactive oxygen species production through JAK2/STAT3 signaling. Hepatology 54(3):979–988. https://doi.org/10.1002/hep.24469
Zhang E, Yin S, Song X, Fan L, Hu H (2016) Glycycoumarin inhibits hepatocyte lipoapoptosis through activation of autophagy and inhibition of ER stress/GSK-3-mediated mitochondrial pathway. Sci Rep 6(1):38138. https://doi.org/10.1038/srep38138
Zhang X, Fan J, Wang S, Li Y, Wang Y, Li S, Luan J, Wang Z, Song P, Chen Q, Tian W, Ju D (2017) Targeting CD47 and autophagy elicited enhanced antitumor effects in non-small cell lung cancer. Cancer Immunol Res 5(5):363–375. https://doi.org/10.1158/2326-6066.CIR-16-0398
Zhao M, Chen J, Zhu P, Fujino M, Takahara T, Toyama S, Tomita A, Zhao L, Yang Z, Hei M, Zhong L, Zhuang J, Kimura S, Li XK (2015) Dihydroquercetin (DHQ) ameliorated concanavalin A-induced mouse experimental fulminant hepatitis and enhanced HO-1 expression through MAPK/Nrf2 antioxidant pathway in RAW cells. Int Immunopharmacol 28(2):938–944. https://doi.org/10.1016/j.intimp.2015.04.032
Zu S, Yang L, Huang J, Ma C, Wang W, Zhao C, Zu Y (2012) Micronization of taxifolin by supercritical antisolvent process and evaluation of radical scavenging activity. Int J Mol Sci 13(7):8869–8881. https://doi.org/10.3390/ijms13078869
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81573332 and 81773620) and Special Research Foundation of State Key Laboratory of Medical Genomics and Collaborative Innovation Center of Systems Biomedicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal care and experiment procedures complied with the Animal Management Rules of China and were approved by the Animal Care Committee of Fudan University.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 591 kb)
Rights and permissions
About this article
Cite this article
Zai, W., Chen, W., Luan, J. et al. Dihydroquercetin ameliorated acetaminophen-induced hepatic cytotoxicity via activating JAK2/STAT3 pathway and autophagy. Appl Microbiol Biotechnol 102, 1443–1453 (2018). https://doi.org/10.1007/s00253-017-8686-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8686-6